Effectiveness of a Group-Based Rehabilitation Program Combining Education with Multimodal Exercises in the Treatment of Patients with Nonspecific Chronic Low Back Pain: A Retrospective Uncontrolled Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design & Participants
2.2. Pre and Post-Treatment Assessment
2.3. Intervention Protocol
2.3.1. Education
2.3.2. Pilates
2.3.3. Resistance Training
2.3.4. Aquatic Exercises
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Improvement in Pain Intensity and Related Functional Disability
4.2. Pain-Related Psychosocial Factors
4.3. Health-Related Quality of Life
4.4. Underlying Mechanisms (Based on Scientific Literature)
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Balague, F.; Mannion, A.F.; Pellise, F.; Cedraschi, C. Non-specific low back pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Fatoye, F.; Gebrye, T.; Odeyemi, I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol. Int. 2019, 39, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Burke, A.L.; Mathias, J.L.; Denson, L.A. Psychological functioning of people living with chronic pain: A meta-analytic review. Br. J. Clin. Psychol. 2015, 54, 345–360. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Kamper, S.J.; Apeldoorn, A.T.; Chiarotto, A.; Smeets, R.J.; Ostelo, R.W.; Guzman, J.; van Tulder, M.W. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ 2015, 350, h444. [Google Scholar] [CrossRef]
- Zaina, F.; Balague, F.; Battie, M.; Karppinen, J.; Negrini, S. Low back pain rehabilitation in 2020: New frontiers and old limits of our understanding. Eur. J. Phys. Rehabil. Med. 2020, 56, 212–219. [Google Scholar] [CrossRef]
- Booth, J.; Moseley, G.L.; Schiltenwolf, M.; Cashin, A.; Davies, M.; Hubscher, M. Exercise for chronic musculoskeletal pain: A biopsychosocial approach. Musculoskelet. Care 2017, 15, 413–421. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Malmivaara, A.; van Tulder, M.W. Exercise therapy for chronic low back pain. Cochrane Database Syst. Rev. 2021, 9, CD009790. [Google Scholar] [CrossRef]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med. 2020, 54, 1279–1287. [Google Scholar] [CrossRef]
