Scientific Appraisal and Therapeutic Properties of Plants Utilized for Veterinary Care in Poonch District of Jammu and Kashmir, India
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Quantitative Data Analysis
- (A).
- Relative Frequency of Citation (RFC): RFC was calculated by using the following formula
- (B).
- (C).
- Informant Consensus Factor (ICF): To assess consistency of familiarity with the medicinal plants, the Informant Consensus Factor (ICF) was used by following [49]. Prior to analysis, all the ailments were categorized following Heinrich et al. [49] and Bhatia et al. [28]. The ICF was calculated by using the formula
- (D).
2.4. Statistical Analysis
2.5. Reverse Pharmacological Correlations
3. Results
3.1. Demography of the Informants
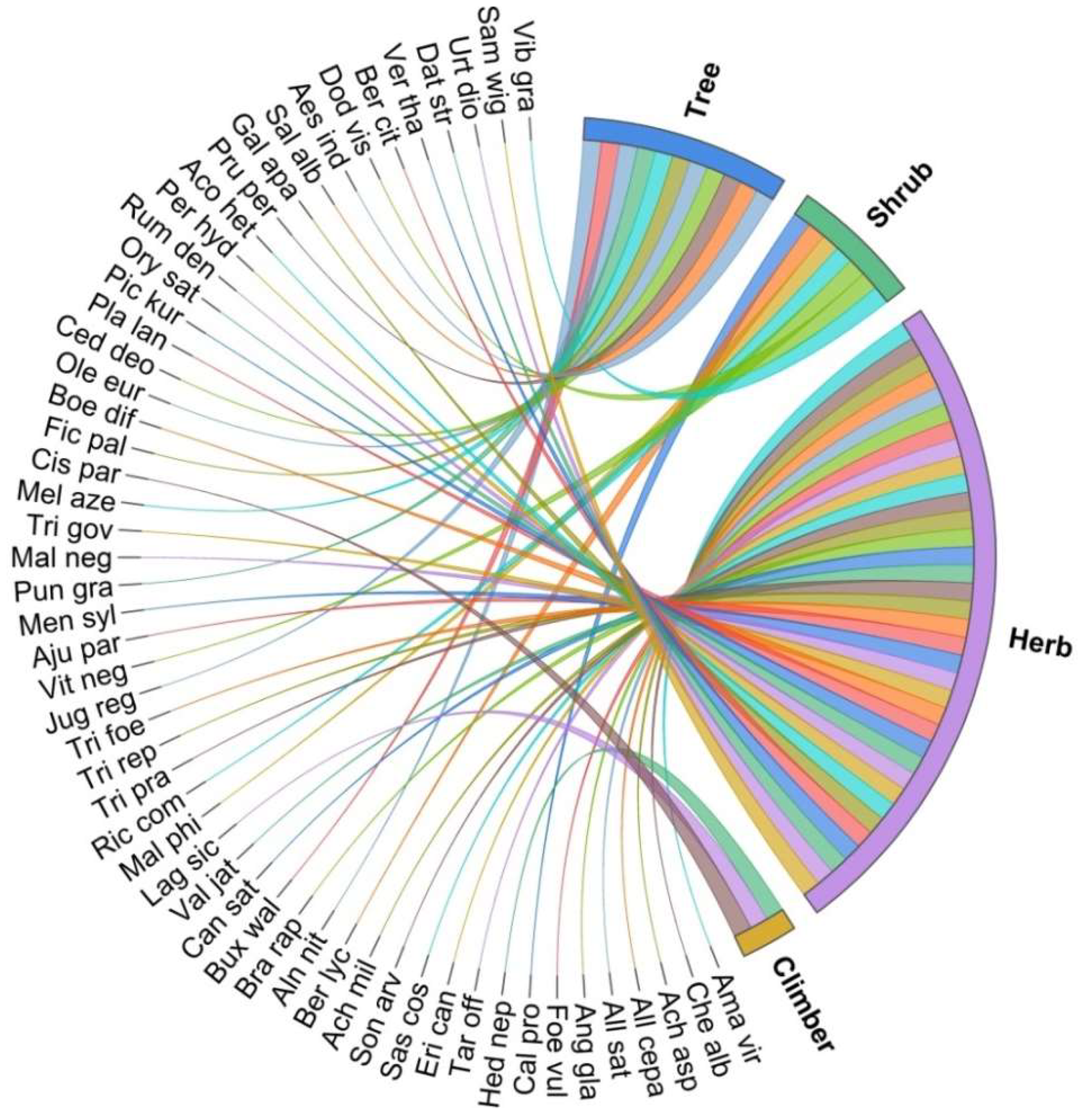
3.2. Medicinal Plant Diversity
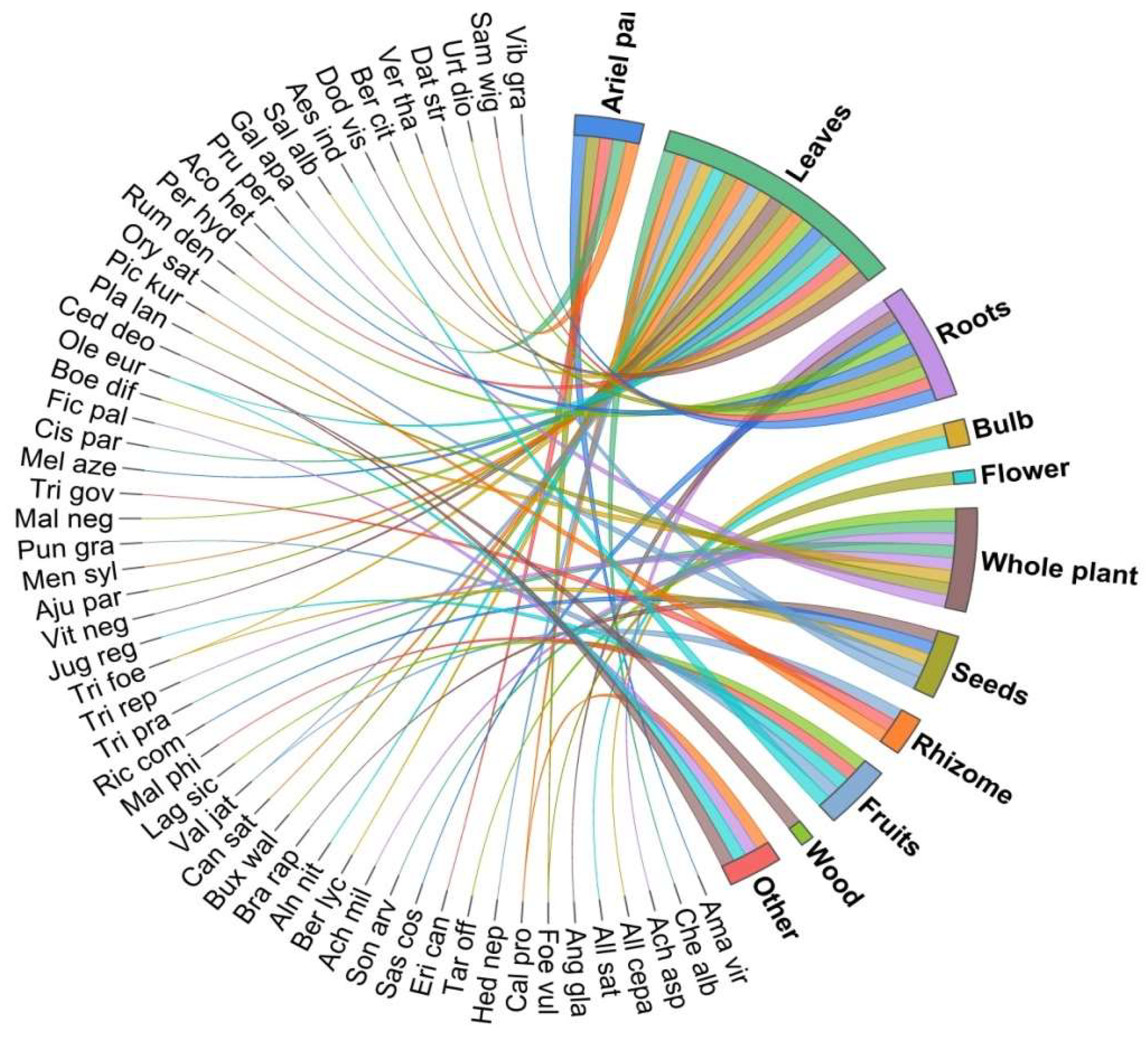
3.3. Utilization Pattern
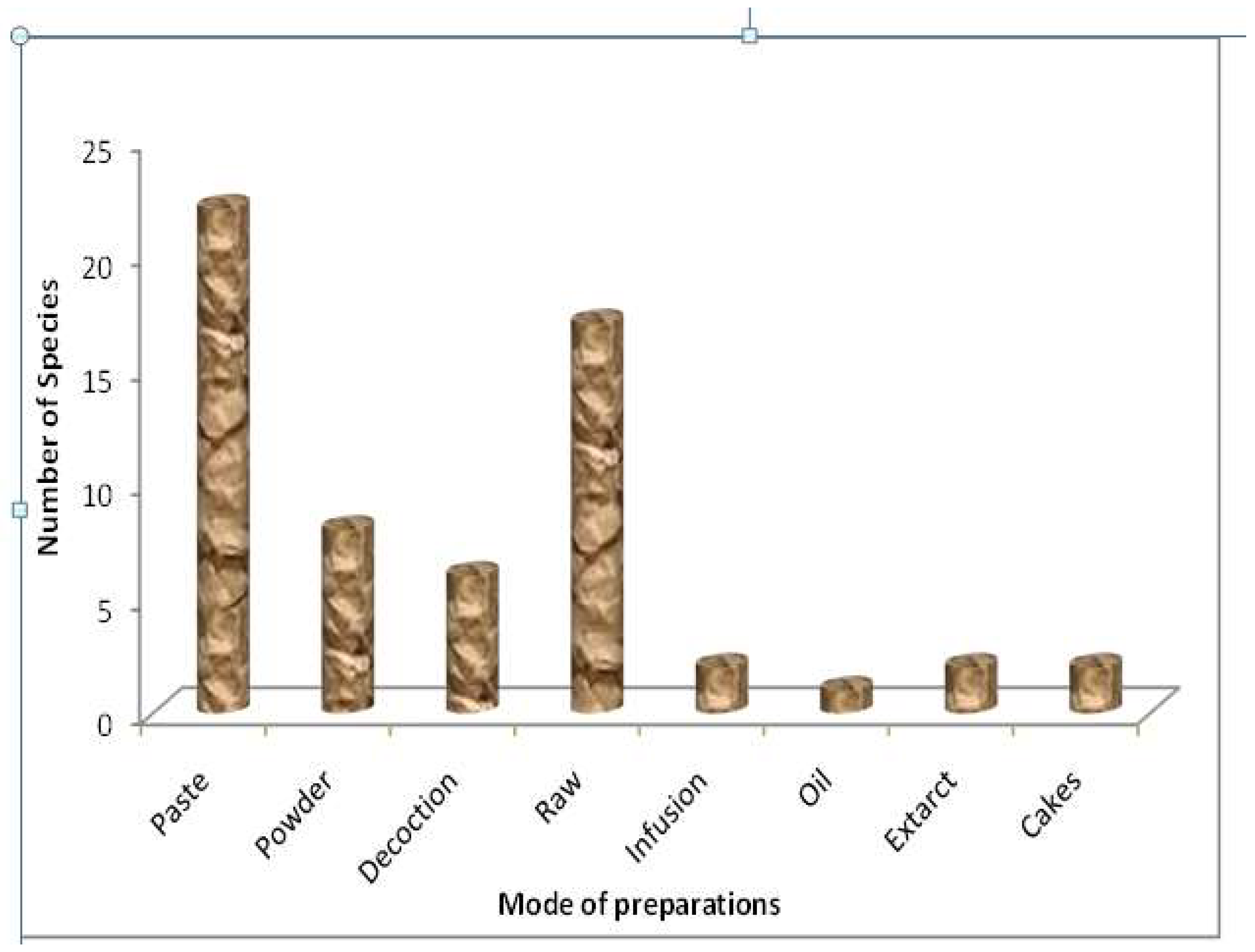
3.4. Methods of Herbal Drug Preparations and Administration
3.5. Quantitative Analysis
3.6. Statistical Analysis
3.7. Reverse Pharmacological Correlation of Ethno-Vetenairy Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balaji, S.N.; Chakravarthi, V.P. Ethnoveterinary practices in India: A review. Vet. World 2010, 3, 549–551. [Google Scholar] [CrossRef]
- Wani, Z.A.; Pant, S. Ethnomedicinal study of plants used to cure skin diseases and healing of wounds in Gulmarg Wildlife Sanctuary (GWLS), Jammu and Kashmir. Indian J. Tradit. Knowl. 2020, 19, 327–334. [Google Scholar]
- Mukherjee, P.K.; Harwansh, R.K.; Bahadur, S.; Banerjee, S.; Kar, A. Evidence-based validation of Indian traditional medicine: Way forward. In From Ayurveda to Chinese Medicine; World Scientific: Singapore, 2017; pp. 137–167. [Google Scholar]
- Nema, N.K.; Dalai, M.K.; Mukherjee, P.K. Ayush herbs and status que in herbal industries. Pharma Rev. 2011, 141, 141–148. [Google Scholar]
- Mukherjee, P.K.; Venkatesh, P.; Ponnusankar, S. Ethnopharmacology and integrative medicine—Let the history tell the future. J. Ayurveda Integr. Med. 2010, 1, 100. [Google Scholar] [CrossRef]
- Sharma, R.; Patki, P. Double-blind, placebo-controlled clinical evaluation of an Ayurvedic formulation (GlucoCare capsules) in non-insulin dependent diabetes mellitus. J. Ayurveda Integr. 2010, 1, 45–51. [Google Scholar]
- Mukherjee, P.K. Quality Control of Herbal Drugs—An Approach to Evaluation of Botanicals, 1st ed.; Business Horizons: New Delhi, India, 2002. [Google Scholar]
- Negi, V.S.; Maikhuri, R.K.; Chandra, A.; Maletha, A.; Dhyani, P.P. Assessing sustainability of farming systems in mountain agroecosystems of Western Himalaya, India. Agroecol. Sustain. Food Syst. 2018, 42, 751–776. [Google Scholar] [CrossRef]
- Negi, V.S.; Maikhuri, R.K.; Rawat, L.S.; Vashishtha, D.P. The livestock production system in a village ecosystem in the Rawain valley, Uttarakhand, Central Himalaya. Int. J. Sustain. Dev. World Ecol. 2010, 17, 431–437. [Google Scholar] [CrossRef]
- Rajkumari, R.; Nirmala, R.K.; Singh, P.K.; Das, A.K.; Dutta, B.K.; Pinokiyo, A. Ethnoveterinary plants used by the Chiru tribes of Manipur, Northeast India. Indian J. Tradit. Knowl. 2014, 13, 368–376. [Google Scholar]
- Negi, V.S.; Maikhuri, R.K.; Rawat, L.S. Paradigm and ecological implication of changing agricultural land-use: A case study from Govind Wildlife Sanctuary, Central Himalaya, India. J. Mt. Sci. 2012, 9, 547–557. [Google Scholar] [CrossRef]
- Parthiban, R.; Vijayakumar, S.; Prabhu, S.; Yabesh, J.G.E.M. Quantitative traditional knowledge of medicinal plants used to treat livestock diseases from Kudavasal taluk of Thiruvarur District, Tamil Nadu, India. Braz. J. Pharm. 2015, 26, 109–121. [Google Scholar] [CrossRef]
- Xiong, Y.; Long, C. An ethnoveterinary study on medicinal plants used by the Buyi people in Southwest Guizhou, China. J. Ethnobiol. Ethnomed. 2020, 16, 46. [Google Scholar] [CrossRef]
- Eshetu, G.R.; Dejene, T.A.; Telila, L.B.; Bekele, D.F. Ethnoveterinary medicinal plants: Preparation and application methods by traditional healers in selected districts of southern Ethiopia. Vet. World 2015, 8, 674–684. [Google Scholar]
- Phondani, P.C.; Maikhuri, R.K.; Kala, C.P. Ethnoveterinary uses of medicinal plants among traditional herbal healers in Alaknanda catchment of Uttarakhand, India. Afr. J. Trad. Compl. Alter. Med. 2010, 7, 195–206. [Google Scholar] [CrossRef]
- Abebe, D.; Ayehu, A. Medicinal Plants and Health Practices of Northern Ethiopia; B.S.P.E.: Addis Ababa, Ethiopia, 1993; p. 511. [Google Scholar]
- Mathias, E.; McCorkle, C.M. Animal health. In Biotechnology: Building on Farmers’ Knowledge; Bunders, J., Haverkort, B., Hiemstra, W., Eds.; MacMillan Education Publishing: Basingstoke, UK, 1997; pp. 22–51. [Google Scholar]
- Raut, A.; Tillu, G.; Vaidya, A.D.B. Reverse pharmacology effectuated by studies of Ayurvedic products for arthritis. Curr. Sci. 2016, 11, 337–342. [Google Scholar] [CrossRef]
- Patwardhan, B.; Mashelkar, R.A. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov. Today 2009, 14, 804–811. [Google Scholar] [CrossRef]
- Surh, Y.J. Reverse pharmacology applicable for botanical drug development—Inspiration from the legacy of traditional medicine. J. Trad. Compl. Med. 2013, 1, 5–7. [Google Scholar]
- Kunwar, R.M.; Nepal, B.K.; Kshhetri, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang districts of Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 1–6. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, V. Ethnobotanical studies in Northwest and Trans-Himalaya: Ethnoveterinary medicines used in Jammu and Kashmir, India. J. Ethnophar. 1989, 27, 63–70. [Google Scholar] [CrossRef]
- Bhat, M.N.; Singh, B.; Surmal, O.; Singh, B.; Shivgotra, V.; Musarella, C.M. Ethnobotany of the Himalayas: Safeguarding medical practices and traditional uses of Kashmir regions. Biology 2021, 10, 851. [Google Scholar] [CrossRef]
- Singh, B.; Singh, S.; Kishor, A.; Singh, B. Traditional usage of medicinal plants in humans and animal health care and their chemical constituents from hills and valleys of Jammu province, Western Himalaya. Indian J. Nat. Prod. Res. 2021, 22, 84–100. [Google Scholar]
- Abass, Z.; Ahmad, J.; Ahmad, I. Socio-economic and educational status of tribal (Gujjar and Bakerwal) of Jammu and Kashmir: An Overview. Int. J. Hum. Soc. Stud. 2015, 3, 35–41. [Google Scholar]
- Wani, Z.A.; Pant, S.; Singh, B. Descriptive study of plant resources in context of ethnomedicinal relevance of indigenous flora; a case study from Rajouri-Poonch region of Himalaya. Ethnobot. Res. Appl. 2021, 21, 1–22. [Google Scholar] [CrossRef]
- Negi, V.S.; Pathak, R.; Thakur, S.; Joshi, R.K.; Bhatt, I.D.; Rawal, R.S. Scoping the Need of Mainstreaming Indigenous Knowledge for Sustainable Use of Bioresources in the Indian Himalayan Region. Environ. Manag. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnophar. 2014, 151, 1005–1018. [Google Scholar]
- Bisht, N.S.; Khajuria, A.K. Ethno-medicinal plants of Tehsil, Kathua, Jammu & Kashmir. J. Mount. Res. 2014, 9, 1–12. [Google Scholar]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef]
- Rao, P.K.; Hasan, S.S.; Bhellum, B.L.; Manhas, R.K. Ethnomedicinal plants of Kathua district, J&K, India. J. Ethnopharmacol. 2015, 171, 12–27. [Google Scholar]
- Kumar, K.; Sharma, Y.P.; Manhas, R.K.; Bhatia, H. Ethnomedicinal plants of Shankaracharya Hill, Srinagar, J&K, India. J. Ethnopharmacol. 2015, 170, 255–274. [Google Scholar]
- Shah, A.; Bharati, K.A.; Ahmad, J.; Sharma, M.P. New ethnomedicinal claims from Gujjar and Bakerwals tribes of Rajouri and Poonch districts of Jammu and Kashmir, India. J. Ethnopharmacol. 2015, 166, 119–128. [Google Scholar] [CrossRef]
- Tali, B.A.; Khuroo, A.A.; Ganie, A.H.; Nawchoo, I.A. Diversity, distribution and traditional uses of medicinal plants in Jammu and Kashmir (J&K) state of Indian Himalayas. J. Herb. Med. 2019, 17, 100280. [Google Scholar]
- Singh, B.; Singh, B.; Kishor, A.; Singh, S.; Bhat, M.N.; Surmal, O.; Musarella, C.M. Exploring plant-based ethnomedicine and quantitative ethnopharmacology in protected area: Ethnobotanical study of medicinal plants utilized by population of Jasrota Hill in Western Himalaya, India. Sustainability 2020, 12, 7526. [Google Scholar] [CrossRef]
- Jan, M.; Mir, T.A.; Ganie, A.H.; Khare, R.K. Ethnomedicinal use of some plant species by Gujjar and Bakerwal community in Gulmarg Mountainous Region of Kashmir Himalaya. Ethnobot. Res. Appl. 2021, 21, 1–23. [Google Scholar] [CrossRef]
- Heinrich, M.; Edwards, S.; Moerman, D.E.; Leonti, M. Ethnopharmacological field studies: A critical assessment of their conceptual basis and methods. J. Ethnopharmacol. 2009, 124, 1–17. [Google Scholar] [CrossRef]
- Edwards, S.; Nebel, S.; Heinrich, M. Questionnaire surveys: Methodological and epistemological problems for field-based ethnopharmacologists. J. Ethnopharmacol. 2005, 100, 30–36. [Google Scholar] [CrossRef]
- Negi, V.S.; Pathak, R.; Sekar, K.C.; Rawal, R.S.; Bhatt, I.D.; Nandi, S.K.; Dhyani, P.P. Traditional knowledge and biodiversity conservation: A case study from Byans Valley in Kailash Sacred Landscape, India. J. Environ. Plan. Manag. 2018, 61, 1722–1743. [Google Scholar] [CrossRef]
- Ribeiroa, R.V.; Bieskia, I.G.C.; Baloguna, S.O.; Martins, D.T.O. Ethnobotanical study of medicinal plants used by Ribeirinhos in the North Araguaia microregion, Mato Grosso. J. Ethnopharmacol. 2017, 205, 69–102. [Google Scholar] [CrossRef]
- Jain, S.K.; Rao, R.R. Handbook of Field and Herbarium Methods; Today and Tomorrows Printers and Publishers: New Delhi, India, 1976. [Google Scholar]
- Singh, N.P.; Singh, D.K.; Uniyal, B.P. Flora of Jammu and Kashmir; Botanical Survey of India; Ministry of Environment and Forests: Calcutta, India, 2002. [Google Scholar]
- Swami, A.; Gupta, B.K. Flora of Udhampur; Bishen Singh Mahendra Pal Singh: Dehradun, India, 1998. [Google Scholar]
- Sharma, B.M.; Kachroo, P. Flora of Jammu and Plants of Neighbourhood Volumes I–II; Bishen Singh Mahendera Pal Singh: Dehradun, India, 1982. [Google Scholar]
