Do Not Lose Your Head over the Unequal Regeneration Capacity in Prolecithophoran Flatworms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection, Determination and Maintenance
2.2. Amputation and In Vivo Observation
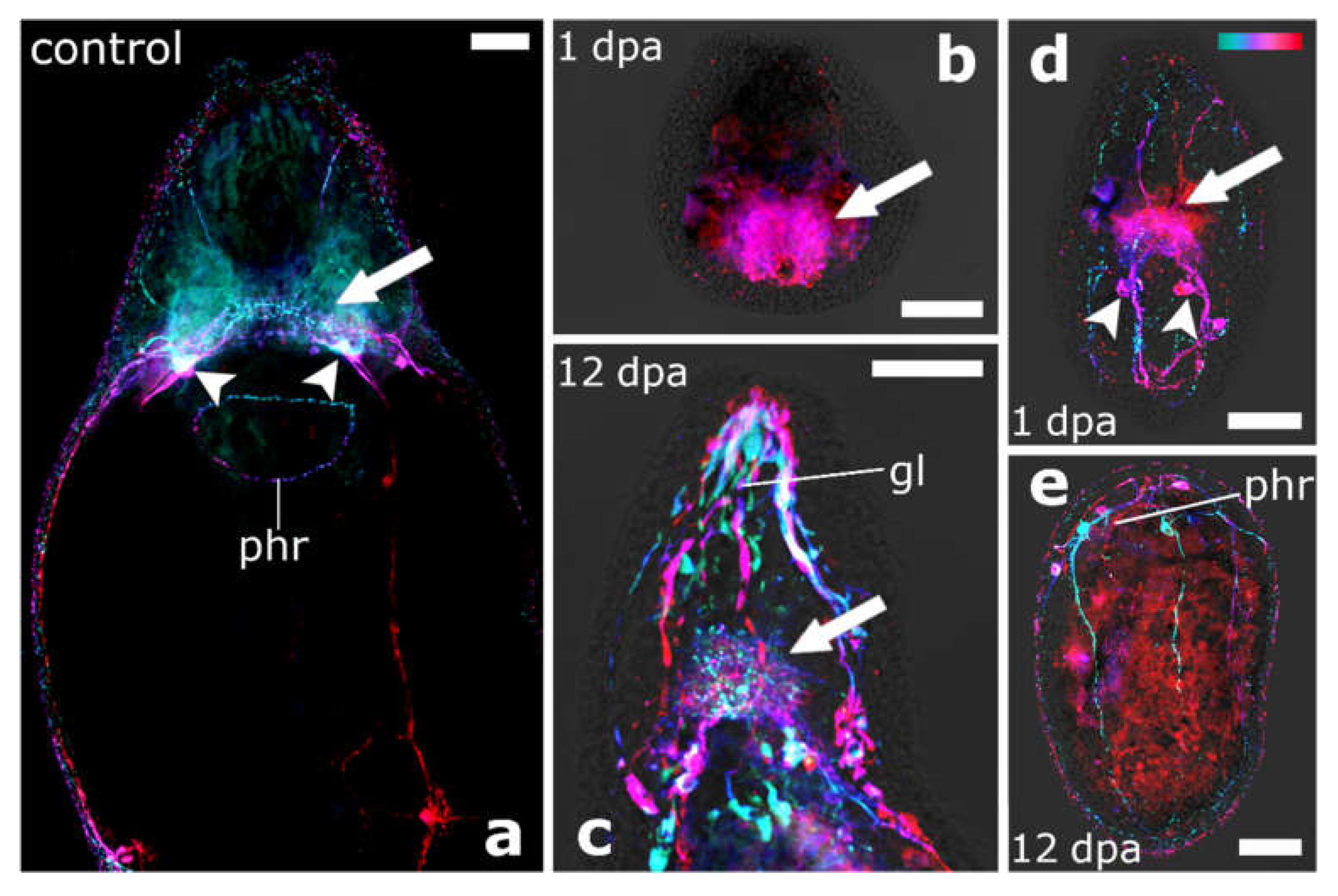
2.3. Immunocytochemistry and Fluorescent Staining
2.4. Histological Sections and Stainings
2.5. Microscopy and Visualisation
2.6. Molecular Phylogeny
3. Results
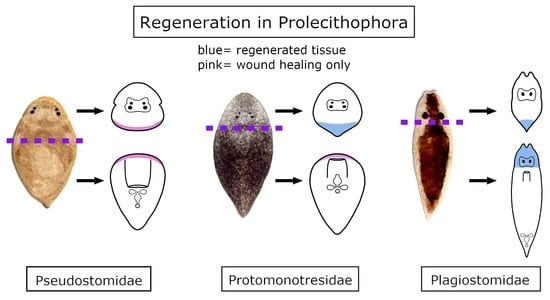
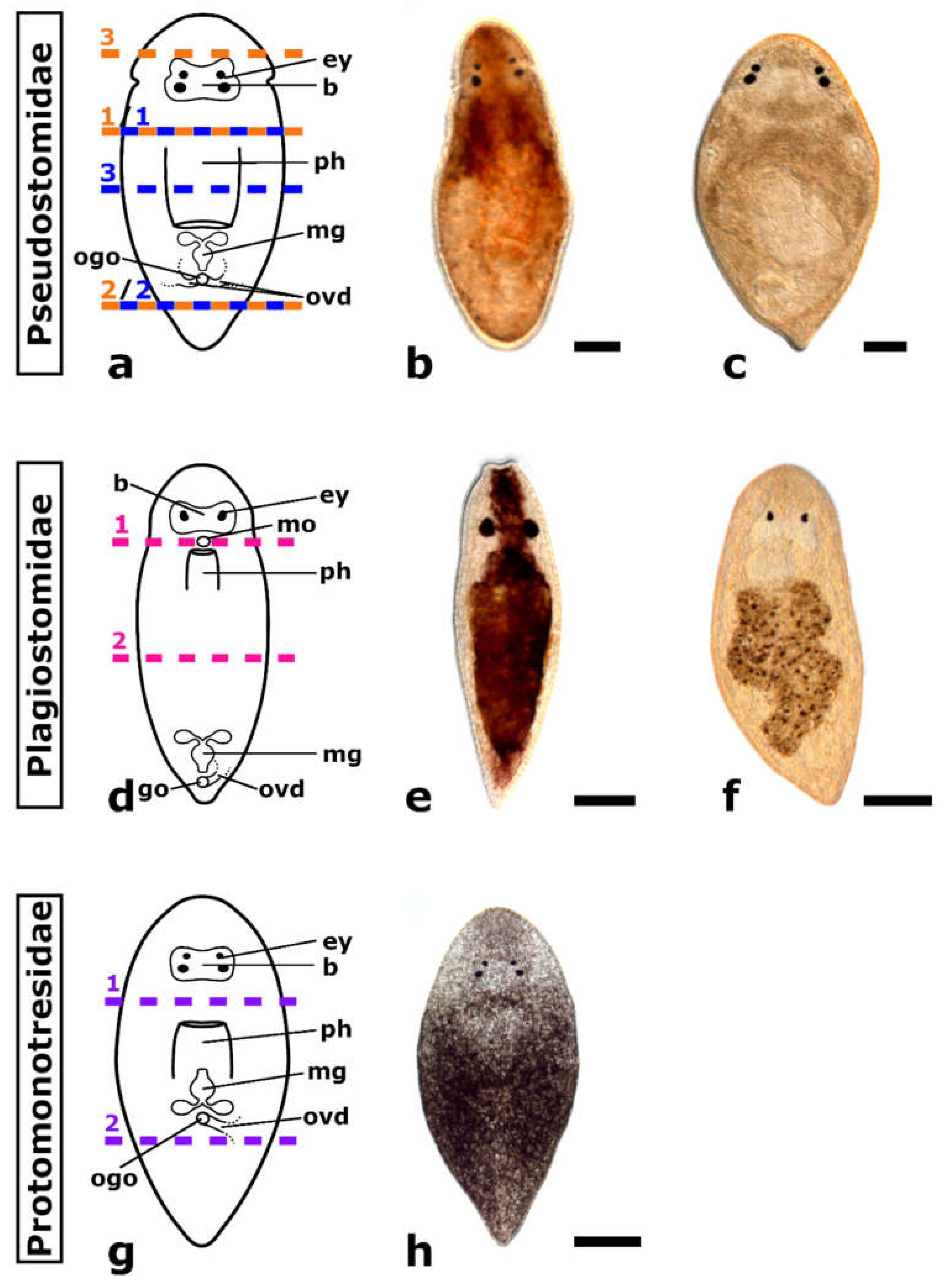
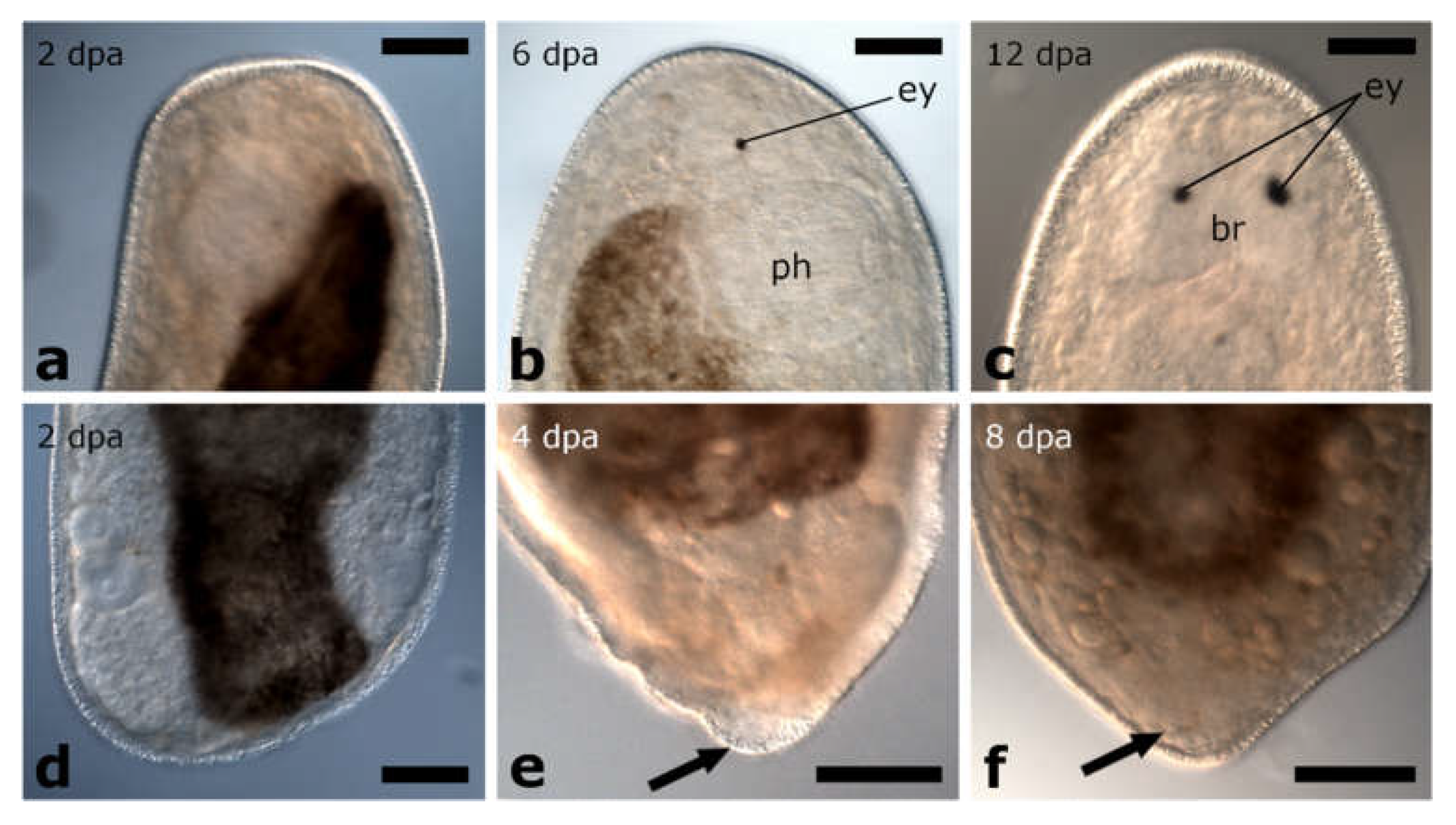
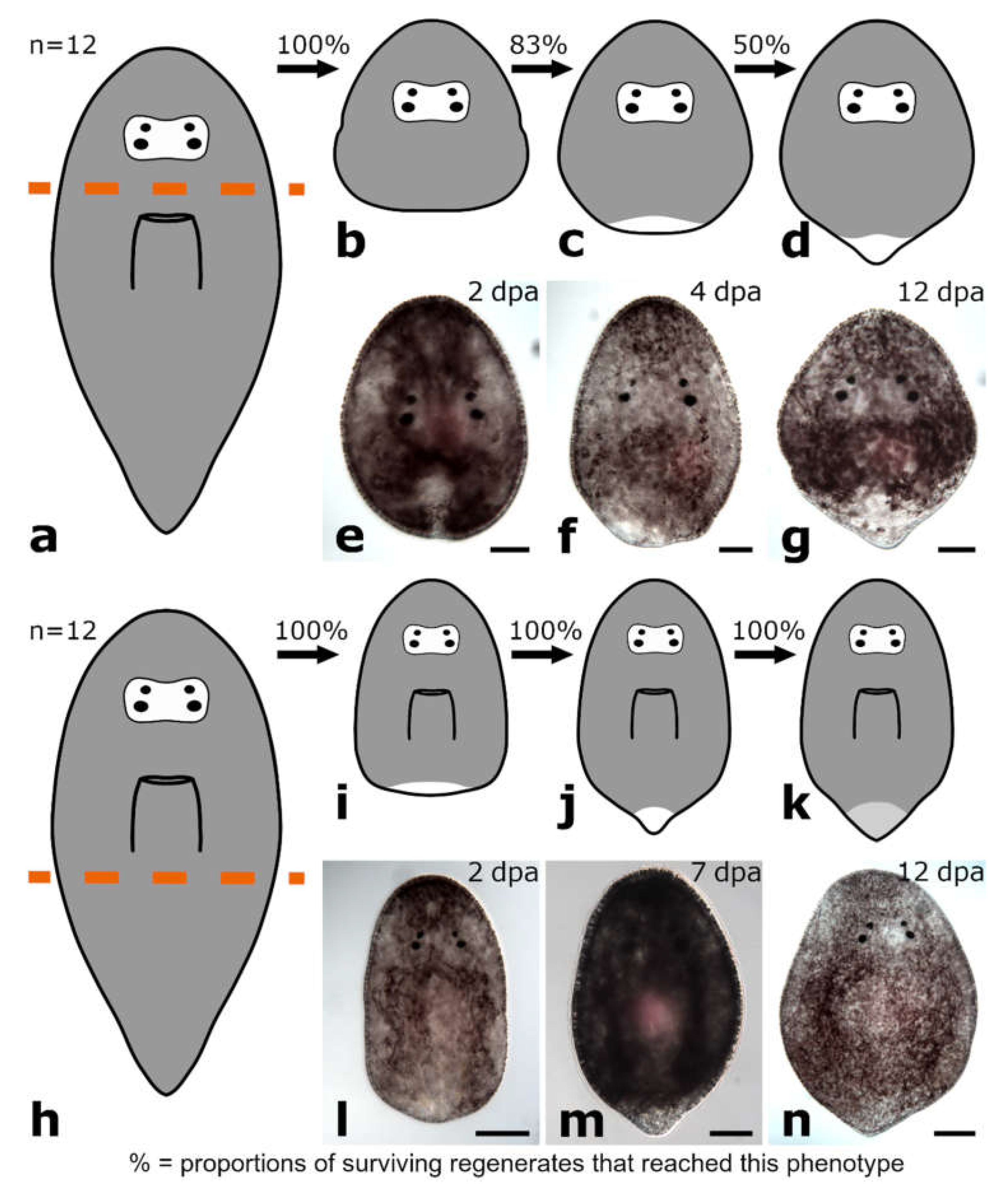
3.1. Regeneration Capacity in Pseudostomidae
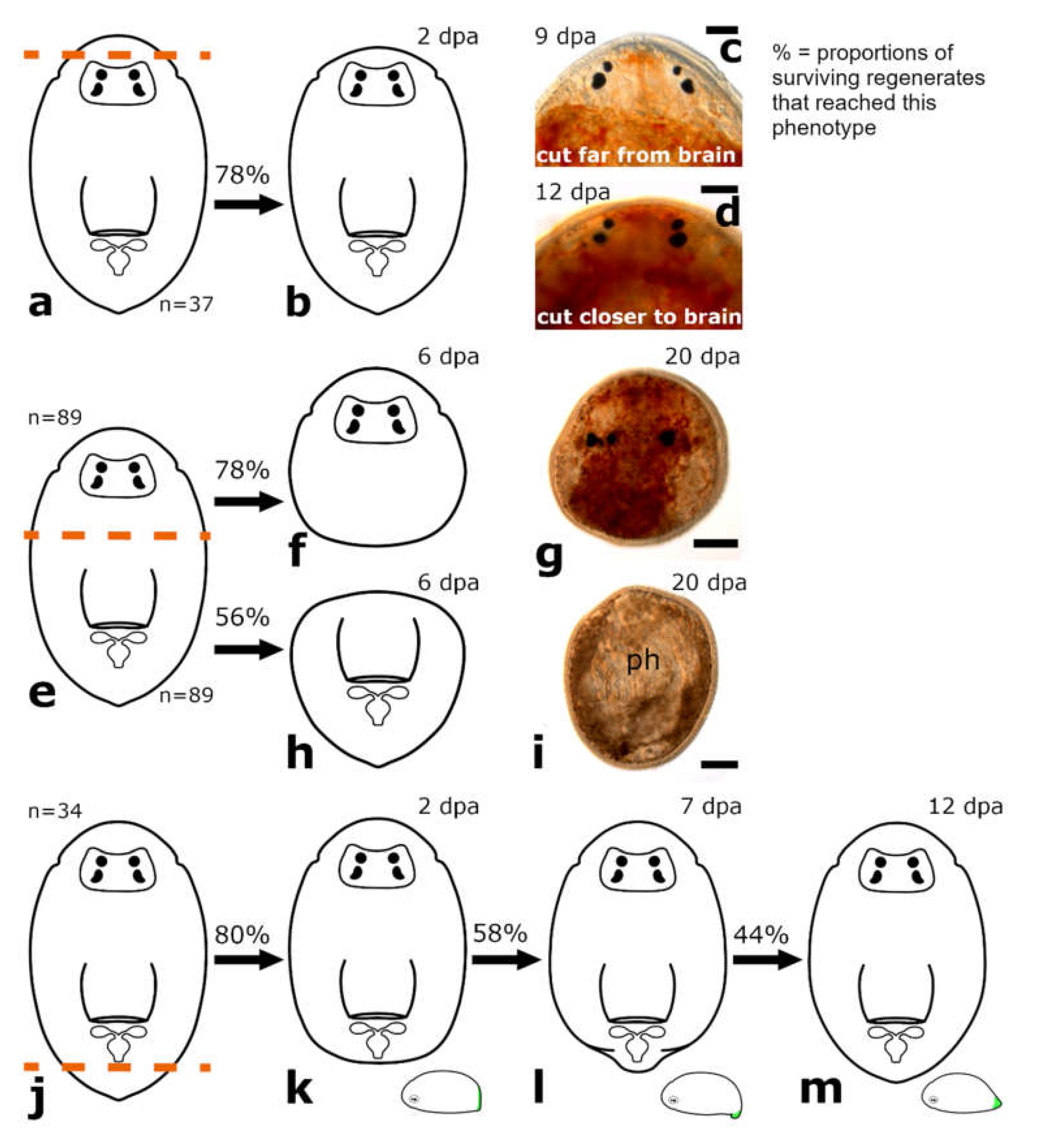
3.1.1. Amputation Anterior to the Brain
Posterior Regeneration
Anterior Regeneration
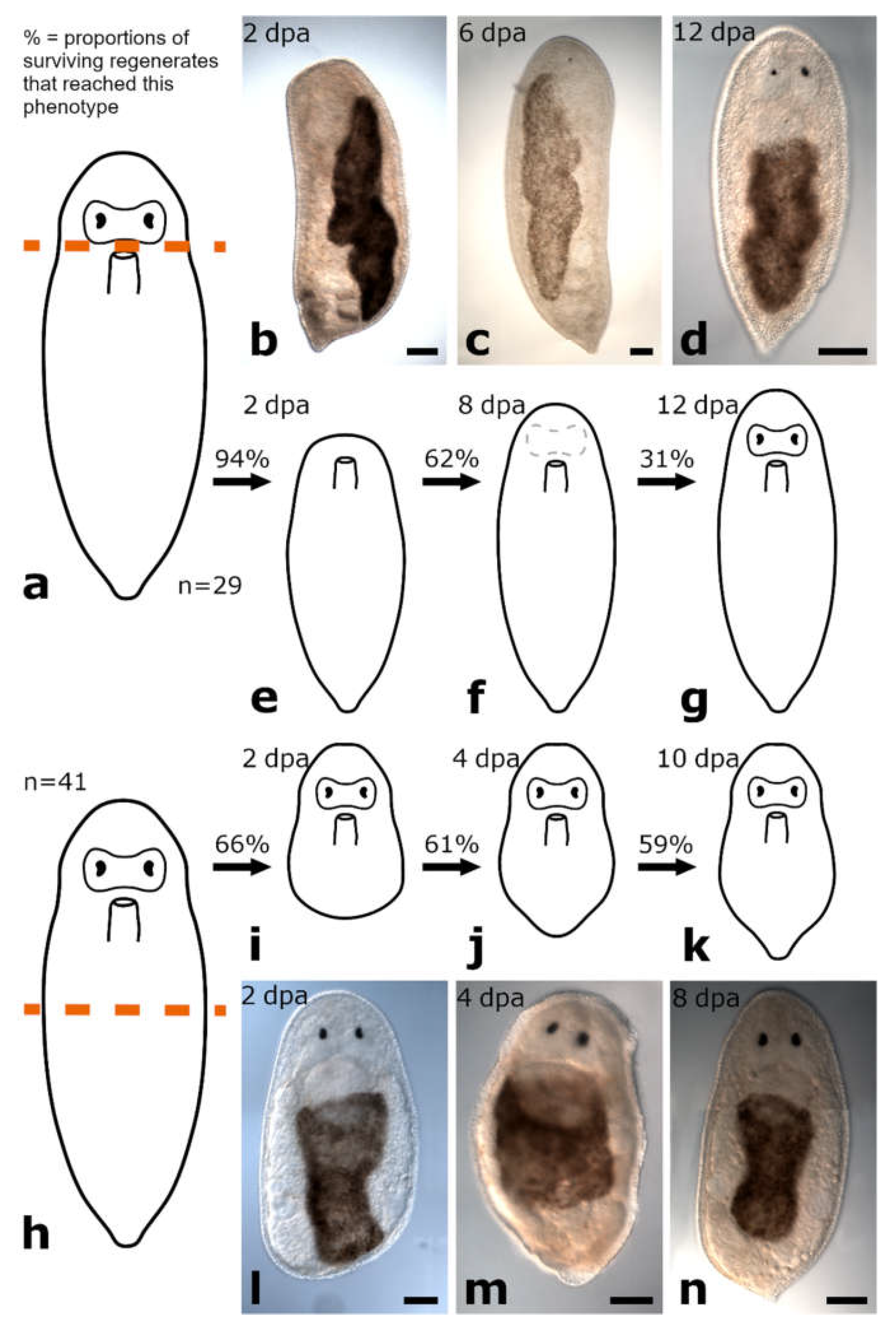
3.1.2. Amputation between Brain and Pharynx
Posterior and Anterior Regeneration in Monoophorum striatum
Posterior and Anterior Regeneration in Cylindrostoma monotrochum
Posterior and anterior Regeneration in Other Pseudostomids
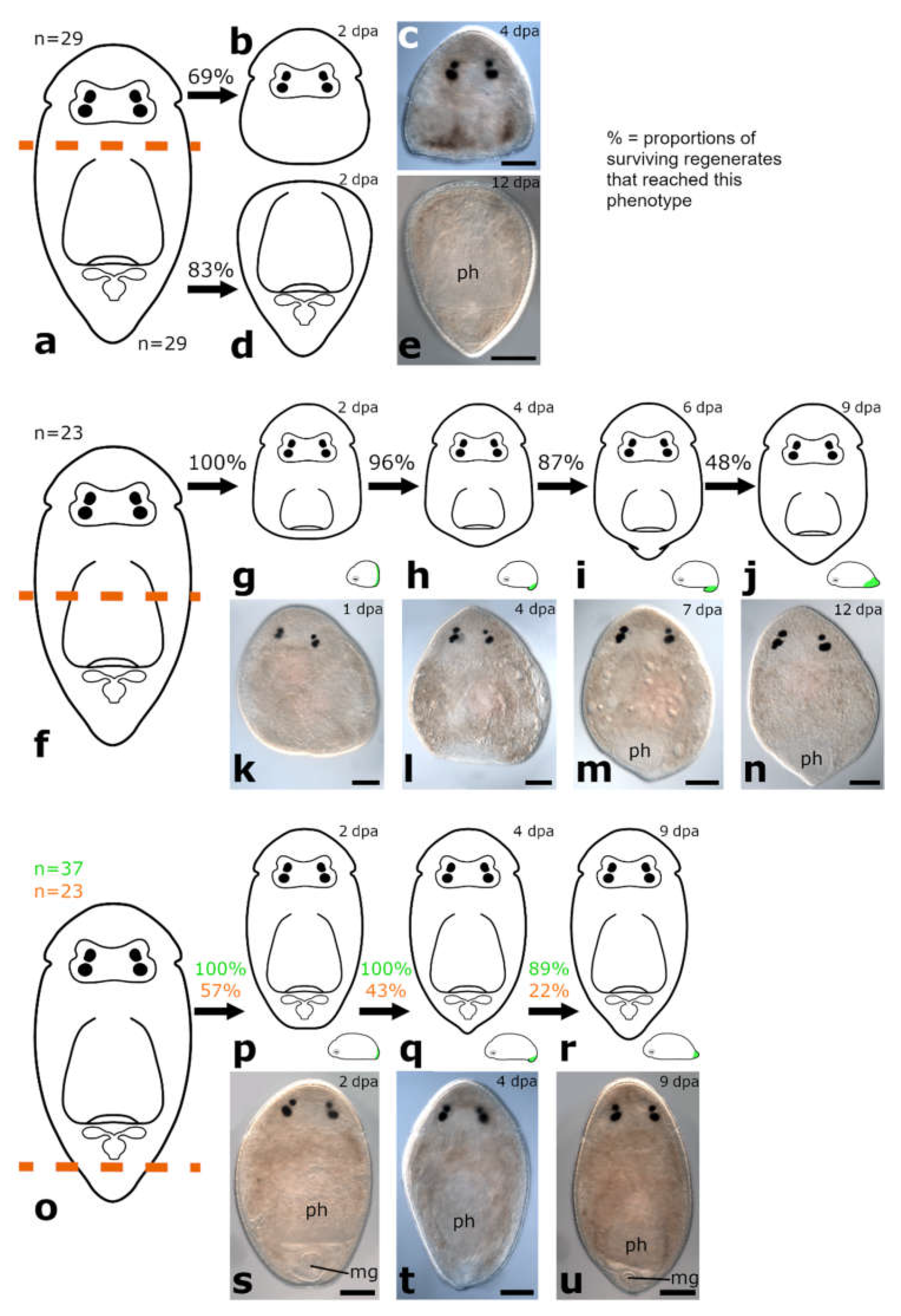
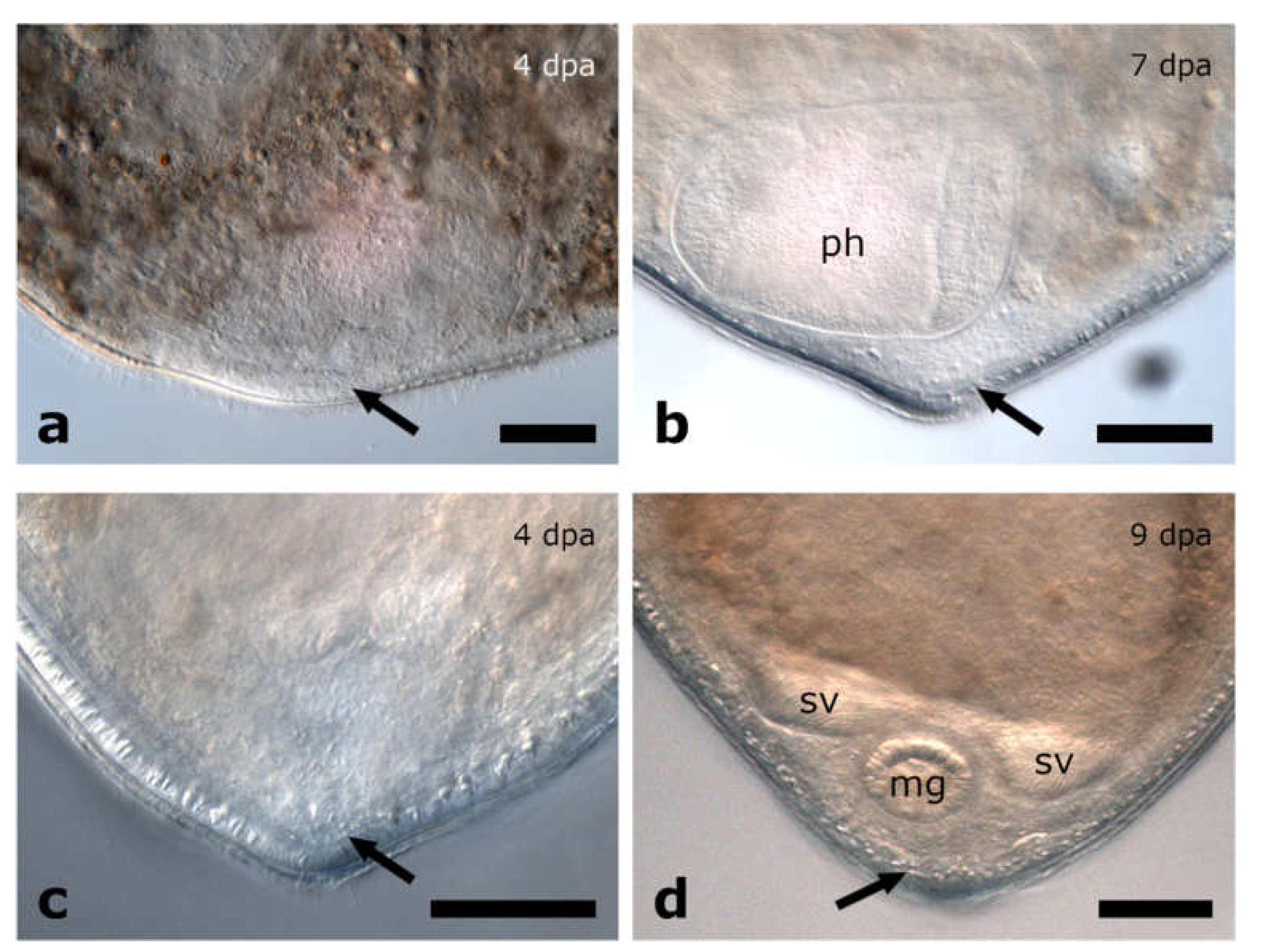
3.1.3. Amputation through the Pharynx
Posterior Regeneration
Anterior Regeneration
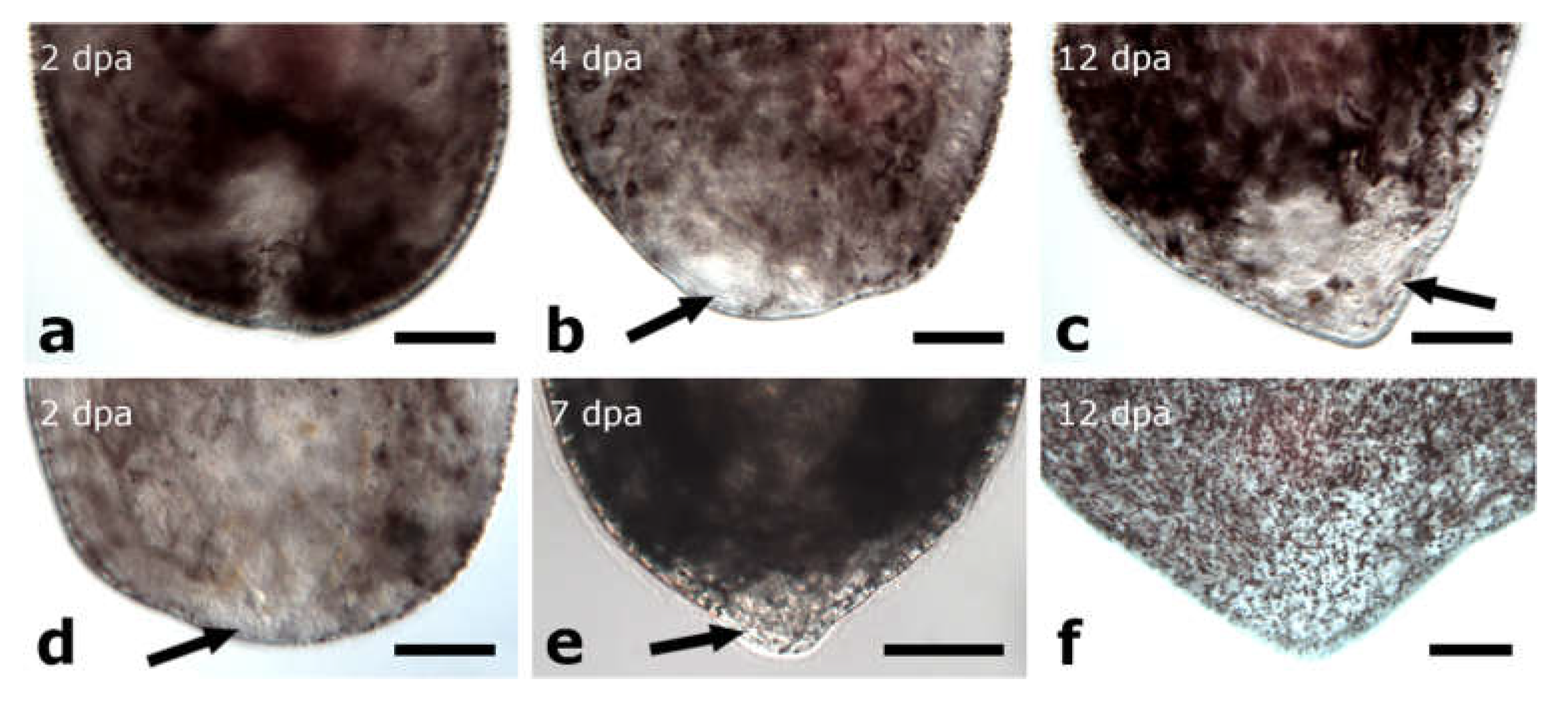
3.1.4. Amputation Posterior to the Pharynx
Posterior Regeneration
Anterior Regeneration
Posterior and Anterior Regeneration in Other Pseudostomids
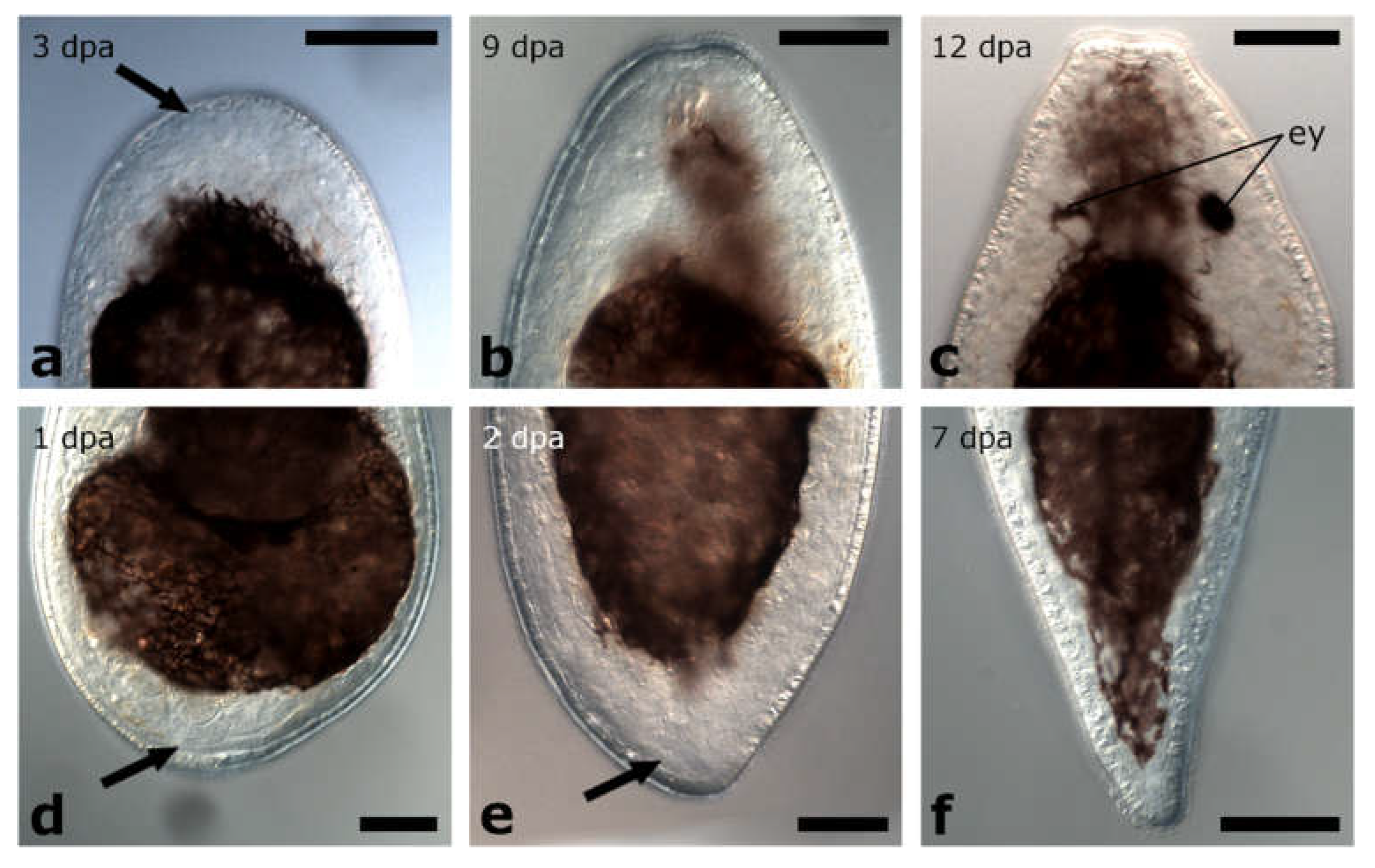
3.2. Regeneration Capacity in Plagiostomidae
3.2.1. Amputation between Brain and Pharynx
Posterior Regeneration
Anterior Regeneration
Posterior and Anterior Regeneration in Other Plagiostomids
3.2.2. Amputation Posterior to the Pharynx
Posterior Regeneration
Anterior Regeneration
Posterior and Anterior Regeneration in Other Plagiostomids
3.3. Regeneration Capacity in Protomonotresidae
3.3.1. Amputation between Brain and Pharynx
Posterior Regeneration