- Louw, A.; Sluka, K.A.; Nijs, J.; Courtney, C.A.; Zimney, K. Revisiting the Provision of Pain Neuroscience Education: An Adjunct Intervention for Patients but a Primary Focus of Clinician Education. J. Orthop. Sports Phys. Ther. 2021, 51, 57–59. [Google Scholar] [CrossRef]
- Monticone, M.; Ferrante, S.; Rocca, B.; Baiardi, P.; Dal Farra, F.; Foti, C. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: Results of a randomized controlled trial. Clin. J. Pain 2013, 29, 929–938. [Google Scholar] [CrossRef]
- Furlan, A.D.; Brosseau, L.; Imamura, M.; Irvin, E. Massage for low-back pain: A systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2002, 27, 1896–1910. [Google Scholar] [CrossRef]
- French, S.D.; Cameron, M.; Walker, B.F.; Reggars, J.W.; Esterman, A.J. Superficial heat or cold for low back pain. Cochrane Database Syst. Rev. 2006, 1, CD004750. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Maher, C.G.; Pinto, R.Z.; Traeger, A.C.; Lin, C.C.; Chenot, J.F.; van Tulder, M.; Koes, B.W. Clinical practice guidelines for the management of non-specific low back pain in primary care: An updated overview. Eur. Spine J. 2018, 27, 2791–2803. [Google Scholar] [CrossRef]
- Gardner, T.; Refshauge, K.; McAuley, J.; Hubscher, M.; Goodall, S.; Smith, L. Combined education and patient-led goal setting intervention reduced chronic low back pain disability and intensity at 12 months: A randomised controlled trial. Br. J. Sports Med. 2019, 53, 1424–1431. [Google Scholar] [CrossRef]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Roland, M.; Fairbank, J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Pulles, A.; Koke, A.J.A.; Strackke, R.P.; Smeets, R. The responsiveness and interpretability of psychosocial patient-reported outcome measures in chronic musculoskeletal pain rehabilitation. Eur. J. Pain 2020, 24, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Hudes, K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J. Can. Chiropr. Assoc. 2011, 55, 222–232. [Google Scholar]