- Phillips, O.; Gentry, A.H. The useful plants of Tambopata, Peru, II: Additional hypothesis testing in quantitative ethnobotany. Econ. Bot. 1993, 47, 33–43. [Google Scholar] [CrossRef]
- Zenderland, J.; Hart, R.; Bussmann, R.; Zambrana, M.P.; Sikharulidze, S.; Kikodze, D.; Tchelidze, D.; Khutsishvili, M.; Batsatsashvili, K. The Use of “Use Value”: Quantifying Importance in Ethnobotany. Econ. Bot. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Phillips, O.; Gentry, A.H.; Reynel, C.; Wilki, P.; Gavez–Durand, C.B. Quantitative ethnobotany and Amazonian conservation. Conserv. Biol. 1994, 8, 225–248. [Google Scholar] [CrossRef]
- Musa, M.S.; Abdelrasool, F.E.; Elsheikh, E.A.; Ahmed, L.A.M.N.; Mahmoud, A.L.E.; Yagi, S.M. Ethnobotanical study of medicinal plants in the Blue Nile State, South-eastern Sudan. J. Med. Plants Res. 2011, 5, 4287–4297. [Google Scholar]
- Heinrich, M.; Ankli, A.; Frei, B.; Weimann, C.; Sticher, O. Medicinal plants in Mexico: Healers’ consensus and cultural importance. Soc. Sci. Med. 1998, 47, 1863–1875. [Google Scholar] [CrossRef]
- Amjad, M.S.; Qaeem, M.F.; Ahmad, I.; Khan, S.; Chaudhari, S.K.; Malik, N.Z.; Shaheen, H.; Khan, A.M. Descriptive study of plant resources in the context of the ethnomedicinal relevance of indigenous flora: A case study from Toli Peer National Park, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2017, 13, e0199149. [Google Scholar] [CrossRef]
- Majeed, M.; Bhatti, K.H.; Amjad, M.S.; Abbasi, A.M.; Bussmann, R.W.; Nawaz, F.; Rashid, A.; Mahmood, A.; Khan, W.M.; Ahmad, K.S. Ethno-veterinary uses of Poaceae in Punjab, Pakistan. PLoS ONE 2020, 15, e0241705. [Google Scholar] [CrossRef]
- Munir, M.; Sadia, S.; Khan, A.; Rahim, B.Z.; Nayyar, B.G.; Ahmad, K.S.; Khan, A.M.; Fatima, I.; Qureshi, R. Ethnobotanical study of Mandi Ahmad Abad, District Okara, Pakistan. PLoS ONE 2022, 17, e0265125. [Google Scholar] [CrossRef]
- Dar, G.H.; Malik, A.H.; Khuroo, A.A. A Contribution to the Flora of Rajouri and Poonch Districts in the Pir Panjal Himalaya (Jammu & Kashmir), India. Check List 2014, 10, 317–328. [Google Scholar]
- Yineger, H.; Kelbessa, E.; Bekele, T.; Lulekal, E. Ethnoveterinary medicinal plants at Bale Mountains National Park, Ethiopia. J. Ethnopharmacol. 2007, 112, 55–70. [Google Scholar] [CrossRef]
- Khattak, N.S.; Nouroz, F.; Rahman, I.; Noreen, S. Ethno veterinary uses of medicinal plants of district Karak, Pakistan. J. Ethnopharmacol. 2015, 171, 273–279. [Google Scholar] [CrossRef]
- Sharafatmandrad, M.; Mashizi, A.K. Ethnopharmacological study of native medicinal plants and the impact of pastoralism on their loss in arid to semiarid ecosystems of southeastern Iran. Sci. Rep. 2020, 10, 15526. [Google Scholar] [CrossRef]
- Okach, D.O.; Nyunja, A.R.O.; Opande, G. Phytochemical screening of some wild plants from Lamiaceae and their role in traditional medicine in Uriri District—Kenya. Int. J. Herb. Med. 2013, 1, 135–143. [Google Scholar]
- Rodriguez-Chavez, J.L.; Egas, V.; Linares, E.; Bye, R.; Hernandez, T.; Espinosa-Garcia, F.J.; Delgado, G. Mexican arnica (Heterotheca inuloides Cass. Asteraceae: Asteraceae): Ethnomedical uses, chemical constituents and biological properties. J. Ethnopharmacol. 2017, 195, 39–63. [Google Scholar] [CrossRef]
- Tewari, D.; Mocan, A.; Parvanov, E.D.; Sah, A.N.; Nabavi, S.M.; Huminiecki, L.; Ma, Z.F.; Lee, Y.Y.; Horbanczuk, J.O.; Atanasov, A.G. Ethnopharmacological approaches for therapy of jaundice: Part II. Highly used plant species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae families. Front. Pharmacol. 2017, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.I.M.M.; Van Staden, J. Ethnobotany, phytochemistry and pharmacology of Arctotis arctotoides (L.f.) O. Hoffm.: A review. J. Ethnopharmacol. 2018, 220, 294–320. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yao, H.; Song, J.; Zhu, Y.; Liu, C.; Chen, S. Evaluating the feasibility of using candidate DNA barcodes in discriminating species of the large Asteraceae family. BMC Evol. Biol. 2010, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Samant, S.S. Ethnobotanical observations in Mornaula Research Forest of Kumoun, West Himalaya, India. Ethnobot. Leafl. 2010, 14, 193–217. [Google Scholar]
- Chekole, G.; Asfaw, Z.; Kelbessa, E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J. Ethnobiol. Ethnomed. 2015, 11, 4. [Google Scholar] [CrossRef]
- Cheikhyoussef, A.; Summers, R.W.; Kahaka, G. Qualitative and quantitative analysis of phytochemical compounds in Namibian Myrothamnus flabellifolius. Int. Sci. Technol. J. Namib. 2015, 5, 71–83. [Google Scholar]
- Malik, K.; Ahmad, M.; Zafar, M.; Ullah, R.; Mehmood, H.M.; Parveen, B.; Rashid, N.; Sultana, S.; Shah, S.N.; Lubna. An ethnomedicinal study of medicinal plants used to treat skin diseases in northern Pakistan. BMC Complement. Altern. Med. 2019, 19, 210. [Google Scholar] [CrossRef]
- Bano, A.; Ahmad, M.; Hadda, T.B.; Saboor, A.; Sultana, S.; Zafar, M.; Khan, M.P.K.; Arshad, M.; Ashraf, M.A. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J. Ethnobiol. Ethnomedicine 2014, 10, 43. [Google Scholar] [CrossRef]
- Yaseen, G.; Ahmad, M.; Sultana, S.; Alharrasi, A.S.; Hussain, J.; Zafar, M.; Rehman, S. Ethnobotany of Medicinal Plants in the Thar Desert (Sindh) of Pakistan. J. Ethnopharmacol. 2015, 163, 43–59. [Google Scholar] [CrossRef]
- Mahishi, P.; Srinivasa, B.H.; Shivanna, M.B. Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J. Ethnopharmacol. 2005, 98, 307–312. [Google Scholar] [CrossRef]
- Bose, D.; Roy, J.G.; Mahapatra, S.D.; Datta, T.; Mahapatra, S.D.; Biswas, H. Medicinal plants used by tribals in Jalpaiguri district, West Bengal, India. J. Med. Stud. 2015, 3, 15–21. [Google Scholar]
- Faruque, M.; Uddin, S.; Barlow, J.; Hu, S.; Dong, S.Q.; Li, X.; Hu, X. Quantitative ethnobotany of medicinal plants used by indigenous communities in the Bandarban district of Bangladesh. Front. Pharmacol. 2018, 9, 40. [Google Scholar] [CrossRef]
- Raj, A.J.; Biswakarma, S.; Pala, N.A.; Shukla, G.; Vineeta; Kumar, M.; Chakravarty, S.; Bussmann, R.M. Indigenous uses of ethnomedicinal plants among forest-dependent communities of Northern Bengal, India. J. Ethnobiol. Ethnomed. 2018, 14, 8. [Google Scholar] [CrossRef]
- Pala, N.A.; Sarkar, B.C.; Shukla, G.; Chettri, N.; Deb, S.; Bhat, J.A.; Chakravarty, S. Floristic composition and utilization of ethnomedicinal plant species in home gardens of the Eastern Himalaya. J. Ethnobiol. Ethnomed. 2019, 15, 14. [Google Scholar] [CrossRef]
- Giday, M.; Asfaw, Z.; Woldu, Z. Medicinal plants of the Meinit ethnic group of Ethiopia: An ethnobotanical study. J. Ethnopharmacol. 2009, 124, 513–521. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, S.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Ayyanar, M.; Ignacimuthu, S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J. Ethnopharmacol. 2011, 134, 851–864. [Google Scholar] [CrossRef]
- Yabesh, J.E.M.; Prabhu, S.; Vijayakumar, S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. J. Ethnopharmacol. 2014, 154, 774–789. [Google Scholar] [CrossRef]
- Rahman, I.U.; Ijaz, F.; Iqbal, Z.; Afzal, A.; Ali, N.; Afzal, M.; Khan, M.A.; Muhammad, S.; Qadir, G.; Asif, M.A. Novel Survey of the Ethno Medicinal Knowledge of Dental Problems in Manoor Valley (Northern Himalaya), Pakistan. J. Ethnopharmacol. 2016, 194, 877–894. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wahile, A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. J. Ethnopharmacol. 2006, 103, 25–35. [Google Scholar] [CrossRef]
- Majid, A.; Ahmad, H.; Saqib, Z.; Rahman, I.; Khan, U.; Alam, J.; Shah, A.H.; Jan, S.A.; Ali, N. Exploring threatened traditional knowledge; ethnomedicinal studies of rare endemic flora from Lesser Himalayan region of Pakistan. Braz. J. Phar. 2019, 29, 785–792. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Osivand, A.; Kpabitey, S.; Amoatey, C.A.; Oikawa, Y.; Fujii, Y. Exploring alternative use of medicinal plants for sustainable weed management. Sustainability 2017, 9, 1468. [Google Scholar] [CrossRef]
- Linh, D.T.T.; Duong, H.T.; Hiep, N.T.; Huyen, P.T.; Khoi, N.M.; Long, D.D. Simultaneous quantification of Hederacoside C and α-hederin in Hedera nepalensis K. Koch using HPLC-UV. VNU J. Sci. Med. Pharm. Sci. 2020, 36. [Google Scholar] [CrossRef]
- Madikizela, B.; Ndhlala, A.R.; Finnie, J.F.; Van Staden, J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. J. Ethnopharmacol. 2012, 141, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Sticher, O.; Heinrich, M. Antiquity of medicinal plant usage in two Macro-Mayan ethnic groups (Mexico). J. Ethnopharmacol. 2003, 88, 119–124. [Google Scholar] [CrossRef]
- Rahman, S.; Ismail, M.; Shah, M.R.; Iriti, M.; Muhammad, S. GC/MS analysis, free radical scavenging, anticancer β glucuronidase inhibitory activities of Trillium govanianum rhizome. Bangladesh J. Pharm. 2015, 10, 577–583. [Google Scholar] [CrossRef]
- Leonti, M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011, 134, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Ch, M.I.; Khan, M.A.; Hanif, W. Ethnoveterinary Medicinal Uses of Plants of from Samahni Valley District Bhimber, (Azad Kashmir) Pakistan. Asian J. Plant Sci. 2006, 5, 390–396. [Google Scholar]
- Sharma, R.; Manhas, R.K.; Magotra, R. Ethnoveterinary remedies of diseases among milk yielding animals in Kathua, Jammu and Kashmir, India. J. Ethnopharmacol. 2012, 141, 265–272. [Google Scholar] [CrossRef]
- Khuroo, A.A.; Malik, A.H.; Dar, A.R.; Dar, G.H.; Khan, Z.S. Ethno-veterinary medicinal uses of some plant species by the Gujjar tribe of the Kashmir Himalaya. Asian J. Plant Sci. 2007, 6, 148–152. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, M.A.; Mujtaba, G.; Hussain, M. Ethnobotanical study about medicinal plants of Poonch valley Azad Kashmir. J. Anim. Plant Sci. 2012, 22, 493–500. [Google Scholar]
- Harsha, V.H.; Shripathi, V.; Hedge, G.R. Ethnoveterinary practices in Uttara Kannada district of Karnataka. Indian J. Tradit. Knowl. 2005, 4, 253–258. [Google Scholar]
- Yadav, S.S.; Bhukal, R.K.; Bhandoria, M.S.; Ganie, S.A.; Gulia, S.K.; Raghav, T.B.S. Ethnoveterinary Medicinal plants of Tosham block of district Bhiwani (Haryana) India. J. Appl. Pharm. Sci. 2014, 4, 40–48. [Google Scholar]