Anterior Regeneration
3.3.2. Amputation Posterior to the Pharynx
Posterior Regeneration
Anterior Regeneration
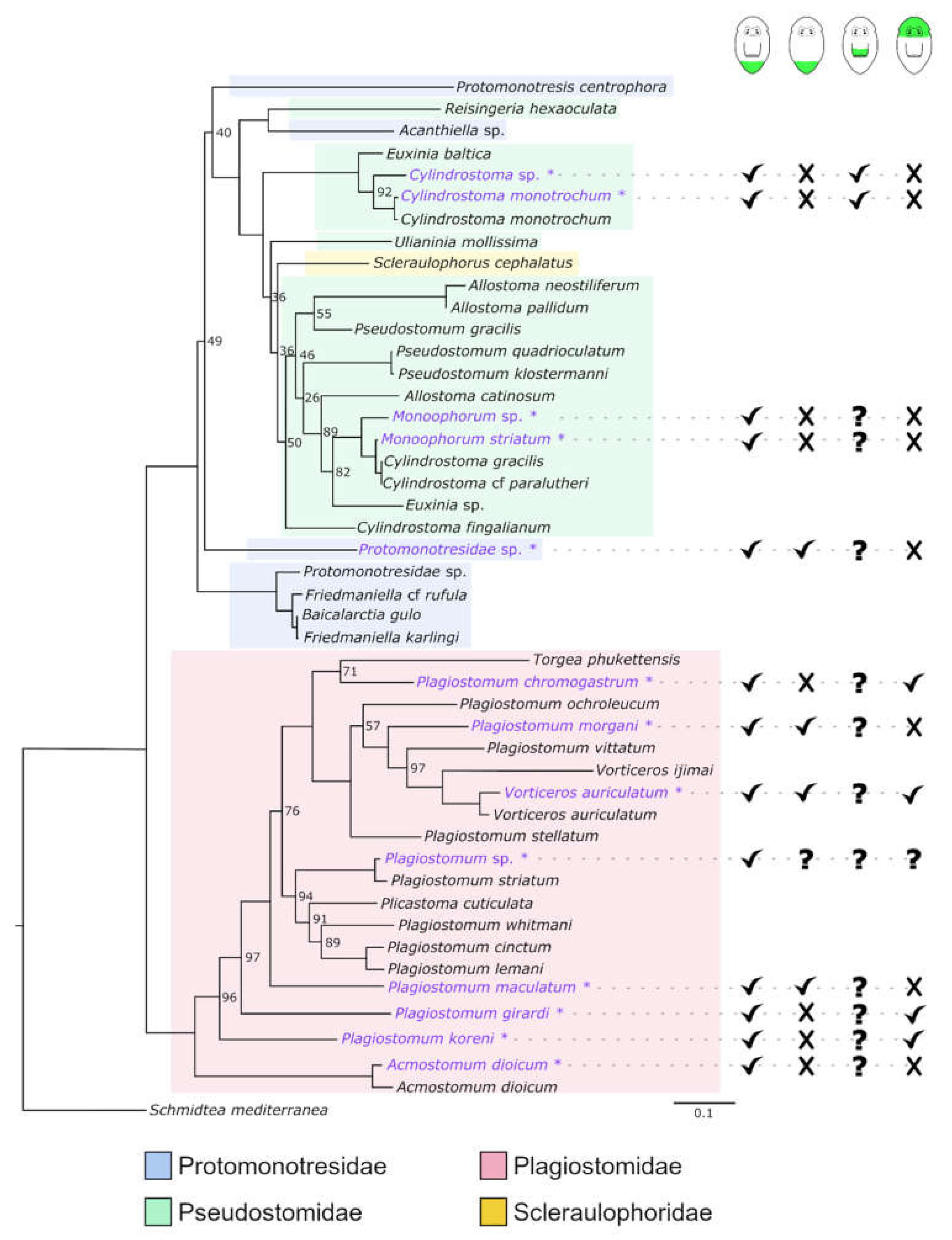
3.4. Molecular Phylogeny
4. Discussion
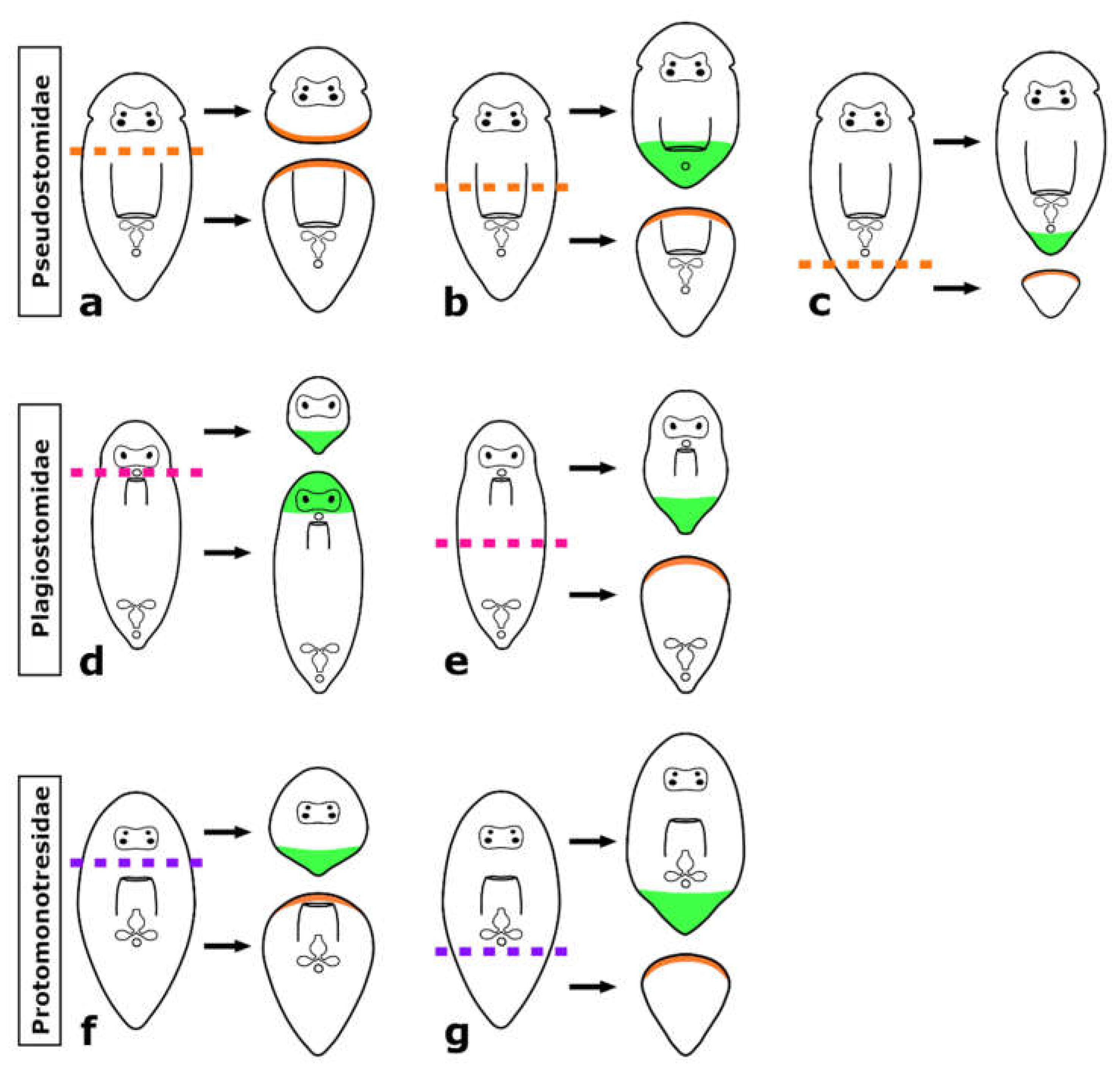
4.1. Comparative Regeneration in Prolecithophora
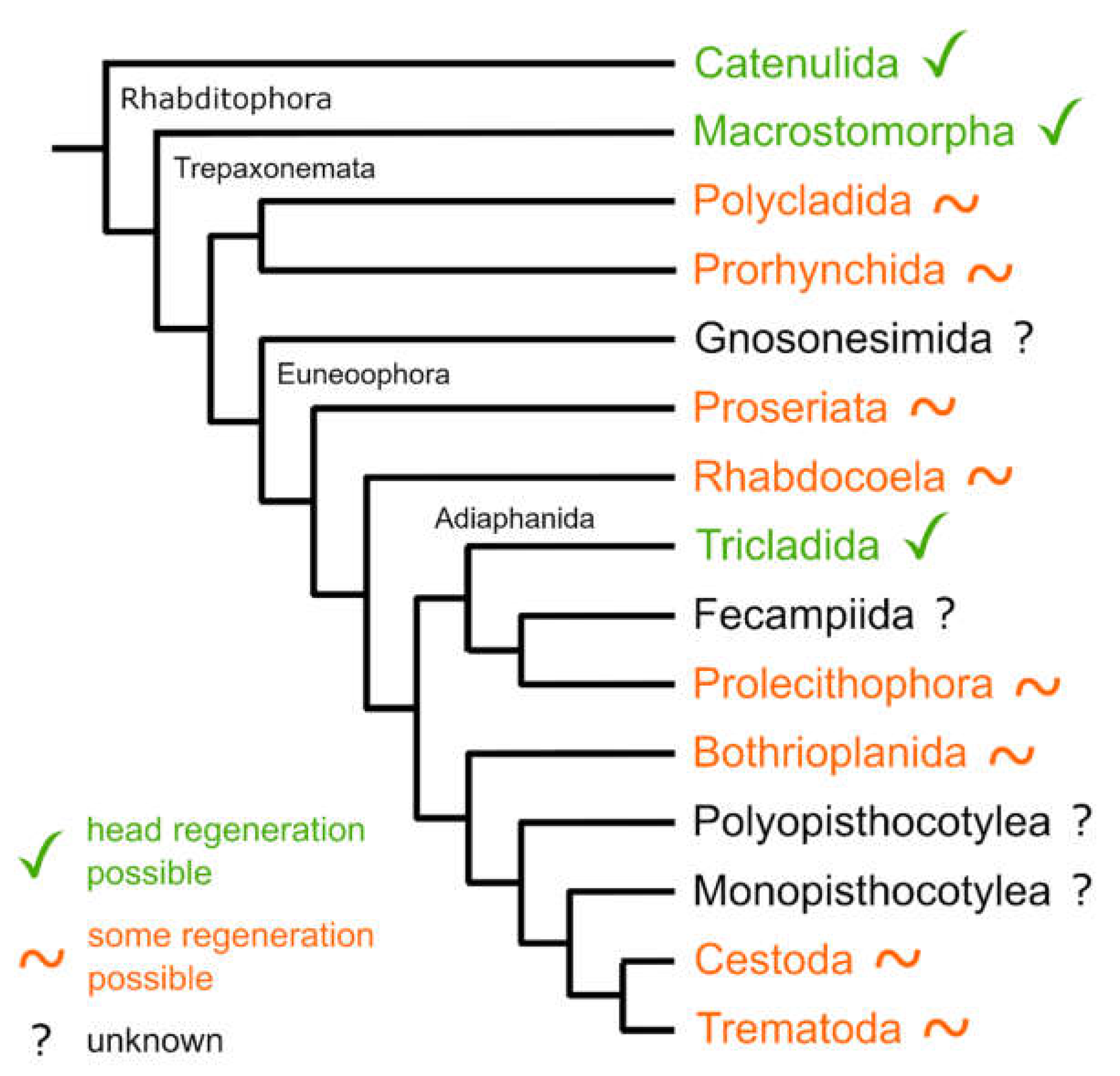
4.2. Molecular Phylogeny
4.3. Regeneration Capacity Is Linked to the Shape and the Size of the Regenerate
4.4. The Brain Root Is Required for Head Regeneration
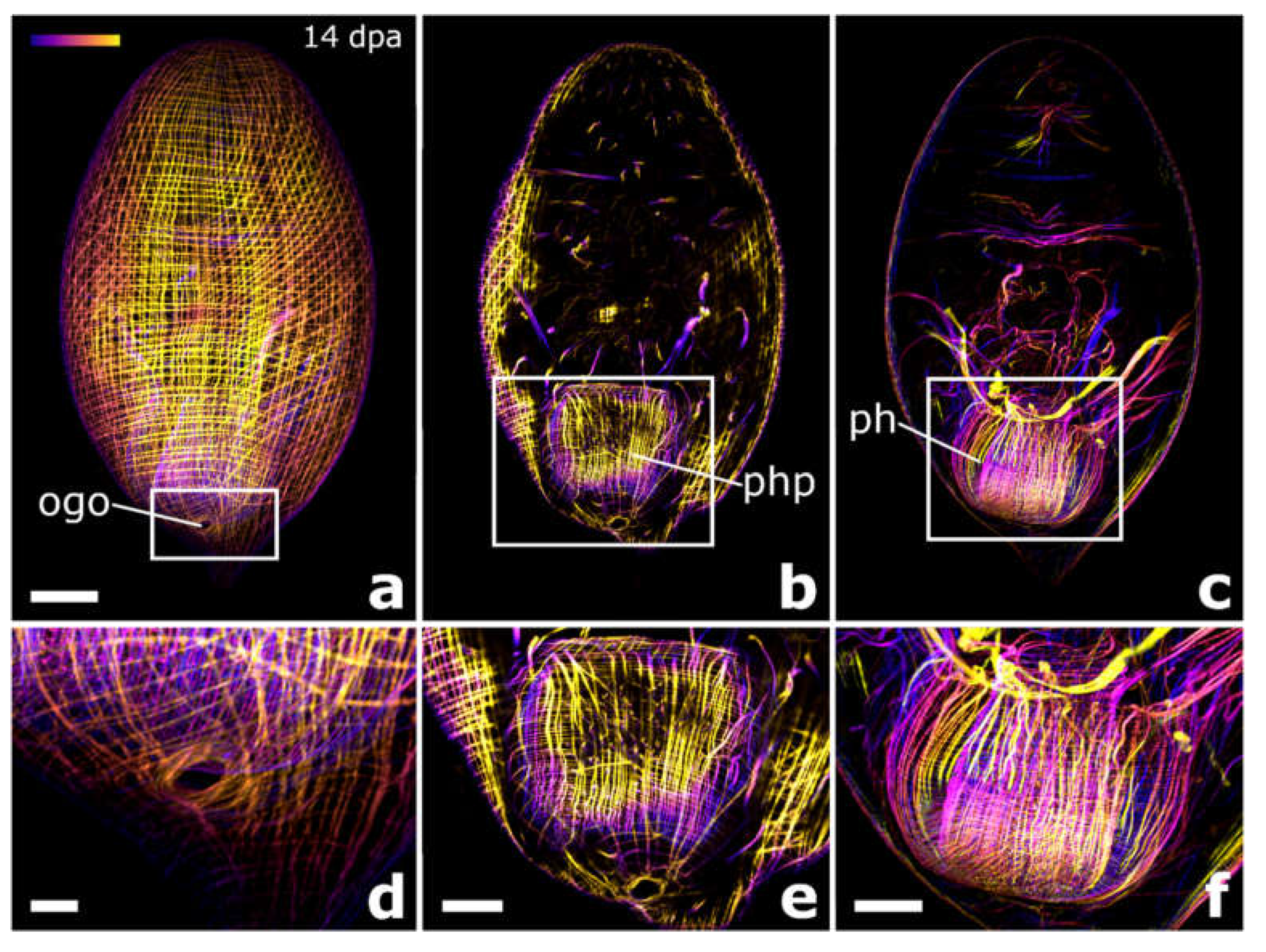
4.5. Why Can the Pharynx Not Regenerate de Novo?
4.6. Comparative Regeneration in Platyhelminthes
4.7. Is the Capacity to Regenerate All Organs an Ancestral Feature in Adiaphanida?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalyell, J.G. Observations on Some Interesting Phenomena in Animal Physiology, Exhibited by Several Species of Planariae: Illustrated by Coloured Figures of Living Animals; Archibald Constable &Co.: Edinburgh, UK, 1814. [Google Scholar]
- Baguñà, J.; Salo, E.; Auladell, C. Regeneration and pattern formation in planarians. III. that neoblasts are totipotent stem cells and the cells. Development 1989, 107, 77–86. [Google Scholar] [CrossRef]
- Agata, K.; Tanaka, T.; Kobayashi, C.; Kato, K.; Saitoh, Y. Intercalary regeneration in planarians. Dev. Dyn. 2003, 226, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Alvarado, A.S. Fundamentals of Planarian Regeneration. Annu. Rev. Cell Dev. Biol. 2004, 20, 725–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, S.A.; Alvarado, A.S. Planarians and the history of animal regeneration: Paradigm shifts and key concepts in biology. In Planarian Regeneration; Humana Press: New York, NY, USA, 2018; pp. 207–239. [Google Scholar]