- Garratt, A.M.; Furunes, H.; Hellum, C.; Solberg, T.; Brox, J.I.; Storheim, K.; Johnsen, L.G. Evaluation of the EQ-5D-3L and 5L versions in low back pain patients. Health Qual. Life Outcomes 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, J.M.; Wertli, M.M.; Ulrich, N.H.; Pichierri, G.; Brunner, F.; Farshad, M.; Porchet, F.; Steurer, J.; Gravestock, I.; Group, L.S. Evaluating the Minimal Clinically Important Difference of EQ-5D-3L in Patients With Degenerative Lumbar Spinal Stenosis: A Swiss Prospective Multicenter Cohort Study. Spine 2020, 45, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.; Bolton, J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J. Manip. Physiol. Ther. 2004, 27, 26–35. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef]
- O’Sullivan, P.B.; Caneiro, J.P.; O’Sullivan, K.; Lin, I.; Bunzli, S.; Wernli, K.; O’Keeffe, M. Back to basics: 10 facts every person should know about back pain. Br. J. Sports Med. 2020, 54, 698–699. [Google Scholar] [CrossRef]
- Butler, D.; Moseley, G. Explain Pain; Noigroup Publications: Adelaide, Australia, 2003. [Google Scholar]
- Nijs, J.; Paul van Wilgen, C.; Van Oosterwijck, J.; van Ittersum, M.; Meeus, M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: Practice guidelines. Man. Ther. 2011, 16, 413–418. [Google Scholar] [CrossRef]
- Caneiro, J.P.; Roos, E.M.; Barton, C.J.; O’Sullivan, K.; Kent, P.; Lin, I.; Choong, P.; Crossley, K.M.; Hartvigsen, J.; Smith, A.J.; et al. It is time to move beyond ‘body region silos’ to manage musculoskeletal pain: Five actions to change clinical practice. Br. J. Sports Med. 2020, 54, 438–439. [Google Scholar] [CrossRef]
- Lin, I.; Wiles, L.; Waller, R.; Caneiro, J.P.; Nagree, Y.; Straker, L.; Maher, C.G.; O’Sullivan, P.P.B. Patient-centred care: The cornerstone for high-value musculoskeletal pain management. Br. J. Sports Med. 2020, 54, 1240–1242. [Google Scholar] [CrossRef]