- Nigam, G.; Sharma, N.K. Ethnoveterinary plants of Jhansi district, Uttar Pradesh. Indian J. Tradit. Knowl. 2010, 9, 664–667. [Google Scholar]
- Aziem, S.; Chamola, B.P.; Mahato, S.; Pala, N.A. Utilization and traditional knowledge of ethnoveterinary medicinal plants in Tehri district of Garhwal Himalaya, India. Int. J. Indig. Med. Plants 2013, 46, 1330–1337. [Google Scholar]
- Narayana, V.L.; Narasimharao, G.M. Plants used in Ethnoveterinary Medicine by Tribals of Visakhapatnam and Vizianagarm Districts, Andhra Pradesh, India. Int. J. Pure Appl. Biosci. 2015, 3, 432–439. [Google Scholar]
- Chen, G.; Yang, M.; Song, Y.; Lu, Z.; Zhang, J.; Huang, H.; Guan, S.; Wu, L.; Guo, D. Comparative analysis on microbial and rat metabolism of ginsenoside Rb1 by high-performance liquid chromatography coupled with tandem mass spectrometry. Biomed. Chromatogr. 2008, 22, 779–785. [Google Scholar] [CrossRef]
- Ahmad, M.; Sultana, S.; Fazl-i-Hadi, S.; Ben Hadda, T.; Rashid, S.; Zafar, M.; Khan, M.A.; Khan, M.P.Z.; Yaseen, G. An Ethnobotanical study of Medicinal Plants in high mountainous region of Chail valley (District Swat-Pakistan). J. Ethnobiol. Ethnomedicine 2014, 10, 36. [Google Scholar] [CrossRef]
- Kumar, A.B.S.; Lakshman, K.; Jayaveera, K.N.; Nandeesh, R.; Manitripathi, S.N.; Krishna, V.; Manjunath, M.; Suresh, M.V. Estimation of Rutin and Quercitin in Amaranthus viridis L. by High Performance Layer Chromatography (HPLC). Ethnobot. Leafl. 2009, 13, 437–442. [Google Scholar]
- Iqbal, M.J.; Hanif, S.; Mahmood, Z.; Anwar, F.; Jamil, A. Antioxidant and antimicrobial activities of Chowlai (Amaranthus viridis L.) leaf and seed extracts. J. Med. Plants Res. 2012, 6, 4450–4455. [Google Scholar]
- Carminate, B.; Martin, G.B.; Barcelos, R.M.; Gontijo, I. Evaluation of antifungal activity of Amaranthus viridis L. (Amaranthaceae) on Fusariosis by Piper nigrum L. and on Anthracnose by Musa sp. Agric. J. 2012, 7, 215–219. [Google Scholar]
- Kumar, A.B.S.; Lakshman, K.; Jayaveera, K.N.; Nandeesh, R.; Manoj, B.; Ranganayakula, D. Comparative in vitro anthelminthic activity of three plants from the Amaranthaceae family. Arch. Biol. Sci. 2010, 62, 185–189. [Google Scholar] [CrossRef]
- Ahmad, M.; Mohiuddin, O.A.; Mehjabeen, N.J.; Anwar, M.; Habib, S.; Alam, S.M.; Baig, I.A. Valuation of spasmolytic and analgesic activity of ethanolic extract of Chenopodium album (Linn.) and its fraction. Med. Plants Res. 2012, 6, 4691–4697. [Google Scholar]
- Akhtar, M.B.; Iqbal, Z.; Khan, M.N. Evaluation of anthelmintic activity of Chenopodiumalbum (Bathu) against nematodes in sheep. Int. J. Agric. Biol. 1999, 1, 121–124. [Google Scholar]
- Bylka, W.; Kowalewski, Z. Flavonoids in Chenopodium album L. and Chenopodium opulifolium L. Herba Pol. 1997, 43, 208–213. [Google Scholar]
- Goyal, B.R.; Goyal, R.K.; Mehta, A.A. Phyto-pharmacology of Achyranthes aspera: A review. Pharmacogn. Rev. 2007, 1, 143–150. [Google Scholar]
- Prasad, S.; Bhattacharya, I.C. Pharmacognostical studies of Achyranthes aspera Linn. J. Sci. Ind. Res. 1961, 20, 46–51. [Google Scholar]
- Kapoor, V.K.; Singh, H. Isolation of betain from Achyranthes aspera Linn. Indian J. Chem. 1966, 4, 461. [Google Scholar]
- Fossen, T.; Pedersen, A.T.; Andersen, O.M. Flavonoids from red onion (Allium cepa). Phytochemistry 1998, 42, 281–285. [Google Scholar] [CrossRef]
- Nasri, S.; Anoush, M.; Khatami, N. Evaluation of analgesic and anti-inflammatory effects of fresh onion juice in experimental animals. Afr. J. Pharm. Pharmacol. 2012, 6, 1679–1684. [Google Scholar]
- Sakakibara, H.; Yoshino, S.; Kawai, Y.; Terao, J. Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Biosci. Biotechnol. Biochem. 2008, 72, 94–100. [Google Scholar] [CrossRef] [PubMed]
- El-Saber, B.G.; Magdy, B.A.; Wasef, G.L.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, M.Y.; Prasad, D.H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M.; Ali, M. Garlic (Allium sativum): A review of its potential use as an anti-cancer agent. Curr. Cancer Drug Targ. 2003, 3, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Mengesha, W. Traditional uses, phytochemistry and pharmacological properties of garlic (Allium sativum) and its biological active compounds. Int. J. Sci. Res. Eng. Technol. 2015, 1, 142–148. [Google Scholar]
- Butola, J.S.; Vashistha, R.K. An overview on conservation and utilization of Angelica glauca Edgew. in three Himalayan states of India. Med. Plants 2013, 5, 171–178. [Google Scholar]
- Chopra, R.N.; Ayer, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of Scientific and Industrial Research (CSIR): New Delhi, India, 1992. [Google Scholar]
- Anonymous. Wealth of India, Revised Volume 1; Publication and Information Directorate, CSIR: New Delhi, India, 1985. [Google Scholar]
- Hoult, J.R.S.; Paya, M. Pharmacological and Biochemical activities of simple coumarins: Natural products with therauptical potential. Gen. Pharmacol. Vasc. Syst. 1996, 27, 713–722. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sanchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography-negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Sharma, K.; Kharb, R.; Kaur, R. Pharmacognostical aspects of Calotropis procera (Ait.) R.Br. Int. J. Pharm. Biol. Sci. 2011, 2, 1–9. [Google Scholar]
- Verma, R.; Satsangi, G.P.; Shrivastava, J.N. Ethno-medicinal profile of different plant parts of Calotropis procera (Ait.) R.Br. Ethnobot. Leafl. 2010, 14, 721–742. [Google Scholar]
- Gupta, S.; Gupta, B.; Kapoor, K.; Sharma, P. Ethnopharmacological potential of Calotropis procera: An overview. Int. Res. J. Pharm. 2012, 3, 19–22. [Google Scholar]
- Quazi, S.; Mathur, K.; Arora, S. Calotropis procera: An overview of its phytochemistry and pharmacology. Indian J. Drugs 2013, 1, 63–69. [Google Scholar]
- Jafri, L.; Saleem, S.; Kondrytuk, T.P.; Haq, I.U.; Ullah, N.; Pezzuto, J.M.; Mirza, B. Hedera nepalensis K. Koch: A Novel Source of Natural Cancer Chemopreventive and Anticancerous Compounds. Phytother. Res. 2016, 30, 447–453. [Google Scholar] [CrossRef]
- Hashmi, W.J.; Ismail, H.; Mehmood, F.; Mirza, B. Neuroprotective, antidiabetic and antioxidant effect of Hedera nepalensis and lupeol against STZ + AlCl3 induced rats model. DARU J. Pharm. Sci. 2018, 26, 179–190. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; Abo-Golayel, M.K.; Abo-Elnaga, G.; Al-beshri, H. Hepatoprotective effects of dandelion (Taraxacum officinale) against induced chronic liver cirrhosis. J. Med. Plants Res. 2013, 7, 1494–1505. [Google Scholar]
- Clare, B.A.; Conroy, R.S.; Spelman, K. The diuretic effect in human subjects of an extract of Taraxacum officinale folium over a single day. J. Altern. Complement. Med. 2009, 15, 929–934. [Google Scholar] [CrossRef]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmocol. 2013, 2, 1–5. [Google Scholar]
- Grauso, L.; Emrick, S.; De Falco, B.; Lanzotti, V.; Bonamoni, G. Common dandelion: A review of its botanical, phytochemical and pharmacological profiles. Phytochem. Rev. 2019, 18, 1115–1132. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Mukhtar, N.; Iqbal, K.; Anis, I.; Malik, A. Sphingolipids from Conyza canadensis. Phytochemistry 2002, 61, 1005–1008. [Google Scholar] [CrossRef]
- Shah, N.Z.; Khan, M.A.; Muhammad, N.; Azeem, S. Antimicrobial and phytotoxic study of Conyza canadensis. J. Med. Plants Res. 2012, 1, 63–67. [Google Scholar]
- Al-Snafi, A.E. Pharmacological and therapeutic importance of Erigeron canadensis (Syn. Conyza canadensis). Indo Am. J. Pharm. Sci. 2017, 4, 248–256. [Google Scholar]
- Govindan, S.V.; Bhattacharaya, S.C. Alantolides and cyclocostunolides from Saussurea lappa. Indian J. Chem. 1977, 15, 956. [Google Scholar]
- Kumar, S.; Ahuja, N.M.; Juawanda, G.S.; Chhabra, B.R. New guaianolides from Saussurea lappa roots. Fitoterapia 1995, 66, 287–288. [Google Scholar]
- Cho, J.Y.; Park, J.; Yoo, E.S.; Baik, K.U.; Jung, J.H.; Lee, J.; Park, M.H. Inhibitory effect of sesquiterpene lactones from Saussurea lappa on tumor necrosis factor-alpha production in murine macrophage like cells. Planta Med. 1998, 64, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Talwar, K.K.; Singh, I.P.; Kalsi, P.S. A serquiterpenoid with plant growth regulatory activity from Saussurea lappa. Phytochemistry 1991, 31, 1336–1338. [Google Scholar]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Saussurea costus: Botanical, chemical and pharmacological review of an ayurvedic medicinal plant. J. Ethnopharmacol. 2007, 110, 279–390. [Google Scholar] [CrossRef]
- Lalla, J.K.; Hamrapurkar, P.D.; Mukherjee, S.A.; Thorat, U.R. Sensitivity of HPTLC v/s HPLC for the analysis of alkaloid-Saussurine. In Proceedings of the 54th Indian Pharmaceutical Congress, Pune, India, 13–15 December 2002; p. 293. [Google Scholar]
- Tandi, J.; Sutrisna, I.N.E.; Pratiwi, M.; Handayani, T.W. Potential test neuropathy Sonchus arvensis L. leaves on male rats (Rattus norvegicus) Diabetes mellitus. Pharmacogn. J. 2020, 12, 1115–1120. [Google Scholar] [CrossRef]
- Xia, D.Z.; Yu, X.F.; Zhu, Z.Y.; Zou, Z.D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901. [Google Scholar] [CrossRef]
- Alkreathy, H.M.; Khan, R.A.; Khan, M.R.; Sahreen, S. CCl4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis. BMC Complement. Altern. Med. 2014, 14, 452. [Google Scholar] [CrossRef]
- Akram, M. Minireview on Achillea millefolium Linn. J. Membr. Biol. 2013, 246, 661–663. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany, bioactivity and biomedical research. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Chandler, R.F.; Hooper, S.N.; Harvey, M.J. Ethnobotany and phytochemistry of yarrow, Achillea millefolium, Compositae. Econ. Bot. 1982, 36, 203–223. [Google Scholar] [CrossRef]
- Parra, S.A.; Gaur, K.; Ranawat, L.S.; Rather, M.I. An overview of various aspects of plant Berberis lycium Royale. Am. J. Pharmacol. Sci. 2018, 6, 19–24. [Google Scholar]