- Gurley, K.A.; Rink, J.C.; Alvarado, A.S. β-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 2008, 319, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Wagner, D.E.; Wang, I.E.; Reddien, P.W. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 2011, 332, 811–816. [Google Scholar] [CrossRef]
- Liu, S.Y.; Selck, C.; Friedrich, B.; Lutz, R.; Vila-Farré, M.; Dahl, A.; Brandl, H.; Lakshmanaperumal, N.; Henry, I.; Rink, J.C. Reactivating head regrowth in a regeneration-deficient planarian species. Nature 2013, 500, 81–84. [Google Scholar] [CrossRef]
- Sikes, J.M.; Newmark, P.A. Restoration of anterior regeneration in a planarian with limited regenerative ability. Nature 2013, 500, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Umesono, Y.; Tasaki, J.; Nishimura, Y.; Hrouda, M.; Kawaguchi, E.; Yazawa, S.; Nishimura, O.; Hosoda, K.; Inoue, T.; Agata, K. The molecular logic for planarian regeneration along the anterior-posterior axis. Nature 2013, 500, 73–76. [Google Scholar] [CrossRef]
- Hosoda, K.; Motoishi, M.; Kunimoto, T.; Nishimura, O.; Hwang, B.; Kobayashi, S.; Yazawa, S.; Mochii, M.; Agata, K.; Umesono, Y. Role of MEKK1 in the anterior-posterior patterning during planarian regeneration. Dev. Growth Differ. 2018, 60, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Molina, M.D.; Saló, E.; Cebrià, F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev. Biol. 2007, 311, 79–94. [Google Scholar] [CrossRef]
- Rink, J.C. Stem cells, patterning and regeneration in Planarians: Self-organization at the organismal scale. In Planarian Regeneration; Humana Press: New York, NY, USA, 2018; pp. 57–172. [Google Scholar]
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farré, M.; Werner, S.; Rink, J.C. Model systems for regeneration: Planarians. Development 2019, 146, dev167684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, B.; Ladurner, P.; Nimeth, K.; Gschwentner, R.; Rieger, R. The regeneration capacity of the flatworm Macrostomum lignano—On repeated