- de Oliveira, N.T.B.; Ricci, N.A.; Dos Santos Franco, Y.R.; Salvador, E.; Almeida, I.C.B.; Cabral, C.M.N. Effectiveness of the Pilates method versus aerobic exercises in the treatment of older adults with chronic low back pain: A randomized controlled trial protocol. BMC Musculoskelet. Disord. 2019, 20, 250. [Google Scholar] [CrossRef]
- Cortell-Tormo, J.M.; Sanchez, P.T.; Chulvi-Medrano, I.; Tortosa-Martinez, J.; Manchado-Lopez, C.; Llana-Belloch, S.; Perez-Soriano, P. Effects of functional resistance training on fitness and quality of life in females with chronic nonspecific low-back pain. J. Back Musculoskelet. Rehabil. 2018, 31, 95–105. [Google Scholar] [CrossRef]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Abadi, F.H.; Sankaravel, M.; Zainuddin, F.F.; Elumalai, G.; Razli, A.I. The effect of aquatic exercise program on low-back pain disability in obese women. J. Exerc. Rehabil. 2019, 15, 855–860. [Google Scholar] [CrossRef]
- Rewald, S.; Mesters, I.; Lenssen, A.F.; Emans, P.J.; Wijnen, W.; de Bie, R.A. Effect of aqua-cycling on pain and physical functioning compared with usual care in patients with knee osteoarthritis: Study protocol of a randomised controlled trial. BMC Musculoskelet. Disord. 2016, 17, 88. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1988. [Google Scholar]
- Kim, K.S.; An, J.; Kim, J.O.; Lee, M.Y.; Lee, B.H. Effects of Pain Neuroscience Education Combined with Lumbar Stabilization Exercise on Strength and Pain in Patients with Chronic Low Back Pain: Randomized Controlled Trial. J. Pers. Med. 2022, 12, 303. [Google Scholar] [CrossRef]
- Pires, D.; Cruz, E.B.; Caeiro, C. Aquatic exercise and pain neurophysiology education versus aquatic exercise alone for patients with chronic low back pain: A randomized controlled trial. Clin. Rehabil. 2015, 29, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Bodes Pardo, G.; Lluch Girbes, E.; Roussel, N.A.; Gallego Izquierdo, T.; Jimenez Penick, V.; Pecos Martin, D. Pain Neurophysiology Education and Therapeutic Exercise for Patients With Chronic Low Back Pain: A Single-Blind Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Romm, M.J.; Ahn, S.; Fiebert, I.; Cahalin, L.P. A Meta-Analysis of Therapeutic Pain Neuroscience Education, Using Dosage and Treatment Format as Moderator Variables. Pain Pract. 