- Shabir, A.; Shahzad, M.; Arfat, Y.; Ali, L.; Aziz, R.S.; Murtaza, G.; Waqar, S.A.; Alamgeer. Berberis lycium Royle: A review of its traditional uses, phytochemistry and pharmacology. Afr. J. Pharm. Pharmacol. 2012, 6, 2346–2353. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.R.; Shah, N.A.; Shah, S.A.; Ismail, H.; Younis, T.; Zahra, Z. Phytochemical, antioxidant and hepatoprotective effects of Alnus nitida bark in carbon tetrachloride challenged Sprague Dawley rats. BMC Complement. Altern. Med. 2016, 16, 268. [Google Scholar] [CrossRef]
- Sajid, M.; Yan, C.; Li, D.; Merugu, B.; Negi, H.; Khan, M.R. Potent anticancer activity of Alnus nitida against lung cancer cells; in vitro and in vivo studies. Biomed. Pharmacother. 2019, 110, 254–264. [Google Scholar] [CrossRef]
- Siddiqui, I.N.; Ahmad, V.U.; Zahoor, A.; Ahmed, A.; Khan, S.S.; Khan, A.; Hassan, Z. Two new diaryl heptanoids from Alnus nitida. Nat. Prod. Commun. 2010, 5, 1787–1788. [Google Scholar]
- Dejanovic, G.M.; Asllanaj, E.; Gamba, M.; Raguindin, P.F.; Itodo, O.A.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; Glisic, M.; et al. Phytochemical characterization of turnip greens (Brassica rapa ssp. rapa): A systematic review. PLoS ONE 2021, 16, e0247032. [Google Scholar] [CrossRef]
- Jan, S.A.; Shinwari, Z.K.; Malik, M.; Ilyas, M. Antioxidant and anticancer activities of Brassica rapa: A review. MOJ Biol. Med. 2018, 3, 175–178. [Google Scholar]
- Nandeesh, R.; Kumar, A.; Lakshman, K.; Swamy, V.B.N.; Khan, S.; Ganapathy, S. Evaluation of wound healing activity of Buxus wallichiana Bail. Asian J. Pharmacodyn. Pharmacokinet. 2010, 10, 59–63. [Google Scholar]
- Ata, A.; Naz, S.; Choudhary, M.I.; Atta-ur-Rahman, N.; Sener, B.; Turkoz, S. New triterpenoidal alkaloids from Buxus sempervirens. Z. Nat. C 2002, 57, 21–28. [Google Scholar]
- Atta-ur-Rahman; Ata, A.; Naz, S.; Choudhary, M.I.; Sener, B.; Turkoz, S. New Steroidal Alkaloids from the roots of Buxus sempervirens. J. Nat. Prod. 1999, 62, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 5, 227–300. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2018, 33, 482–503. [Google Scholar] [CrossRef]
- Prajapati, R.P.; Kalariya, M.; Parmar, S.K.; Sheth, N.R. Phytochemical and pharmacological review of Lagenaria siceraria. J. Ayurveda Integr. Med. 2010, 1, 266–272. [Google Scholar] [CrossRef]
- Tanaka, R.; Nakata, T.; Yamaguchi, C.; Wada, S.; Yamada, T.; Tokuda, H. Potential anti-tumor-promoting activity of 3-Hydroxy-D: A-friedooleanan-2-one from the stem bark of Mallotus philippinensis. Planta Med. 2008, 74, 413–416. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, M.; Kumar, P.; Bhatti, R.C.; Kaur, R.; Sharma, N.K.; Singh, A.N. Mallotus philippensis (Lam.) Mull. Arg. A review on its pharmacology and phytochemistry. J. Herbmed Pharm. 2021, 10, 31–50. [Google Scholar] [CrossRef]
- Marwat, S.K.; Rehman, F.; Khan, E.A.; Baloch, M.S.; Sadiq, M.; Ullah, I.; Javaria, S.; Shaheen, S. Review-Ricinus communis-Ethnomedicinal uses and pharmacological activities. Pak. J. Pharm. Sci. 2017, 30, 1815–1827. [Google Scholar]
- Pope, G.S.; Elcoate, P.V.; Simpson, S.A.; Andrews, D.G. Isolation of an oestrogenic isoflavone (biochanin A) from redclover. Chem. Ind. 1953, 10, 1092. [Google Scholar]
- Sabudak, T.; Guler, N. Trifolium L. A review on its phytochemical and pharmacological profile. Phytother. Res. 2009, 23, 439–446. [Google Scholar] [CrossRef]
- Oleszek, W.; Stochmal, A. Triterpene saponins and flavonoids in the seeds of Trifolium species. Phytochemistry 2002, 6, 165–170. [Google Scholar] [CrossRef]
- Toppo, F.A.; Anand, R.; Pathak, A.K. Pharmacological actions and potential uses of Trigonella foenum graecum L. A review. Asian J. Pharm. Clin. Res. 2009, 2, 29–38. [Google Scholar]
- Arunabha, M.; Bhattacharjee, C. Trigonella foenum-graecum: A review of its traditional uses, phytochemistry and pharmacology. Int. J. Adv. Sci. Res. 2019, 5, e5217. [Google Scholar]
- Gupta, A.; Behl, T.; Panichayupakaranan, P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents, nutritional, pharmacological and therapeutic importance of Juglans regia—A review. IOSR J. Pharm. 2018, 8, 1–21. [Google Scholar]
- Suva, M.A. A Brief Review on Vitex negundo Linn: Ethnobotany, Phytochemistry and Pharmacology. Planta Act. 2014, 1, 1. [Google Scholar]
- Rastogi, T.; Kubde, M.; Farooqui, I.A.; Khadabadi, S.S. A review on ethnomedicinal uses and phyto-pharmacology of anti-inflammatory herb Vitex negundo. Int. J. Pharm. Sci. Res. 2017, 1, 23–28. [Google Scholar]
- Nirja, R.; Sharma, M.L. Antidiabetic and antioxidant activity of ethanolic extract of Ajuga parviflora Benth. (Lamiaceae) vern. Neelkanthi, Neelbati. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 232–238. [Google Scholar]
- Khan, P.M.; Malik, A.; Ahmad, S.; Nawab, H.F. Withanolides from Ajuga parviflora. J. Nat. Prod. 1999, 62, 1290–1292. [Google Scholar] [CrossRef]
- Rahman, N.; Ahmad, M.; Riaz, M.; Mehjabeen, J.N.; Ahmad, R. Phytochemical, antimicrobial, insecticidal and brine shrimp lethality bioassay of the crude methanolic extract of Ajuga parviflora Benth. Pak. J. Pharm. Sci. 2013, 26, 751–756. [Google Scholar] [PubMed]
- Rastogi, R.P.; Mehrotra, B.N. Compendium of Indian Medicinal Plants; Central Drug Research Institute: New Delhi, India, 1991. [Google Scholar]
- Jamal, A.; Siddiqui, A.; Tajuddin, A.; Jafri, M.A. A review on gastric ulcer remedies used in Unani System of medicine. Nat. Prod. Rad. 2006, 5, 153–159. [Google Scholar]
- Akram, M.; Uzair, M.; Malik, N.S.; Mahmood, A.; Sarwer, N.; Madni, A.; Asif, H.M. Mentha arvensis Linn. A review article. J. Med. Plants Res. 2011, 5, 4499–4503. [Google Scholar]
- Jain, V.; Muruganathan, G.; Deepak, M.; Vishwanatha, L.G.; Manohar, D. Isolation and standardization of various phytochemical constituents from methanolic extracts of fruit rinds of Punica granatum. Nat. Med. 2011, 9, 414–420. [Google Scholar]
- Mehta, D.; Mehta, M. Punica granatum L. (Punicaceae): Lifeline for Modern Pharmaceutical Research. J. Ethnopharmacol. 2012, 4, 185–199. [Google Scholar]
- Jayaprakash, A. Punica granatum: A review of phytochemicals, antioxidant and antimicrobial properties. J. Acad. Ind. Res. 2017, 5, 132–138. [Google Scholar]
- Keyrouz, E.; El Feghali, P.A.R.; Jaafar, M.; Nawas, T. Malva neglecta: A natural inhibitor of bacterial growth and biofilm formation. J. Med. Plants Res. 2017, 11, 380–386. [Google Scholar]
- Seyyednejad, S.M.; Koochak, H.; Darabpour, E.; Motamedi, H. A survey on Hibiscus rosa-sinensis, Alcea rosea L and Malva neglecta Wall. as antibacterial agents. Asian Pac. J. Trop. Med. 2010, 3, 351–355. [Google Scholar] [CrossRef]
- Saleem, U.; Akhtar, R.; Anwar, F.; Shah, M.A.; Chaudary, Z.; Ayaz, M.; Ahmad, B. Neuroprotective potential of Malva neglecta is mediated via down regulation of cholinesterase and modulation of oxidative stress markers. Metab. Brain Dis. 2012, 36, 889–900. [Google Scholar] [CrossRef]
- Saleem, U.; Khalid, S.; Zaib, S.; Anwar, F.; Ahmad, B.; Ullah, I.; Zeb, A.; Ayaz, M. Phytochemical analysis and wound healing studies on ethnomedicinally important plant Malva neglecta Wall. J. Ethnopharmacol. 2020, 249, 112401. [Google Scholar] [CrossRef]
- Radha, S.P.; Pundir, A. Review of Ethnomedicinal plant: Trillium govanianum Wall. ex D. Don. Int. J. Theor. Appl. Sci. 2019, 11, 4–9. [Google Scholar]
- Vishnukanta, A.C.; Rana, A. Melia azedarach: A phytopharmacological review. Pharmacogn. Rev. 2008, 2, 173–179. [Google Scholar]
- Rani, M.; Suhag, P.; Kumar, R.; Singh, R.; Kalidhar, S.B. Chemical component and biological efficacy of Melia azedarach stems. J. Med. Aromat. Plant Sci. 1999, 21, 1043–1047. [Google Scholar]
- Merra, P.S.; Kalidhar, S.B. Phytochemical investigation of Melia azedarach leaves. J. Med. Aromat. Plant Sci. 2003, 25, 397–399. [Google Scholar]
- Kumari, S.; Anmol; Bhatt, V.; Suresh, P.S.; Sharma, U. Cissampelos pareira L. A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 274, 113850. [Google Scholar] [CrossRef]
- Singh, S.; Nishteswar, K. Review on Cissampelos pareira and Cyclea peltata (Patha Dwaya)—Phyto-Pharmacological Perspectives. Int. J. Ayurvedic Med. 2013, 4, 282–289. [Google Scholar] [CrossRef]
- Alqasoumi, S.I.; Basudan, O.A.; Al-Rehaily, A.J.; Abdel-Kader, M.S. Phytochemical and pharmacological study of Ficus palmata growing in Saudi Arabia. Saudi Pharm. J. 2014, 22, 460–471. [Google Scholar] [CrossRef]
- Maurya, R.; Sathiamoorthy, B.; Mundkinajeddu, D. Flavonoids and phenol glycosides from Boerhavia diffusa. Nat. Prod. Res. 2007, 21, 126–134. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Justin, M.; Valentao, P.; Andrade, P.B.; Llorach, R.; Rodrigues, A.; Seabra, R.M.; Leitao, A. Characterization of the phenolic profile of Boerhaavia diffusa L. by HPLC-PAD-MS/MS as a tool for quality control. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 451–458. [Google Scholar] [CrossRef]
- Pereira, D.M.; Faria, J.; Gaspar, L.; Valentao, P.; Andrade, P.B. Boerhaavia diffusa: Metabolite profiling of a medicinal plant from nyctaginaceae. Food Chem. Toxicol. 2009, 47, 2142–2149. [Google Scholar] [CrossRef]
- Kapil, S.P.; Sanjivani, R.B. Ethnomedicinal uses, phytochemistry and pharmacological properties of the genus Boerhavia. J. Ethnopharmacol. 2016, 182, 200–220. [Google Scholar]
- Chaudhary, A.K.; Ahmad, S.; Mazumder, A. Cedrus deodara (Roxb.) Loud. A Review on its Ethnobotany, Phytochemical and Pharmacological Profile. Pharmacogn. Res. 2011, 3, 12–17. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Sarikurkcu, C.; Kocak, M.S.; Calapoglu, M.; Uren, M.C.; Ceylan, O. Plantago lanceolata as a source of health-beneficial phytochemicals: Phenolics profile and antioxidant capacity. Food Biosci. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, A.M.B.; Wan Sulaiman, M.W.A.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Sharma, N.; Pathania, V.; Singh, B.; Gupta, R.C. Intraspecific variability of main phytochemical compounds in Picrorhiza kurroa Royle ex Benth. from North Indian higher altitude Himalayas using reversed phase high-performance liquid chromatography. J. Med. Plants Res. 2012, 6, 3181–3187. [Google Scholar]
- Vasas, A.; Orban-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef]