regeneration, rejuvenation, and the minimal size needed for regeneration. Dev. Genes Evol. 2006, 216, 565–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirks, U.; Gruber-Vodicka, H.R.; Egger, B.; Ott, J.A. Proliferation pattern during rostrum regeneration of the symbiotic flatworm Paracatenula galateia: A pulse-chase-pulse analysis. Cell Tissue Res. 2012, 349, 517–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girstmair, J.; Schnegg, R.; Telford, M.J.; Egger, B. Cellular dynamics during regeneration of the flatworm Monocelis sp. (Proseriata, Platyhelminthes). Evodevo 2014, 5, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertemes, P.; Grosbusch, A.L.; Egger, B. No head regeneration here: Regeneration capacity and stem cell dynamics of Theama mediterranea (Polycladida, Platyhelminthes). Cell Tissue Res. 2020, 379, 301–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadt, T.; Prantl, V.; Grosbusch, A.L.; Bertemes, P.; Egger, B. Regeneration of the flatworm Prosthiostomum siphunculus (Polycladida, Platyhelminthes). Cell Tissue Res. 2021, 383, 1025–1041. [Google Scholar] [CrossRef]
- Egger, B.; Gschwentner, R.; Rieger, R. Free-living flatworms under the knife: Past and present. Dev. Genes Evol. 2007, 217, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Norén, M.; Jondelius, U. The phylogenetic position of the Prolecithophora (Rhabditophora, “Platyhelminthes”). Zool. Scr. 2002, 31, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Keller, J. Die ungeschlechtliche Fortpflanzung der Süßwasserturbellarien. Jena. Z. Für Med. Und Nat. 1894, 28, 370–407. [Google Scholar]
- Monti, R. Studi sperimentali sulla rigenerazione nei rabdoceli marini (Plagiostoma Girardii Graff). Rend. Inst. Lombardo 1900, 33. [Google Scholar]