2021, 21, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Elfving, B.; Andersson, T.; Grooten, W.J. Low levels of physical activity in back pain patients are associated with high levels of fear-avoidance beliefs and pain catastrophizing. Physiother. Res. Int. 2007, 12, 14–24. [Google Scholar] [CrossRef]
- Smeets, R.J.; Vlaeyen, J.W.; Kester, A.D.; Knottnerus, J.A. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J. Pain 2006, 7, 261–271. [Google Scholar] [CrossRef]
- Augelli, N.V.; Lucas, R.J.; Howells, G.A. Hepatic resections: An eight year experience at a community hospital. Am. Surg. 1988, 54, 373–375. [Google Scholar]
- Soer, R.; Reneman, M.F.; Speijer, B.L.; Coppes, M.H.; Vroomen, P.C. Clinimetric properties of the EuroQol-5D in patients with chronic low back pain. Spine J. 2012, 12, 1035–1039. [Google Scholar] [CrossRef]
- Steiger, F.; Wirth, B.; de Bruin, E.D.; Mannion, A.F. Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur. Spine J. 2012, 21, 575–598. [Google Scholar] [CrossRef]
- Belavy, D.L.; Van Oosterwijck, J.; Clarkson, M.; Dhondt, E.; Mundell, N.L.; Miller, C.T.; Owen, P.J. Pain sensitivity is reduced by exercise training: Evidence from a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 100–108. [Google Scholar] [CrossRef]
- Sluka, K.A.; Frey-Law, L.; Hoeger Bement, M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018, 159 (Suppl. S1), S91–S97. [Google Scholar] [CrossRef]
- Hayden, J.A.; Ellis, J.; Ogilvie, R.; Stewart, S.A.; Bagg, M.K.; Stanojevic, S.; Yamato, T.P.; Saragiotto, B.T. Some types of exercise are more effective than others in people with chronic low back pain: A network meta-analysis. J. Physiother. 2021, 67, 252–262. [Google Scholar] [CrossRef]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.; Menezes Costa, L.C.; Ostelo, R.W.; Macedo, L.G. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev. 2016, 1, CD012004. [Google Scholar] [CrossRef]