- Huq, A.K.M.M.; Jamal, J.A.; Stanslas, J. Ethnobotanical, Phytochemical, pharmacological and toxicological aspects of Persicaria hydropiper (L.) Delarbre. Evid.-Based Complement. Altern. Med. 2014, 2014, 782830. [Google Scholar] [CrossRef]
- Adams, S.J.; Kuruvilla, G.R.; Krishnamurthy, K.V.; Nagarajan, M.; Venkatasubramanian, P. Pharmacognostic and phytochemical studies on Ayurvedic drugs Ativisha and Musta. Braz. J. Pharm. 2013, 23, 398–409. [Google Scholar] [CrossRef]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Latif, A.; Ali, M.; Khan, F.A.; Tahir, M.N.; Shaheen, F.; Wadood, A.; Ahmad, M. Antioxidant and anticholinesterase potential of diterpenoid alkaloids from Aconitum heterophyllum. Bioorgan. Med. Chem. 2017, 25, 3368–3376. [Google Scholar] [CrossRef]
- Nisar, M.; Obaidullah; Ahmad, M.; Wadood, N.; Lodhi, M.A.; Shaheen, F.; Choudhary, M.I. New diterpenoid alkaloids from Aconitum heterophyllum Wall: Selective butyrylcholinestrase inhibitors. J. Enzym. Inhib. Med. Chem. 2009, 24, 47–51. [Google Scholar] [CrossRef]
- Wani, Z.A.; Pant, S. Aconitum heterophyllum Wall. ex Royle: An Endemic, Highly Medicinal and Critically Endangered Plant Species of Northwestern Himalaya in Peril. Curr. Tradit. Med. 2021, 7, 2–7. [Google Scholar] [CrossRef]
- Bento, C.; Concalves, A.C.; Silva, B.; Silva, R.S. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev. Int. 2020, 38, 1703–1734. [Google Scholar] [CrossRef]
- Vlase, L.; Mocan, A.; Hanganu, D.; Benedec, D.; Gheldiu, A. Comparative study of polyphenolic content, antioxidant and antimicrobial activity from Galium species (Rubiaceae). Dig. J. Nanomater. Biostruct. 2014, 9, 1085–1094. [Google Scholar]
- Morimoto, M.; Tanimoto, K.; Sakatani, A.; Komai, K. Antifeedant activity of an anthraquinone aldehyde in Galium aparine L. against Spodoptera litura F. Phytochemistry 2002, 60, 163–166. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and medicinal importance of Galium aparine: A Review. Indo Am. J. Pharm. Sci. 2018, 5, 1739–1744. [Google Scholar]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, N.; El-Shazly, A.M. Phytochemsirty, pharmacology and medicinal uses of plants of Genus Salix: An updated review. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, G.S. Evaluation of immunomodulatory activity of Aesculus indica. Int. J. PharmTech Res. 2009, 1, 132–134. [Google Scholar]
- Zahoor, M.; Shafiq, S.; Ullah, H.; Sadiq, A.; Ullah, F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018, 19, 5. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. A review of Dodonaea viscosa: A potential medicinal plant. IQSR J. Pharm. 2017, 7, 10–21. [Google Scholar] [CrossRef]
- Ahmad, M.; Butt, M.A.; Zhang, G.; Sultana, S.; Tariq, A.; Zafar, M. Bergenia ciliata: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. Biomed. Pharmacother. 2018, 97, 708–721. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-ul-Haq, M.; Jaffar, H.Z.E. Common mullein, pharmacological and chemical aspects. Braz. J. Pharmacogn. 2013, 23, 948–959. [Google Scholar] [CrossRef]
- Sayyed, A.; Shah, M. Phytochemistry, pharmacological and traditional uses of Datura stramonium L. review. J. Pharmacogn. Phytochem. 2014, 2, 123–125. [Google Scholar]
- Asgarpanah, J.; Mohajerani, R. Phytochemistry and pharmacologic properties of Urtica dioica L. J. Med. Plants Res. 2012, 6, 5714–5719. [Google Scholar]
- Chashoo, I.A.; Kumar, D.; Bhat, Z.A.; Khan, N.A.; Kumar, V.; Nowshehri, J.A. Antimicrobial activities of Sambucus wightiana Wall. ex Wight & Arn. J. Pharm. Res. 2012, 5, 2467–2468. [Google Scholar]
- Wang, X.; Shi, H.M.; Li, X.B. Chemical Constituents of Plants from the Genus Viburnum. Chem. Biodivers. 2010, 7, 267–593. [Google Scholar] [CrossRef]
- Muhammad, A.; Ghaisuddin; Anwar, S.; Naveed, M.; Ashfaq, A.K.; Bina, S.S. Evaluation of Viburnum grandiflorum for its in-vitro pharmacological screening. Afr. J. Pharm. Pharmacol. 2012, 6, 1606–1610. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A. Allicin: Chem & Biol Prop. Molecules 2014, 18, 12591–12618. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mat. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Habtemariam, S. Rutin as a Natural Therapy for Alzheimer’s Disease: Insights into its Mechanisms of Action. Curr. Med. Chem. 2016, 23, 860–873. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agarwal, P.; Rajput, S. A review of Quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Wal, P.; Wal, A.; Sharma, G.; Rai, A.K. Biological activities of Lupeol. Syst. Rev. Pharm. 2011, 2, 96–103. [Google Scholar] [CrossRef]
- Jhonston, G.A.R.; Chebib, M.; Duke, R.K.; Fernandez, S.P.; Hanrahan, J.R.; Hinton, T.; Mewett, K.N. Herbal Products and GABA Receptors. In Encyclopedia of Neuroscience; Academia Press: Ghent, Belgium, 2009; pp. 1095–1101. [Google Scholar]
- Shukla, R.; Panday, V.; Vadnera, G.P.; Lodhi, S. Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Academia Press: Ghent, Belgium, 2019; pp. 293–322. [Google Scholar]
- Wang, S.C.; Lu, M.C.; Chen, H.L.; Tseng, H.; Ke, Y.; Wu, Y.C.; Yang, P. Cytotoxicity of calotropin is through caspase activation and downregulation of anti-apoptotic proteins in K562 cells. Cell Biol. Int. 2009, 33, 1230–1236. [Google Scholar] [CrossRef]
- Cheng, L.; Xia, T.; Wang, Y.; Zhou, W.; Liang, X.; Xue, J.; Shi, L.; Wang, Y.; Ding, Q.; Wang, M. The anticancer effect and mechanism of α-hederin on breast cancer cells. Int. J. Oncol. 2014, 45, 757–763. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhang, Z.; Chen, Z.; Zhang, Z.; Tian, C.; Liu, M.; Jiang, G. The antibacterial activity and mechanism of action of Luteolin against Trueperella pyogenes. Infect. Drug Resist. 2020, 13, 1697–1711. [Google Scholar] [CrossRef]
- Qi, S.; Quan, L.Q.; Cui, X.Y.; Li, X.; Zhao, X.D.; Li, R.T. A natural compound obtained from Valeriana jatamansi selectively inhibits glioma stem cells. Oncol. Lett. 2019, 19, 1384–1392. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam, T.G.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A Key Emphasis to its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, N.; Zhang, J.; Liu, S.; Liu, Y.; Zheng, D. PKCδ protects human breast tumor MCF-7 cells against tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis. J. Cell. Biochem. 2005, 96, 522–532. [Google Scholar] [CrossRef]
- Borris, R.P. Natural products research: Perspectives from a major pharmaceutical company. J. Ethnopharmacol. 1996, 51, 29–38. [Google Scholar] [CrossRef]
- Pieters, L.; Vlietinck, A.J. Bioguided isolation of pharmacologically active plant components, still a valuable strategy for the finding of new lead compounds? J. Ethnopharmacol. 2005, 100, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Said, A.; Naeem, N.; Siraj, S.; Khan, T.; Javed, A.; Rasheed, H.M.; Sajjad, W.; Shah, K.; Wahid, F. Mechanisms underlying the wound healing and tissue regeneration properties of Chenopodium album. 3 Biotech 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Birdane, F.M.; Cemek, M.; Birdane, Y.O.; Gülçin, I.; Büyükokuroğlu, E. Beneficial effects of vulgare ethanol-induced acute gastric mucosal injury in rat. World J. Gastroenterol. 2007, 13, 607–611. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Eloff, J.N. Methods for evaluating efficacy of ethnoveterinary medicinal plants. In Ethnoveterinary Botanical Medicine: Herbal Medicines for Animal Health; Katerere, D.R., Luseba, D., Eds.; CRC Press: London, UK, 2010; pp. 1–24. [Google Scholar]

| S. No. | Variable | Category | No. of Informants | % Age |
|---|---|---|---|---|
| 1 | Gender | Male | 38 | 64.40 |
| Female | 21 | 35.59 | ||
| 2 | Marital status | Married | 37 | 62.7 |
| Unmarried | 22 | 37.3 | ||
| 2 | Age (in years) | 20–35 | 15 | 25.42 |
| 36–50 | 23 | 38.98 | ||
| Above 50 | 21 | 35.59 | ||
| 3 | Educational Qualification | Illiterate | 19 | 32.20 |
| Primary | 13 | 22.03 | ||
| Middle | 10 | 16.95 | ||
| Secondary | 8 | 13.56 | ||
| Hr. Sec. | 5 | 8.47 | ||
| Above | 4 | 6.78 |
| Taxa/Local Name | Family/ Voucher Number | Life Form | Ethno-Veterinary Uses | Part(s) Used | Preparation | Method of Use | FC | RFC | UV |
|---|---|---|---|---|---|---|---|---|---|
| Amaranthus viridis L. {Ganar} | Amaranthaceae/BGSBU-01 | H | Tonic | AP | Raw | Aerial parts of the plant are cut into small pieces and mixed with wheat husk. This mixture is fed preferably twice a day for two weeks. | 23 | 0.38 | 0.35 |
| Chenopodium album L. {Bathua} | Amaranthaceae/BGSBU-02 | H | Wound healing | L | Paste | Paste prepared from the leaves boiled in mustard oil is applied externally. | 32 | 0.53 | 0.58 |
| Achyranthes aspera L. {Phutkanda} | Amaranthaceae/BGSBU-03 | H | Fever | R | Paste | Root paste is given orally. | 39 | 0.65 | 0.68 |
| Poisonous bite | R | Infusion | Infusion of root is given to cattle. | ||||||
| Allium cepa L. {Gandha} | Amaryllidaceae/ BGSBU-04 | H | Loss of appetite | B | Paste | Paste of bulbs mixed with salt is fed to the cattle. | 37 | 0.61 | 0.58 |
| Stimulate oestrus cycle | B | Paste | Crushed bulbs are mixed with salt and given to cows. | ||||||
| Skin infection | B | Paste | Paste prepared from the crushed bulbs is applied on the infected body part. | ||||||
| Allium sativum L. {Thoom} | Amaryllidaceae/ BGSBU-05 | H | Deworming | B | Paste | Paste of crushed bulbs is mixed with flour and given orally. | 23 | 0.38 | 0.31 |
| Angelica glauca Edgew. {Chora} | Apiaceae/BGSBU-06 | H | Cold | R | Decoction | Decoction of root is given to cattle thrice a day. | 31 | 0.51 | 0.54 |
| Diarrhea | R | Paste | Root paste is given to cattle. | ||||||
| Alopecia | R | Paste | Root paste is applied externally. | ||||||
| Foeniculum vulgare Mill. {Sounf} | Apiaceae/BGSBU-07 | H | Indigestion | AP | Decoction | Aerial parts are boiled in water and are fed to the animal for 2–3 days | 43 | 0.71 | 0.78 |
| Constipation | F | Decoction | Decoction prepared by boiling fruits in water is given to cattle. | ||||||
| Calotropis procera (Aiton) W.T.Aiton {Aak} | Apocynaceae/ BGSBU-08 | S | Removal of retained placenta | Ltx | Raw | Tail of buffaloes is dipped for 4–5 min into latex. | 38 | 0.63 | 0.61 |
| Deworming | L | Raw | Green leaves are given as feedstuff daily. | ||||||