- Laumer, C.E.; Hejnol, A.; Giribet, G. Nuclear genomic signals of the “microturbellarian” roots of platyhelminth evolutionary innovation. Elife 2015, 4, e05503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumer, C.E.; Giribet, G. Phylogenetic relationships within Adiaphanida (phylum Platyhelminthes) and the status of the crustacean-parasitic genus Genostoma. Invertebr. Biol. 2017, 136, 184–198. [Google Scholar] [CrossRef]
- Egger, B.; Lapraz, F.; Tomiczek, B.; Müller, S.; Dessimoz, C.; Girstmair, J.; Škunca, N.; Rawlinson, K.A.; Cameron, C.B.; Beli, E.; et al. A transcriptomic-phylogenomic analysis of the evolutionary relationships of flatworms. Curr. Biol. 2015, 25, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, B.; Bachmann, L.; Fromm, B. Atp8 is in the ground pattern of flatworm mitochondrial genomes. BMC Genom. 2017, 18, 414. [Google Scholar] [CrossRef] [Green Version]
- Böhmig, L. Untersuchungen über rhabdocöle Turbellarien. II. Plagiostomina und Cylindrostomina Graff. Z. Für Wiss. Zool. 1891, 51, 167–479. [Google Scholar]
- Jondelius, U.; Norén, M.; Hendelberg, J. The Prolecithophora. In Interrelationships of the Platyhelminthes; Taylor & Francis: London, UK, 2001; pp. 74–80. [Google Scholar]
- Noren, M. Four New Plagiostomidae (Prolecithophora:Platyhelminthes) from Phuket, Thailand, with a Re-Evaluation of Torgeidae Jondelius, 1997, and Paramultipeniata Kulinich, 1974; Phuket Marine Biological Center Research Bulletin: Phuket, Thailand, 2004. [Google Scholar]
- von Graff, L. Acoela und Rhabdocoela. Plathelminthes III. Turbellaria. In Bronn’s Klassen und Ordnungen des Tierreichs Volume IV, Abt. 1c; Winter’sche Verlagsbuchhandl: Leipzig, Germany, 1904. [Google Scholar]
- von Graff, L. Turbellaria II. Rhabdocoelida; Verlag von R. Friedländer und Sohn: Berlin, Germany, 1913. [Google Scholar]
- Rieger, R.; Tyler, S.; Smith, J.P.S.; Rieger, G. Platyhelminthes: Turbellaria. In Microscopic Anatomy of Invertebrates; Harrison, F.W., Bogitsch, B.J., Eds.; Wiley-Liss: New York, NY, USA, 1991; Volume 3, pp. 7–140. [Google Scholar]