- Yamato, T.P.; Maher, C.G.; Saragiotto, B.T.; Hancock, M.J.; Ostelo, R.W.; Cabral, C.M.; Menezes Costa, L.C.; Costa, L.O. Pilates for low back pain. Cochrane Database Syst. Rev. 2015, 7, CD010265. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Di Blasi, Z.; Harkness, E.; Ernst, E.; Georgiou, A.; Kleijnen, J. Influence of context effects on health outcomes: A systematic review. Lancet 2001, 357, 757–762. [Google Scholar] [CrossRef]
- Hall, A.M.; Ferreira, P.H.; Maher, C.G.; Latimer, J.; Ferreira, M.L. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: A systematic review. Phys. Ther. 2010, 90, 1099–1110. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Purtill, H.; Kennedy, N.; Conneely, M.; Hurley, J.; O’Sullivan, P.; Dankaerts, W.; O’Sullivan, K. Comparative Effectiveness of Conservative Interventions for Nonspecific Chronic Spinal Pain: Physical, Behavioral/Psychologically Informed, or Combined? A Systematic Review and Meta-Analysis. J. Pain 2016, 17, 755–774. [Google Scholar] [CrossRef]
| Original Cohort (n = 23 Patients) | |||
|---|---|---|---|
| Mean | ±SD | (Range) | |
| n | (%) | ||
| Patient characteristics | |||
| Sex | |||
| Women | 17 | (73.9%) | |
| Men | 6 | (26.1%) | |
| Professionally active | 17 | (70.8%) | |
| Age | 45.6 | ±11.6 | (29.0–64.0) |
| BMI | 23.7 | ±3.4 | (18.9–30.1) |
| Weight (kg) | 67.8 | ±13.3 | (50.0–102.0) |
| Height (m) | 1.69 | ±0.10 | (1.55–1.85) |
| Pre-Treatment (n = 23 Patients) | Post-Treatment (n = 23 Patients) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | ±SD | (Range) | Mean | ±SD | (Range) | p-Value | |
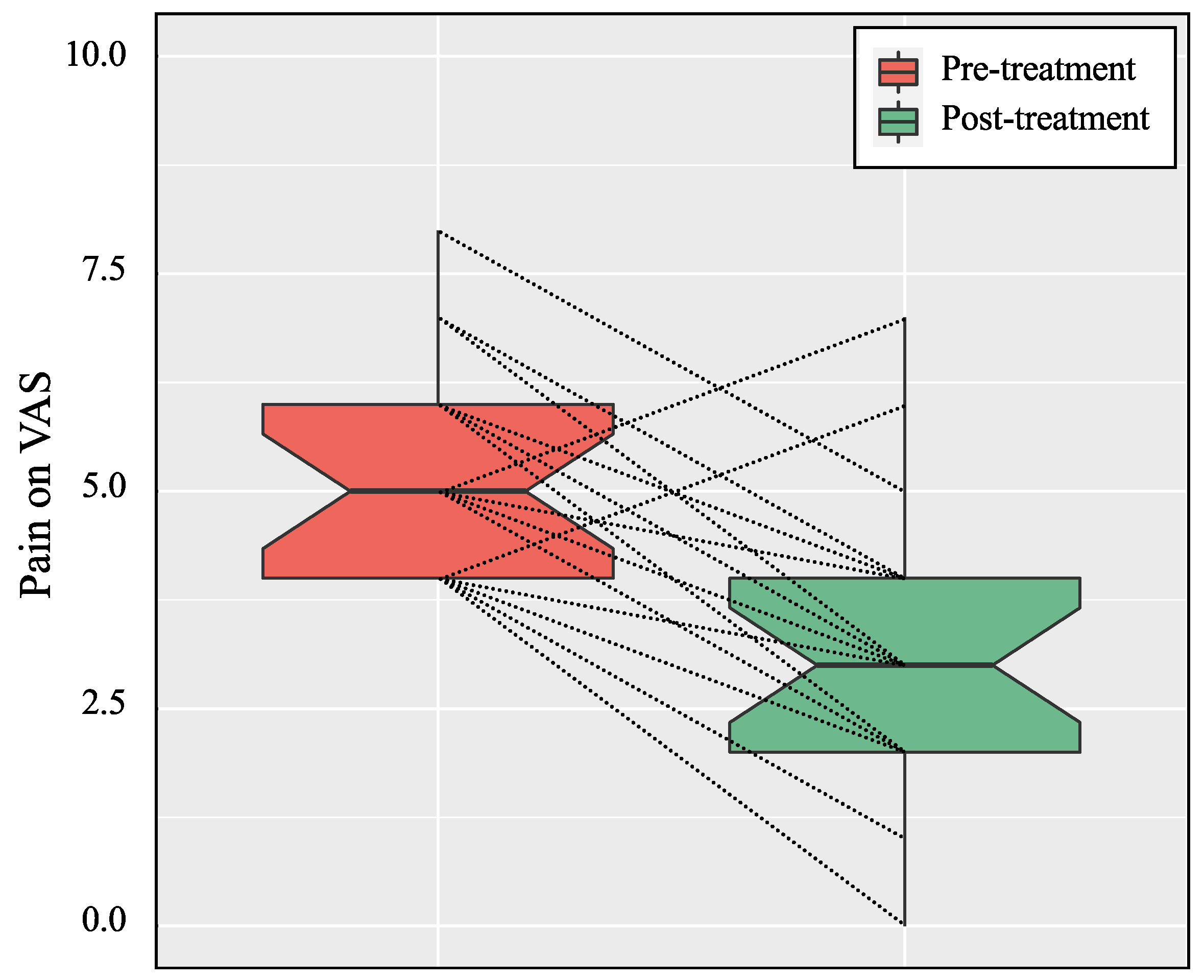
| Pain on VAS | 5.3 | ±1.2 | (4.0–8.0) | 3.1 | ±1.6 | (0.0–7.0) | <0.001 ** |
| Roland Morris | 8.8 | ±3.3 | (2.0–17.0) | 4.0 | ±3.7 | (0.0–13.0) | <0.001 ** |
| PCS | 24.5 | ±9.4 | (3.0–40.0) | 11.7 | ±7.9 | (2.0–26.0) | <0.001 * |
| TSK | 41.5 | ±9.2 | (27.0–62.0) | 32.7 | ±7.0 | (20.0–47.0) | <0.001 * |
| EQ-5D-3L score | 0.59 | ±0.14 | (0.31–0.78) | 0.73 | ±0.07 | (0.57–0.85) | <0.001 ** |
| EQ-5D-3L on VAS | 54.8 | ±16.8 | (20.0–90.0) | 67.0 | ±15.2 | (40.0–90.0) | 0.004 * |
| Final Cohort (n = 23 Patients) | |||||