| increase milk production | L | Raw | Dried leaves are given as feedstuff especially in case of goat to increase the milk quantity. | ||||||
| Hedera nepalensis K.Koch {Harbembel} | Araliaceae/BGSBU-09 | C | Leech removing | L | Extract | Leaf extract is put in the nostrils. | 41 | 0.68 | 0.65 |
| Taraxacum officinale F.H.Wigg. {Handh} | Asteraceae/BGSBU-10 | H | Enhance milk production | WP | Raw | Whole plant is fed to cattle with other feeds. | 45 | 0.75 | 0.78 |
| Stretch of bones and ligaments | AP | Decoction | Decoction prepared by boiling aerial parts into water in 1:1 ratio is given for about 15 days. | ||||||
| Erigeron canadensis L. {Kuttey Haddi} | Asteraceae/BGSBU-11 | H | Indigestion | AP | Paste | Aerial parts of the plants are crushed and the paste is fed to the cattle. | 29 | 0.49 | 0.47 |
| Saussurea costus (Falc.) Lipsch. {Kuth} | Asteraceae/BGSBU-12 | H | Tonic | R | Powder | Root powder mixed with crushed onion bulbs, gur (raw sugar) and water is fed to the cattle. | 21 | 0.36 | 0.38 |
| Sonchus arvensis Linn. {Sonchal} | Asteraceae/BGSBU-13 | H | Enhance milk production | WP | Raw | For increasing milk production, fresh plants are fed to cattle. | 39 | 0.66 | 0.71 |
| Achillea millefolium L. {Chou} | Asteraceae/BGSBU-14 | H | Deworming | WP | Raw | Whole plants are fed to animals. | 42 | 0.71 | 0.67 |
| Berberis lycium Royle {Simboo} | Berberidaceae/ BGSBU-15 | S | Wound healing | L | Paste | Leaves are chewed and this paste is applied on the wounds. | 35 | 0.59 | 0.63 |
| Alnus nitida (Spach) Endl. {Sarol} | Betulaceae/BGSBU-16 | T | Foot and mouth disease | L | Paste | Dried leaves are mixed with oil and applied on the affected parts. | 31 | 0.53 | 0.51 |
| Brassica rapa L. {Sariyoon} | Brassicaceae/BGSBU-17 | H | Enhance milk production | Sd | Cakes | Seed cakes locally called Khahl is fed to lactating cows and buffaloes to enhance milk production. | 48 | 0.81 | 0.87 |
| Vigor maintenance of bulls | Sd | Cakes | Mixture of seed cakes and rice husk is fed to bulls. | ||||||
| Skin Infection | Sd | Paste | Seeds are ground and mixed with mustard oil. This paste is applied externally on infected parts for a week. | ||||||
| Buxus wallichiana Baill. {Chikhri} | Buxaceae/BGSBU-18 | T | Skin infection | L | Decoction | Decoction of fresh or dried leaves is given orally. | 24 | 0.41 | 0.45 |
| Cannabis sativa L. {Bhang} | Cannabaceae/ BGSBU-19 | H | Removal of lice and ticks | L | Paste | Paste of crushed leaves is applied externally. | 31 | 0.53 | 0.51 |
| Valeriana jatamansi Jones ex Roxb. {Balo} | Caprifoliaceae/ BGSBU-20 | H | Fatigue | Rh | Powder | Dried rhizomes are ground to fine powder which is dissolved in about 200–300 mL of normal water and is given to the cattle in the morning for about a week. | 29 | 0.49 | 0.53 |
| Diarrhea | L | Raw | Fresh leaves are used directly or their extract to cure diarrhea. | ||||||
| Lagenaria siceraria (Molina) Standl. {Doberi} | Cucurbitaceae/ BGSBU-21 | C | Yoke Galls | F | Paste | Fruits are burned. Ash is mixed with luke warm mustard oil and the paste is applied on yoke galls of bulls. | 22 | 0.37 | 0.31 |
| Mallotus philippensis (Lam.) Mull. Arg. {Kamila} | Euphorbiaceae/ BGSBU-22 | T | Deworming | F | Powder | Powdered dry fruits are mixed with flour and given to animals for 2–3 days. | 29 | 0.49 | 0.47 |
| Ricinus communis L. {Arand} | Euphorbiaceae/ BGSBU-23 | S | Dysentery | Sd | Paste | Seeds are crushed in small quantity, mixed with fodder and given to cattle. | 30 | 0.51 | 0.53 |
| Trifolium pratense L. {Shatul} | Fabaceae/BGSBU-24 | H | Enhance milk production | WP | Raw | Whole plant is fed to cattle | 45 | 0.76 | 0.79 |
| Trifolium repens L. {Srieh} | Fabaceae/BGSBU-25 | H | Enhance milk production | WP | Raw | Whole plant is fed to cattle | 39 | 0.66 | 0.63 |
| Trigonella foenum-graecum L. {Methi} | Fabaceae/BGSBU-26 | H | Diarrhea | L Sd | Raw | Leaves and seeds are fed to animal for 3–4 days. | 34 | 0.58 | 0.62 |
| Juglans regia Linn. {Khor} | Juglandaceae/ BGSBU-27 | T | Enhancing Milk Production | F | Cakes | The oil cakes obtained by grinding of fruit kernels are fed to cows to enhance their milk production. | 26 | 0.44 | 0.40 |
| Vitex negundo L. {Banno} | Lamiaceae/BGSBU-28 | S | Fever | L | Paste | Young leaves are crushed and given orally. | 24 | 0.41 | 0.38 |
| Ajuga parviflora Benth. {Jan-i-adam} | Lamiaceae/BGSBU-29 | H | Weakness | L | Infusion | Water extract of fresh leaves is given to cattle | 30 | 0.51 | 0.53 |
| Indigestion | L | Decoction | Decoction is given to animals orally | ||||||
| Fever | L | Decoction | Decoction is given to animals orally | ||||||
| Mentha sylvestris L. {Pootno} | Lamiaceae/BGSBU-30 | H | Deworming | L | Raw | Leaves are fed to live stock | 27 | 0.46 | 0.41 |
| Punica granatum L. {Daruno} | Lythraceae/BGSBU-31 | T | Jaundice | F | Decoction | Decoction of fruit exocarp is given orally. | 37 | 0.63 | 0.71 |
| Malva neglecta Wall. {Sonchal} | Malvaceae/BGSBU-32 | H | Constipation | L | Paste | Soaked leaves are crushed, mixed with cow-butter and fed to newly born calves. | 28 | 0.47 | 0.53 |
| Detachment of placenta in Cows | L | Paste | Paste is fed to cows to facilitate the detachment and expulsion of placenta after delivery. | ||||||
| Trillium govanianum Wall. ex D.Don {Trae patri} | Melanthiaceae/ BGSBU-33 | H | Deworming | Rh | Paste | Crushed rhizome is given to the cattle. | 16 | 0.27 | 0.21 |
| Melia azedarach L. {Dreck} | Meliaceae/BGSBU-34 | T | Foot and mouth disease | L | Paste | Fresh leaves are crushed with sugar and water. The paste so formed is given orally to the cattle for 2–3 days. | 31 | 0.53 | 0.48 |
| Cissampelos pareira L. | Menispermaceae/ BGSBU-35 | C | Eye problems | L | Infusion | Infusion of leaves is put in eyes | 21 | 0.36 | 0.38 |
| Ficus palmata Forssk. {Kemeri} | Moraceae/BGSBU-36 | T | Wounds | BK | Raw | Bark is applied on wound for quick healing. | 36 | 0.61 | 0.55 |
| Fracture | Bk | Raw | Bark is wrapped around broken bones. | ||||||
| Boerhavia diffusa L. {Itt-sitt} | Nyctaginaceae/ BGSBU-37 | H | Removal of retained placenta | WP | Raw | Whole plants are fed twice a day. | 28 | 0.47 | 0.45 |
| Olea europaea Subsp. cuspidata (Wall. ex. G. Don) Cif. {Kaou} | Oleaceae/BGSBU-38 | T | Deworming | L | Raw | Fresh leaves are given for deworming. | 39 | 0.66 | 0.58 |
| Fracture | Bk | Raw | Fresh stem bark is tied over broken bones. | ||||||
| Cedrus deodara (Roxb. ex D. Don.) Don. {Dyar} | Pinaceae/BGSBU-39 | T | Vomiting | W | Oil | Small pieces of wood are heated in a vessel which causes an oil to ooze out from them. This oil is given to live stock in vomiting. | 27 | 0.46 | 0.51 |
| Hair fall in Goats | W | Oil | Oil is also applied externally to cure hair fall in goats. | ||||||
| Removal of ticks and lice | W | Oil | Oil is also applied externally. | ||||||
| Plantago lanceolata Linn. {Chamch-e-pater} | Plantaginaceae/ BGSBU-40 | H | Yoke galls | WP | Paste | Paste is applied externally. | 29 | 0.49 | 0.54 |
| Picrorhiza kurroa Royle. ex Benth. {Koudh} | Plantaginaceae/ BGSBU-41 | H | Pneumonia | Rh | Powder | Dried rhizome powder mixed with wheat flour, gur and water is fed to cattle. | 16 | 0.27 | 0.29 |
| Tapeworm | Rh | Paste | Paste is given orally. | ||||||
| Oryza sativa Linn. {Chaval} | Poaceae/BGSBU-42 | H | Detachment and expulsion of placenta | Sd | Raw | Grains are fed to cows after delivery to facilitate the detachment and expulsion of placenta. | 35 | 0.59 | 0.56 |
| Constipation | Sd | Paste | Paste of rice flour is made which is given to sheep. | ||||||
| Rumex dentatus L. {Hullo} | Polygonaceae/ BGSBU-43 | H | Gaseous bloats | R | Paste | Fresh roots are crushed, salt is added and small balls are made which are given to cattle. | 33 | 0.56 | 0.51 |
| Cough | R | Paste | Paste is fed to cattle. | ||||||
| Sprained body parts | R | Paste | Paste is mixed with salt and is given orally. | ||||||
| Persicaria hydropiper L.) Delarbre {Pipla} | Polygonaceae/ BGSBU-44 | H | Tongue infection | L | Raw | Chopped leaves are applied on the tongue. | 25 | 0.42 | 0.44 |
| Aconitum heterophyllum Wallich ex Royle {Patris} | Ranunculaceae/ BGSBU-45 | H | Flatulence | R | Powder | Root powder is given with water. | 17 | 0.29 | 0.34 |
| Prunus persica (Linn.)Batsch.{Aarou} | Rosaceae/BGSBU-46 | T | Wound healing | L | Paste | Paste is applied externally. | 25 | 0.42 | 0.47 |
| Galium aparine L. | Rubiaceae/BGSBU-47 | H | Wound healing | WP | Paste | Paste is applied externally. | 21 | 0.36 | 0.29 |
| Salix alba L. {Beeso} | Salicaceae/BGSBU-48 | T | Deworming | Bk L | Decoction | Bark decoction and leaves are given to animals. | 32 | 0.54 | 0.56 |
| Aesculus indica (Wall. ex Camb.)Hook. {Bankhori} | Sapindaceae/BGSBU-49 | T | General weakness | F | Paste | Crushed fruits mixed with onion and salt are fed to cattle. | 31 | 0.53 | 0.47 |
| Dodonaea viscosa Jacquin {Sanatha} | Sapindaceae/BGSBU-50 | S | Deworming | L | Extract | Leaf extract is given to cattle. | 20 | 0.34 | 0.39 |
| Bergenia ciliata (Haw.) Sternb. {Bat mevo} | Saxifragaceae/ BGSBU-51 | H | Diarrhoea | R | Powder | Dried roots are powdered which is given to cattle with luke-warm water. | 30 | 0.51 | 0.46 |
| Enhance milk production | Paste | Paste is fed to the cattle. | |||||||
| Verbascum thapsus L. {Gidharh tamako} | Scrophulariaceae/ BGSBU-52 | H | Flatulence | AP | Decoction | Decoction is prepared by boiling aerial parts in water for about 2 h, is added to the paddy husk and given to the cattle to cure flatulence. | 26 | 0.44 | 0.3 |
| Datura stramonium L. {Daturo} | Solanaceae/BGSBU-53 | H | Leech removing | Sd | Raw | Dried seeds are heated on fire to release smoke which is used to expel leeches from the nasal cavity. | 21 | 0.36 | 0.31 |
| Urtica dioica L. {Kiyarie} | Urticaceae/BGSBU-54 | H | Fractured bones | R | Paste | Root paste is applied on the fractured bones for early healing. | 23 | 0.39 | 0.47 |
| Sambucus wightiana Wall. | Viburnaceae/BGSBU-55 | H | Foot and mouth disease | R | Paste | Paste is applied externally. | 27 | 0.46 | 0.39 |