- Karling, T.G. Zur Morphologie und Systematik der Alloeocoela Cumulata und Rhabdocoela Lecithophora (Turbellaria). Acta Zool. Fenn. 1940, 26, 1–260. [Google Scholar]
- Tyler, S.; Hooge, M.; Bush, L.M. Turbellarian Taxonomic Database. Version 2.07. 2006. Available online: http://turbellaria.umaine.edu (accessed on 6 September 2022).
- WoRMS Editorial Board. World Register of Marine Species (WoRMS)’. 2022. Available online: https://www.marinespecies.org (accessed on 6 September 2022).
- Westblad, E. Marine “Alloeocoels” (Turbellaria) from North Atlantic and the Mediterranean coasts. II. Ark. För Zool. 1956, 9, 131–174. [Google Scholar]
- Timoshkin, O.A. Taxonomic revision of the relict turbellarian group prolecithophora protomonotresidae from lake Baikal (Plathelminthes): Description of Porfirievia n.gen., six new species of the genus and notes on the phylogeny of the baicalarctiinae. In New Scope Boreal Ecosystes in East Siberia Proccedings of the International Workshop, Tokyo, Japan, 23–25 November 1994; DIWPA: Otsu, Japan, 1997; pp. 151–178. [Google Scholar]
- Riedl, R. Neue Turbellarien aus dem mediterranen Felslittoral. Ergebnisse der “Unterwasser-Expedition Austria 1948–1949”. Zool. Jahrbücher. Abt. Für Syst. Geogr. Und Biol. Der Tiere 1954, 82, 154–244. [Google Scholar]
- Romeis, B. Mikroskopische Technik; R. Oldenburg: München, Germany, 1968. [Google Scholar]
- The GIMP Development Team. GIMP. 2019. Available online: https://www.gimp.org (accessed on 6 September 2022).
- Inkscape Project. Inkscape. 2020. Available online: https://inkscape.org (accessed on 6 September 2022).