|---|---|---|---|---|---|
| Net Improvement | Responders * | ||||
| Mean | ±SD | (Range) | N | (%) | |
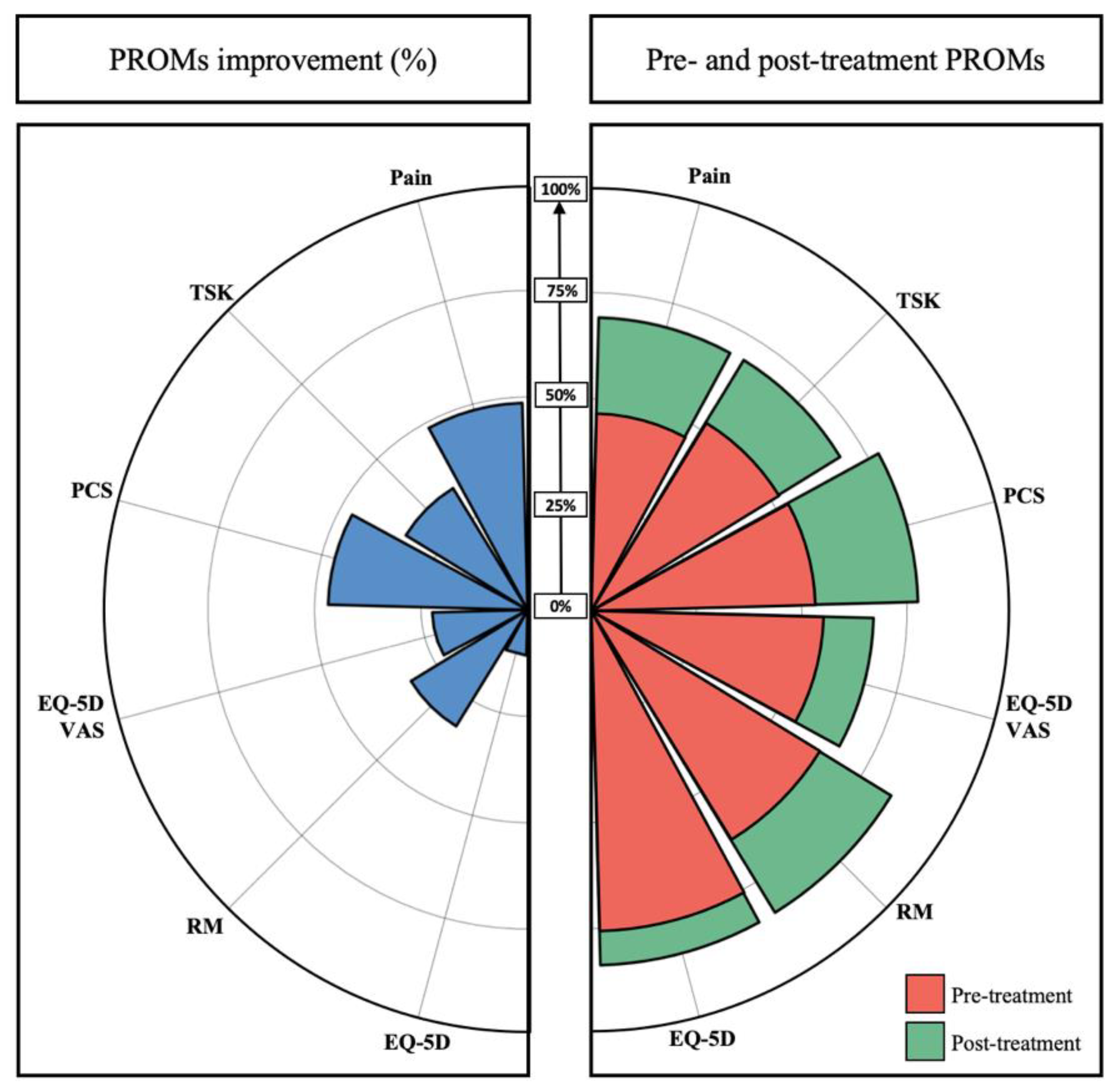
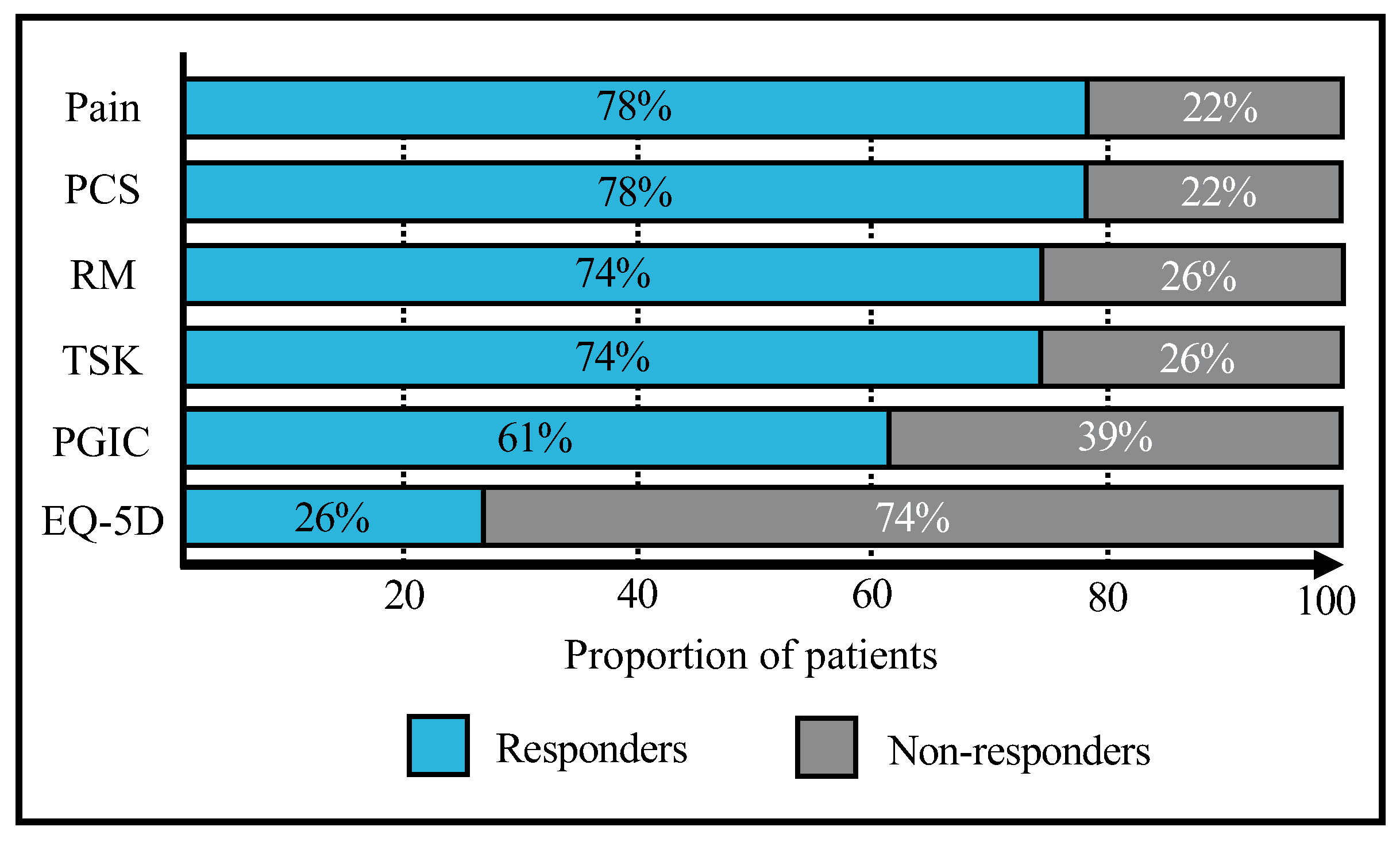
| Pain on VAS (MCID = 2.0 pts) | 2.3 | ±1.7 | (−2.0–4.0) | 18 | (78.3%) |
| Roland Morris (MCID = 5.0 pts) | 4.9 | ±2.9 | (0.0–10.0) | 17 | (73.9%) |
| PCS (MCID = 5.8 pts) | 12.9 | ±9.8 | (−7.0–36.0) | 18 | (78.3%) |
| TSK (MCID = 4.5 pts) | 8.9 | ±6.8 | (−2.0–23.0) | 17 | (73.9%) |
| EQ-5D-3L score (MCID = 0.190 pts) | 0.13 | ±0.14 | (0.00–0.46) | 6 | (26.1%) |
| PGIC (MCID = 5 pts) | 4.4 | ±1.6 | (1.0–7.0) | 14 | (60.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, C.; Sayegh, S.; Faundez, A.; Fourchet, F.; Bothorel, H. Effectiveness of a Group-Based Rehabilitation Program Combining Education with Multimodal Exercises in the Treatment of Patients with Nonspecific Chronic Low Back Pain: A Retrospective Uncontrolled Study. Biology 2022, 11, 1508. https://doi.org/10.3390/biology11101508
Martins C, Sayegh S, Faundez A, Fourchet F, Bothorel H. Effectiveness of a Group-Based Rehabilitation Program Combining Education with Multimodal Exercises in the Treatment of Patients with Nonspecific Chronic Low Back Pain: A Retrospective Uncontrolled Study. Biology. 2022; 11(10):1508. https://doi.org/10.3390/biology11101508
Chicago/Turabian StyleMartins, Cristiano, Souheil Sayegh, Antonio Faundez, François Fourchet, and Hugo Bothorel. 2022. "Effectiveness of a Group-Based Rehabilitation Program Combining Education with Multimodal Exercises in the Treatment of Patients with Nonspecific Chronic Low Back Pain: A Retrospective Uncontrolled Study" Biology 11, no. 10: 1508. https://doi.org/10.3390/biology11101508
APA StyleMartins, C., Sayegh, S., Faundez, A., Fourchet, F., & Bothorel, H. (2022). Effectiveness of a Group-Based Rehabilitation Program Combining Education with Multimodal Exercises in the Treatment of Patients with Nonspecific Chronic Low Back Pain: A Retrospective Uncontrolled Study. Biology, 11(10), 1508. https://doi.org/10.3390/biology11101508