| Viburnum grandiiflorum Wall ex. Wt & Arn. {Kuch} | Viburnaceae/BGSBU-56 | S | Wound healing | R | Powder | Powdered roots mixed with mustard oil is applied externally. | 37 | 0.63 | 0.64 |
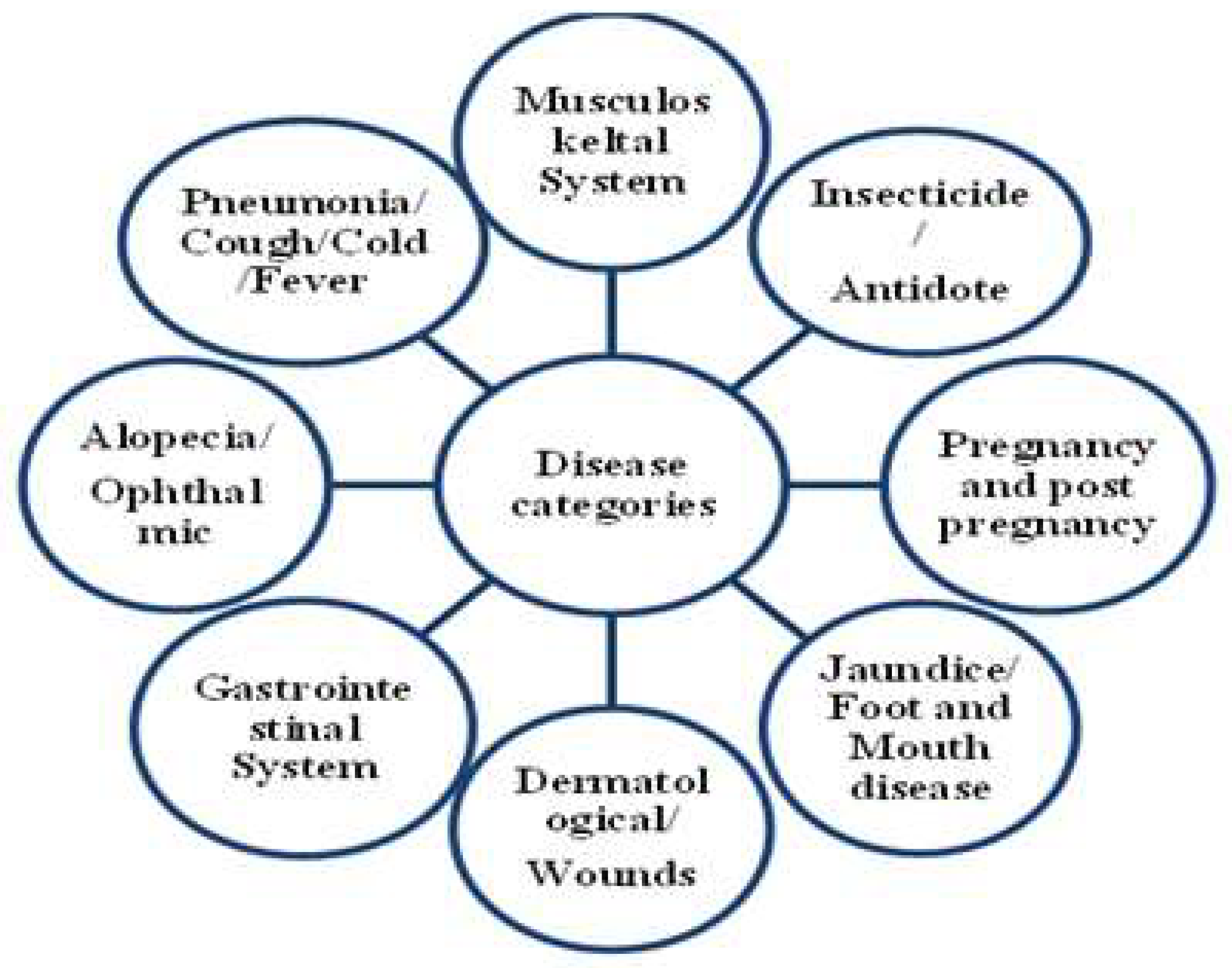
| Disease Category | Ailments Included | Nur | Nt | ICF |
|---|---|---|---|---|
| Musculoskeltal System | Body weakness, Stretch of bones and ligaments, fatigue, Fracture, sprains | 196 | 10 | 0.95 |
| Pneumonia/Cough/Cold/Fever | Pneumonia, Cough, Cold, Fever | 88 | 6 | 0.94 |
| Dermatological/wounds | Skin diseases, wounds, yolk galls | 268 | 9 | 0.97 |
| Alopecia/Ophthalmic | Hair loss, eye disease | 47 | 3 | 0.93 |
| Gastrointestinal System | Indigestion, vomiting, diarrhea, dysentery, flatulence, tongue infection, gaseous bloats, constipation, loss of appetite | 315 | 16 | 0.95 |
| Jaundice/Foot and Mouth disease | Jaundice, Foot and Mouth disease | 126 | 4 | 0.97 |
| Pregnancy and post pregnancy | Stimulate oestrus cycle, Removal of retained placenta, increased milk production, Vigor maintenance | 322 | 12 | 0.96 |
| Insecticide/Antidote | Poisonous bite, deworming, removing of lice and ticks, removing of leaches | 345 | 15 | 0.95 |
| Area | Study Year | No. of Recorded Plant Species | Plants with Similar Use | Plants with Dissimilar Use | Total Species Common in Both Areas | Species Enlisted Only in Aligned Area | Species Enlisted Only in Study Area | % of Plants with Similar Use | % of Plants with Dissimilar Use | Jaccard Index | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kashmir Himalaya | 2007 | 24 | 5 | 2 | 7 | 17 | 49 | 20.8 | 8.3 | 11.9 | [88] |
| Kathua district of J&K | 2012 | 72 | 5 | 8 | 13 | 59 | 43 | 6.9 | 11.1 | 14.6 | [87] |
| Samahni valley, district Bhimber (Azad Kashmir) Pakistan | 2006 | 54 | 9 | 6 | 15 | 39 | 41 | 16.7 | 11.1 | 23.1 | [86] |
| Poonch valley, Azad Kashmir | 2012 | 19 | 3 | 3 | 6 | 13 | 50 | 15.8 | 15.8 | 10.5 | [89] |
| Tosham block of district Bhiwani (Haayana) | 2014 | 52 | 3 | 2 | 5 | 47 | 51 | 5.8 | 3.8 | 5.4 | [91] |
| Jhansi district, UP | 2010 | 47 | 0 | 7 | 7 | 40 | 49 | 0 | 14.9 | 8.5 | [92] |
| Tehri district of Garwal Himalaya | 2013 | 35 | 0 | 5 | 5 | 30 | 51 | 0 | 14.3 | 6.6 | [93] |
| Uttara Kannada district of Karnataka | 2005 | 24 | 0 | 1 | 1 | 23 | 55 | 0 | 4.2 | 1.3 | [90] |
| Visakhapatnam and Vizianagarm districts, AP | 2015 | 61 | 0 | 3 | 3 | 58 | 53 | 0 | 4.9 | 2.8 | [94] |
| Kudavasal taluk of Thiruvarur district, TN | 2016 | 54 | 0 | 6 | 6 | 48 | 50 | 0 | 11.1 | 6.5 | [12] |
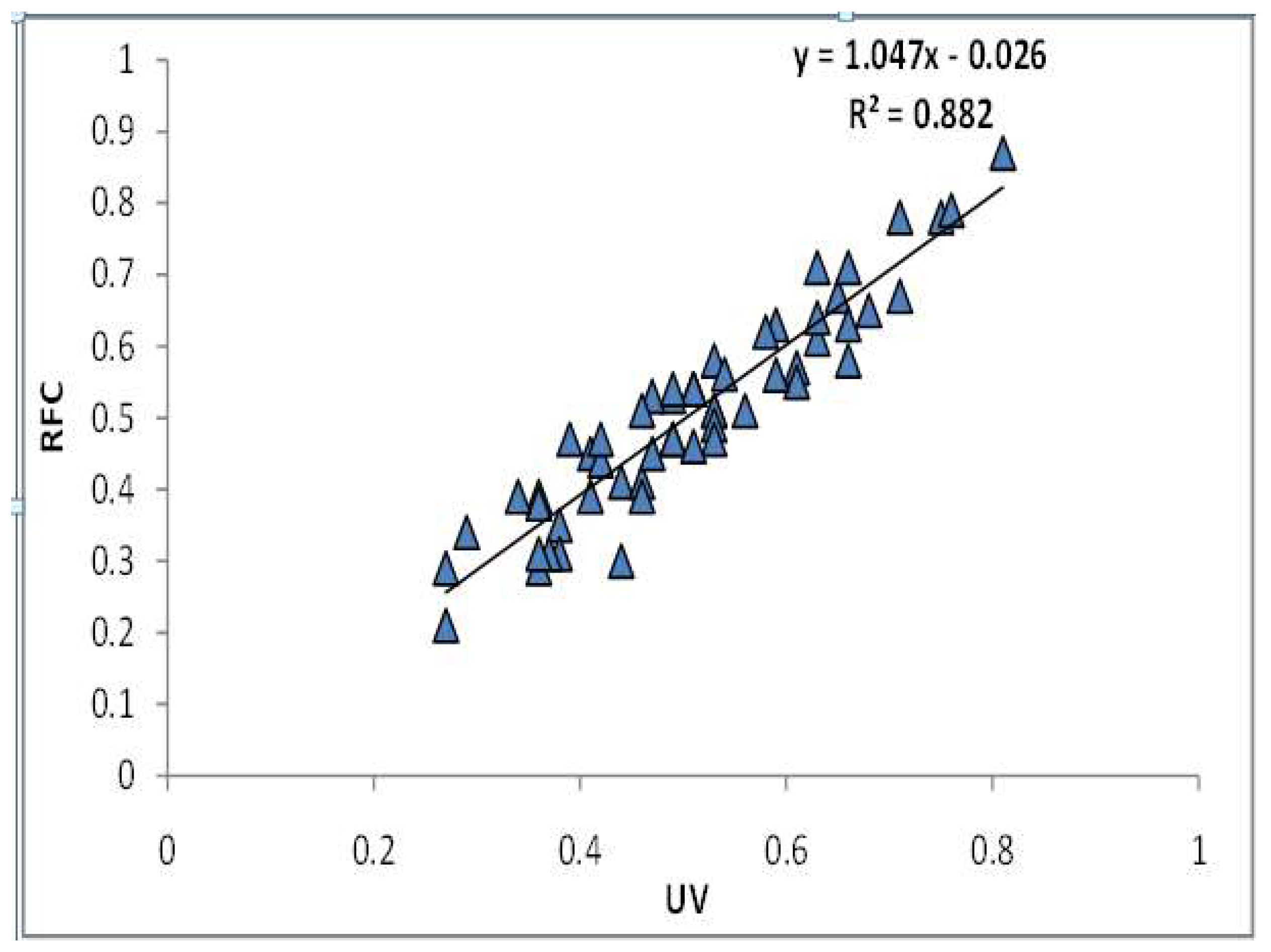
| Correlation between RFC and UV | RFC | UV | |
|---|---|---|---|
| RFC | Pearson Correlation | 1 | 0.940 ** |
| Sig. (2-tailed) | 0.000 | ||
| N | 56 | 56 | |
| UV | Pearson Correlation | 0.940 ** | 1 |
| Sig. (2-tailed) | 0.000 | ||
| N | 56 | 56 | |
| Taxa | Phytochemicals | Pharmacological Activities | Reference(s) |
|---|---|---|---|
| Amaranthus viridis | rutin, quercetin, spinosterol, amasterol | antifungal, hepatoprotective, anthelminthic, antioxidant, antimicrobial, antidiabetic, antipyretic, anti-inflammatory | [97,98,99,100] |
| Chenopodium album | 3-O-glycosides of caempferol, quercetin, andisoramnetin, kaempferol-3-O-(4-β-D-xylopyranosyl)-α-L-rhamnopyranoside-7-O-α-L-rhamno-pyranoside,3-O-(4-β-D-apiofuranosyl)-α-L- rhamnopyranoside-7-O-α-L rhamnopyranoside,3,7-di-O-α-L-rhamnopyranoside, 3-O-glucopyranoside, quercetin 3,7-di-O-β-D-glucopyranoside, 3-O-glucosylglucuronide, 3-O-α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside,3-O-β-D-glucopyranoside, kaempferol-3-O-arabinoglucoside, quercetin, quercetin 3-O-xylosylglucoside, quercetin-3-orhamnoglucoside | antiviral, antifungal, anti-inflammatory, antiallergic, antiseptic, antipruritic, anti-nociceptic, sperm immobilizing immunomodulating, antiparasitic, antispasmodic, antibacterial, anti-helminthic, hypotensive, spasmolytic, hepatoprotective | [101,102,103] |
| Achyranthes aspera | betaine, achyranthine, hentriacontane, ecdysterone, achyranthes saponins A, B, C, D, α-Lrhamnopyranosyl-(1→4)-(β-Dglucopyranosuluronic acid)-(1→3)-oleanolic acid, trigmasta-5, 2-dien-3-β-ol, trans-13-docasenoic acid, n-hexacosanyl-n-decaniate, hexacos-17-enoic acid | antiviral, anticarcinogenic, spermicidal, hepatoprotective, nephroprotective, antidiabetic, anti-inflammatory, immunomodulatory, antimicrobial, antiparasitic, anti-allergic, anti-oxidant, hypolipidemic | [104,105,106] |
| Allium cepa | quercetin 3,7,4′-O-β-triglucopyranoside, quercetin 3,4′-O-β-diglucopyranoside, taxifolin 4′-O-β-glucopyranoiside | analgesic, antidiabetic, antioxidant, antidepressant, aphrodisiac, antihyperlipidemic | [107,108,109] |
| Allium sativum | alliin, allicin, ajoenes, vinyldithiins, quercetin | anticarcinogenic, antioxidant, antidiabetic, reno-protective, anti-atherosclerotic, antibacterial, antifungal, antihypertensive, antiviral, antifungal, antiprotozoal, antioxidant, anti-inflammatory, and anticancer | [110,111,112] |
| Angelica glauca | aleric acid, angelic acid, angelisine, phellandrene, coumarins, bergapten, linalool, borneol, anthotoxin, umbelliferene | cardioactive, carminative, digestive, sudorific, expectorant and stomachic, antipsoriatic, anti-bacterial, antifungal | [113,114,115,116,117] |
| Foeniculum vulgare | trans-anethole, fenchone, methylchavicol, eriodictyol-7-rutinoside, quercetin-3-rutinoside, rosmarinic acid, quercetin-3-glucuronide, isoquercitrin, quercetin-3-arabinoside, kaempferol-3-glucuronide, kaempferol-3-arabinoside, isorhamnetin glucoside, 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-O-caffeoylquinic acid, 1,3-O-di-caffeoylquinic acid, 1,4-O-di-caffeoylquinic acid, 1,5-O-di-caffeoylquinic acid | antimicrobial, antiviral, anti-inflammatory, antimutagenic, antinociceptive, antipyretic, antispasmodic, antithrombotic, apoptotic, cardiovascular, chemomodulatory, antitumor, hepatoprotective, hypoglycemic, hypolipidemic, and memory enhancing property | [118] |
| Calotropis procera | voruscharin, uscharidin, uzarigenin, calotroposide, calactin, calotoxin, uscharin, ascleposide, calotropagenin, coroglaucigenin, calotropin, proceroside, proceragenin, syriogenin, rutin, cyaindin-3-rhamnoglucoside, cycloart-23-en-3β, 25-diol, cyclosadol, multiflorenol, procestrol, quercetin-3- rutinoside, β-sitosterol, β-sitost-4en-3one, stigmasterol, cyanidin-3-rhamnoglucose, ascorbic acid, calactin, calotoxin, calatropagenin, calotropin, polysaccharide containing D-arabinose, D-glucose, D-glucosamine L-rhamnose, calotropagenin, 3-proteinase, calotropin, α-calotropeol, 3-epimoretenol, gigantin, giganteol, isogiganteol, α-lactuceryl acetate, α-lactuceryl isovalerate, lupeol, proceroside, proceragenin, syriogenin, taraxast-20α- (30)-en-(4-methyl-3-pentenoate), 3′-thiazoline cardenolide uscharidin, uzarigenin, voruscharin, β-sitosterol, α- and β-amyrin, lupeol, taraxasteryl acetate, α-and β-calotropeol, 3-epimoretenol, multiflorenol, cyclosadol, several triterpene esters, β-sitosterol, stigmasterol, calotropin, procerain, procerain-B, calactinic acid, choline, O-pyrocatechuic acid, β-sitosterol, taraxasterol, calotroprocerol A, calotroproceryl acetate A, calotroprocerone A, calotroproceryl acetate B | analgesic, antinociceptive, anticonvulsant, antimalarial, anthelminthic, antioxidant, antidiabetic, anticancer, antimicrobial, anti-inflammatory, immunomodulatory, antipyretic, antidiarrheal, antidematogenic, antiplasmodial | [119,120,121,122] |
| Hedera nepalensis | lupeol, hederacoside C, α-hederin | anticancer, neuroprotective, antidiabetic, antioxidant | [123,124] |
| Taraxacum officinale | stigmasterol, campesterol, syringing, dihydrosyringin, dihydroconiferin, luteolin 7 glucoside, luteolin 7 diglucosides, cichoriin, aesculin, dicaffeoyltartaric acid, rutin, hiperoside, quercetin | antibacterial, antioxidant, anticancer, diuretic, hepatoprotective, antiviral, anti-inflammatory | [125,126,127,128,129] |
| Erigeron canadensis | onyzolide, conyzapyranone A, conyzapyranone B, 4 Z,8 Z-matricaria-γ-lactone, 4 E,8 Z-matricaria-γ-lactone, 9,12,13-trihydroxy-10(E)-octadecenoic acid, epifriedelanol, friedeline, taraxerol, simiarenol, spinasterol, stigmasterol, β-sitosterol, quercetin-7-O-beta-D-galacto pyranoside, quercetin, luteolin, apigenin, 5,7,4’-trihydroxy-3’-methoxy flavone, quercetin-3-alpha-rhamnopyranoside, quercetin-3-O-beta-D-glucopyranoside, apigenin-7-O-beta-D-gluco pyranoside, luteolin-7-O-beta-D-glucuronide methyl ester,4’-hydroxy baicalein-7-O-beta-D-glucopyranoside, baicalein, rutin | antimicrobial, antioxidant, anticoagulant, anti-inflammatory, anticancer, antifungal | [130,131,132] |
| Saussurea costus | costunolide, dehydrocostuslactone, costic, palmitic, linoleic acids, cyclocostunolide, alantolactone, isoalantolactone, isodehydrocostus lactone, iso-zaluzanin-C, guiainolides, 12-methoxydihydrodehydrocostuslactone, 4-methoxydehydrocostus lactone, saussurealdehyde, isodehydrocostus-lactone-15-aldehyde, cynaropicrin, reynosin, santamarine, Saussureal, pregnenolone, sitosterol, daucosterol, syrine, chlorogenic acid, saussurine | anti-inflammatory, anticancer, hepatoprotective, immunomodulatory, anti-ulcer, antimicrobial, hypoglycemic, antiparasitic | [133,134,135,136,137,138] |
| Sonchus arvensis | alkaloids, flavonoids, phenols, saponins and tannins | anti-fatigue activity, antioxidant, hepatoprotective, kidney-protective, antidiabetic, antibacterial | [139,140,141] |
| Achillea millefolium | borneol, camphene, azulene, carophyllene, 1,8-cineole, p-cymene, eugenol, farnesene, limonene, myrcene, α-pinene, β-pinene, salicylic acid, achillicin, achillin, terpinolene, α-thujone, artemetin, casticin, isorhamnetin, luteolin, rutin | antibacterial, antifungal, antiparasitic, hemostyptic, anti-inflammatory, antispasmodic, antioxidant, anticancer, hepatoprotective, | [142,143,144] |
| Berberis lycium | berberine, berbamine, chinabine, karakoramine, palmatine, balauchistanamine, gilgitine, jhelumine, punjabine, sindamine, chinabine acetic acid, maleic acid, ascorbic acid, baberine, berbericine hydrochloride, berbericine hydroiodide, oxyberberine, umbellatine | antidiabetic, hepatoprotective, anti-hyperlipidemic, antimutagenic, antioxidant, antidiarrheal, anti-arrhythmic, anti-depressant, anti-microbial, anti-protozoal | [145,146] |