- Lima, R.T.; Sousa, D.; Paiva, A.M.; Palmeira, A.; Barbosa, J.; Pedro, M.; Pinto, M.M.; Sousa, E.; Vasconcelos, M.H. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, I.L.; Cuadrado, D.; Aguado, M.T.; Noreña, C.; Egger, B. Polyclad phylogeny persists to be problematic. Org. Divers Evol. 2019, 19, 585–608. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molecular Evolution, Phylogenetics and Epidemiology. Available online: http://tree.bio.ed.ac.uk/ (accessed on 10 October 2022).
- Westblad, E. Marine “Alloeocoels” (Turbellaria) from North Atlantic and the Mediterranean coasts. I. Ark. För Zool. 1955, 7, 490–529. [Google Scholar]
- Marcus, E. Turbellaria brasileiros (8). Boletins da Faculdade de Philosophia, Sciencias e Letras, Universidade de São Paulo. Zoologia 1950, 15, 5–191. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Coward, S.J. On the minimal size of a planarian capable of regeneration. Trans. Am. Microsc. Soc. 1974, 93, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Birkholz, T.R.; van Huizen, A.v.; Beane, W.S. Staying in shape: Planarians as a model for understanding regenerative morphology. Semin. Cell Dev. Biol. 2019, 87, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Grosbusch, A.L.; Bertemes, P.; Egger, B. The adult musculature of two pseudostomid species reveals unique patterns for flatworms (Platyhelminthes, Prolecithophora). J. Morphol. 2019, 280, 1393–1404. [Google Scholar] [CrossRef]
- Scimone, M.L.; Atabay, K.D.; Fincher, C.T.; Bonneau, A.R.; Li, D.J.; Reddien, P.W. Muscle and neuronal guidepost-like cells facilitate planarian visual system regeneration. Science 2020, 368, eaba3203. [Google Scholar] [CrossRef]
- Pechlaner, R. Die Regenerationsfähigkeit von Otomesostoma auditivum (Forel et Duplessis). [Turbellaria]. Wilhelm Roux'Archiv Für Entwickl. Der Org. 1957, 150, 105–114. [Google Scholar] [CrossRef]
- Grosbusch, A.L.; Bertemes, P.; Egger, B. The serotonergic nervous system of prolecithophorans shows a closer similarity to fecampiids than to triclads (Platyhelminthes). J. Morphol. 2021, 282, 574–587. [Google Scholar] [CrossRef]
- Kotikova, E.A.; Timoshkin, O.A. The structure of the nervous system of Lecithoepitheliata and Prolecithophora from the Baikal Lake. Proc. Zool. Inst. Leningr. 1987, 167, 97–110. [Google Scholar]
- Egger, B.; Gschwentner, R.; Hess, M.W.; Nimeth, K.T.; Adamski, Z.; Willems, M.; Rieger, R.; Salvenmoser, W. The caudal regeneration blastema is an accumulation of rapidly proliferating stem cells in the flatworm Macrostomum lignano. BMC Dev. Biol. 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, T.; Vizoso, D.B.; Schulte, G.; Littlewood, D.T.J.; Waeschenbach, A.; Schärer, L. The first multi-gene phylogeny of the Macrostomorpha sheds light on the evolution of sexual and asexual reproduction in basal Platyhelminthes. Mol. Phylogenet. Evol. 2015, 92, 82–107. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grosbusch, A.L.; Bertemes, P.; Kauffmann, B.; Gotsis, C.; Egger, B. Do Not Lose Your Head over the Unequal Regeneration Capacity in Prolecithophoran Flatworms. Biology 2022, 11, 1588. https://doi.org/10.3390/biology11111588
Grosbusch AL, Bertemes P, Kauffmann B, Gotsis C, Egger B. Do Not Lose Your Head over the Unequal Regeneration Capacity in Prolecithophoran Flatworms. Biology. 2022; 11(11):1588. https://doi.org/10.3390/biology11111588
Chicago/Turabian StyleGrosbusch, Alexandra L., Philip Bertemes, Bob Kauffmann, Clemens Gotsis, and Bernhard Egger. 2022. "Do Not Lose Your Head over the Unequal Regeneration Capacity in Prolecithophoran Flatworms" Biology 11, no. 11: 1588. https://doi.org/10.3390/biology11111588
APA StyleGrosbusch, A. L., Bertemes, P., Kauffmann, B., Gotsis, C., & Egger, B. (2022). Do Not Lose Your Head over the Unequal Regeneration Capacity in Prolecithophoran Flatworms. Biology, 11(11), 1588. https://doi.org/10.3390/biology11111588