| Alnus nitida | Diarylheptanoids | anticancer, anti-inflammatory, anti-influenza, hepatoprotective, antitumor and anti-oxidant | [147,148,149] |
| Brassica rapa | lutein, β-carotene, glucobrassicin, 4-methoxyglucobrassicin, 1-methoxyglucobrassicin, isothiocyanates, nitriles, thiocyanates, epithionitriles, oxazolidine, aconitic, citric, ketoglutaric, malic, shikimic, fumaric, oxalic, ascorbic, succinic and glutamic acids | anticancer, antioxidant, anti-inflammatory, chemopreventive | [150,151] |
| Buxus wallichiana | buxemenol E, buxaltine H, Buxiramin D, buxatinebuxandrine F, buxidine F, (+)-16a, 31-diacetylbuxadine, semperviraminol, buxamine F | bitter tonic, diaphoretic, anti-rheumatic, vermifuge, anti-helminthic, analgesic, purgative diuretic, antiepileptic, antileprotic, hemorrhoids | [152,153,154] |
| Cannabis sativa | cannabigerol, cannabichromene, cannabidiol, tetrahydrocannabinol, 9-tetrahydrocannabivarin, annabicyclol, annabinol, D-limonene, beta-myrcene, alpha-pinene, caryophyllene oxide, D-linalool, beta-caryophyllene | anticonvulsant, antibiotic, antifungal, anti-inflammatory, analgesic, anxiolytic, antipsychotic, antioxidant, antispasmodic, anti-emetic, sedative | [155] |
| Valeriana jatamansi | baldrinal, homobaldrinal, decyl baldrinal, valtroxal, isovalepotriate, acetoxyvalepotriate, isovalemxyhydroxy-dihydrovatrate, rupesin, linarin, linarin-isovalerianate, linarin-2-O-methylbutyrate, hispidulin, hesperetin-7-O-β-rutinoside, hesperidin, kaempferol 3-O-β-D-glucopyranoside, quercetin 3-O-β-D-glucopyranoside, kaempferol, quercetin, 7-O-β-D-glucopyranoside, apigenin 7-O-β-D- glucopyranoside, lariciresinol,, pinsepiol, syringaresinol, pinoresinol, berchemol, podophyllotoxin, hydroxyvalerenic acid, acetoxyvalerenic acid, valerenic acid | neuroprotective, sedative, cytotoxic, cardio-protective, anxiolytic, antidepressant, anti-inflammatory, antispasmolytic, hepatoprotective, antioxidant | [156] |
| Lagenaria siceraria | β-carotene, 22-deoxocurcubitacin-d, 22-deoxoisocurcubitacin d, avenasterol, codisterol, elesterol, isofucasterol, stigmasterol, sitosterol, compesterol, spinasterol, 7-0-glucosyl-6-C-glucoside apigenin, 6-C-glucoside apigenin, 6-C-glucoside luteolin, 7,4′-O-diglucosyl- 6-C-glucoside apigenin | analgesic, anti-inflammatory, antihyperlipidemic, diuretic, hepatoprotective, anthelminthic, antibacterial, immunomodulatory, antistress, hepatoprotective, antioxidant | [157] |
| Mallotus philippensis | rottlerin, mallotoxin, iso rottlerin, crotoxigenin, betulin, friedelin, kamaladiol-3-acetate, lipeol, tannic acid, 3-hydroxy-D-A-friedoolean-3-en-2-one, 2β-hydroxy-D-A-friedooleanan-3-one, 3α-hydroxy-D-A-friedooleanan-2-one, betulin-3 acetate lupeol acetate, berginin acetylaleuritote acid, sitosterol, crotoxigenin, coroghcignin, homorottlerin | antioxidant, antimicrobial, anticancer, anti-viral, anti-inflammatory, hepatoprotective, anti-helminthic, analgesic | [158,159] |
| Ricinus communis | kaempferol-3-O-β-D-xyylopyranoside, Kaempferol-3-O-β-rutinoside, Kaempferol-3-O-β-D-glucopy-ranoside, Quercetin, Ricin, Rutin, Ellagic acid, Epicatechin, Fucosterol, Stigmasterol, α-pinene, 1,8-pinene, 1,8-cineole, β-caryophyllene, Ricinine, demetilricinine, Triricinolein, Lupeol | abortifacient, analgesic, antiasthmatic, anti-fertility, contraceptive, antidiabetic | [160] |
| Trifolium pratense | biochanin A, benzaldehyde, (Z)-β-caryophyllene, 3-methyl-1-butanol, 3-octanone, Z)-β-caryophyllene, β-farnesene, 6,10,14-trimethyl-pentadecanone, 3-methylbutyl butanoate, 3-methyl-1-butanol,3-methylbutanoic acid, ethyl-2-methylpentanoate | estrogenic, antiproliferative, antioxidant | [161,162] |
| Trifolium repens | 4′,5,6,7,8-pentahydroxy-3-methoxyflavone and 5,6,7,8-tetrahydroxy-3-methoxyflavone, 3,7-dihydroxy-4′-methoxyflavone, 5,6,7,8-tetrahydroxy-4′-methoxyflavone, 3,5,6,7,8-pentehydroxy-4′-methoxyflavone, 6-hydroxy-kaempferol, 4′,5,6,7,8-pentahydroxyflavone, 3,4′-dimethoxykaempferol, quercetin and kaempferol, chalcone, chalcanol glucosides, repensin A and repensin B, gallocatechin, epigallocate-chin, gallocatechin-(4α-8)-epigallocatechin | antioxidant, antifungal, anticancer, antiaging, hepatoprotective, anti-inflammatory, antidiabetic | [163] |
| Trigonella foenum-graecum | disogenin, gitogenin, neogitogenin, homorientin saponaretin, neogigogenin, trigogenin, trigonelline and choline | immunomodulatory, antioxidant, chemo preventive, anticancer, antidiabetic, gastro protective, anti-inflammatory, antipyretic, anthelmintic, antigenotoxic, anti-plasmodial, hypocholesterolemic, antiseptic, aphrodisiac, astringent, bitter, demulcent, emollient, expectorant | [164,165] |
| Juglans regia | quercetin, and caffeic acid, paracomaric acid, juglone, ascorbic acid, quercetin arabinoside, quercetin xyloside and quercetin rhamnoside, Glutelins, globulins, albumin and prolamins, glansrins A, B and C, casuarinin, stenophyllarin | antioxidant, antidiabetic, antimicrobial, and hepatoprotective, antifungal, anti-hypertensive, renal protective, anti-inflammatory, antinociceptive, anticancer | [166,167] |
| Vitex negundo | friedelin, casticin, artemetin, terpinen-4-ol, α-terpineol, sabenine, globulol, spathulenol, β-farnesene, farnesol, α-pinene, β-pinene, linalool, terpinyl acetate, caryophyllene epoxide, caryophyllenol, vitexicarpin, viridiflorol, 5-odesmethylnobiletin, gardenin A, gardenin corymbosin, terpinen-4-ol, α-copaene, β-caryophyllene, β-elemene, camphene, α-thujene, α-pinene, sebinene, α-elemene, δ-elemene, β-elemene, β-eudesmol, camphene, careen, 1,8-cineol, α-phellendrene, β-phellendrene, α-guaiene, neral, geranial, bornyl acetate, nerolidol, β-bisabolol, cedrol, agnuside, lagundinin, viridiflorol, betulinic acid, ursolic acid, dimethoxyflavonone, vitexoside, agnuside, R-dalbergiphenol, negundin A, negundin B, vitexin cafeate, epifriedelinol | anxiolytic, analgesic, anti-inflammatory, neuroprotective, antibacterial, antipyretic, anticancer, antioxidant | [168,169] |
| Ajuga parviflora | ajugin A, ajugin B | antidiabetic, antioxidant, antibacterial | [170,171,172] |
| Mentha sylvestris | menthol, menthone, isomenthone, limonene, neomenthol, methyl acetate, beta-caryopyllene, piperitone, alpha- and beta-pipene, acacetin, chrysoeriol, diosmin, eriocitrin (eriodictoyl-7-o-rutinoside), hesperidin, hesperidoside, isorhoifolin, linarin, luteolin, menthoside, methyl rosmarinate, rutin, tilianine, narirutin, nodifloretin, caffeic acid, lithospermic acid, rosmarinic acid, protocatechuic acid, protocatechuic aldehyde, phytosterols, β-sitosterol, daucosterol; aloe-emodin, chrysophanol, emodin | carminative, stimulant, stomachic, aromatic, antiseptic, antispasmodic, sudorific, emmenagogue, anesthetic, anodyne | [173,174,175] |
| Punica granatum | punicalin, punicalagin, gallic acid, ellagic acid, cyaniding, delphinidin, uteolin, quercetin, kaempferol, naringenin, estrone, estriol, testosterone, betasistosterol, coumesterol, gammatocopherol, punicie acid, campesterol, stigmasterol | antioxidant, antibacterial, anticancer, anti-inflammatory, anticoagulant, antimutant, cardioprotective, antifungal, antidiabetic | [176,177,178] |
| Malva neglecta | hydrotyrosol, coumaroylhexoside, kaempferol-3-(p-coumaroyldiglucoside)-7-glucoside, quercetin-3-O-rutinoside, epicatechin-3-O-(4-O-methyl)-gallate, oleic acid, taurine, ethylene dimercaptan, isoeugenol, patchoulane, methyl 12-methyltetradecanoate, isopropyl myristate | antimicrobial, antioxidant, anti-inflammatory, anti-ulcerogenic, hepatoprotective, neuroprotective | [179,180,181,182] |
| Trillium govanianum | pennogenin, diosgenin, borassoside E, govanoside A | analgesic, anti-inflammatory, antifungal, antioxidant, anticancer, antispasmodic, diuretic | [183] |
| Melia azedarach | surinol, melianin b, sendanolactone, 3-α-hydroxy-4, 4,14alpha-trimethyl-5-αpreg-8-en-20-one, ochinin acetate, 4-methoxy-1-vinyl-beta-carboline, 4,8-dimethoxy-1-vinyl-beta-carboline, kuline, kulactone, kulolactone, kulinone, nimbinene, azaridine, paraisine, isochuanliansu, 6 H-β-hydroxy-4-stigmasten-3-one, 6βhydroxy-4-campesten-3-one, quercetrin, quercetin-3-0-β-rutinoside, kaempferol3-0-β rutinoside, rutin, kaempferol-3-L-rhamno-Dglucoside, azaridine, bakayanin, bakalactone, margosine, azadirone, azadiradione, epoxyazadiradione, ohchinol, ohchinin, ohchinolal, ohchinolides A and B, nimbolinin B, 1-desacetylnimboline B, nimbolidins A and B, triterpene B, meliacins A1,A2, B1, B2, sendanin, sendenal, 1-cinnamoylmelianolone, meliantriol, melianone, melianol, lupeol, β-sitosterol, catechin, vanillin, cinnamic acid, salannal, meliacarpinin E | analgesic, immunomodulatory, antifeedant, antifungal, antibacterial, antiviral, cytotoxic, anthelminthic, antilithic, antifertility | [184,185,186] |
| Cissampelos pareira | hayatine, hayatinine, hayatidine, quercitol, sterol, eepeerine, berberine, cissampeline, pelosine, cycleamine, menismin iodine, cissamin chloride, pareirin, cissamine chloride, cissampareine, dehydrodicentrine, dicentrine and insularine | antipyretic, anti-inflammatory, antiarthritic, antiulcer, antidiabetic, anticancer, antifertility, antimicrobial, antioxidant, antivenom, antimalarial, and immunomodulatory | [187,188] |
| Ficus palmata | germanicol acetate, vanillic acid, psoralenoside methyl ether, rutin | hepatoprotective, nephroprotective, antiulcer and anticoagulant | [189] |
| Boerhavia diffusa | eupalitin, eupalitin-3-O-β-Dgalactopyranos, eupatilin-7-O-β-Dgalactopyranoside, eupatilin 7-O-α-Lrhamnopyranosyl (1→2) α-Lrhamnopyranosyl (1→6)- β-Dgalactopyranoside, quercetin-3-O- β-Dglucopyranoside- 7-O- β-Dglucopyranoside, -3-O-α-Lrhamnopyranosyl (1→6)-β-Dgalactopyranoside, kaempferol, quercetin, borhavone, punarnavoside, alkamide, ferulic acid, gentisic acid, caffeoyltartaric acid, boerhaavic acid, boeravinone, coccineon, isomenthone, limonene, menthol, phellandrene, safranal, α-pinene, geranylacetone, eugenol, stigmasterol, campesterol, β-sitosterol | antidiabetic, immunosuppressive, nephroprotective, antilithiatic, antioxidant, hepatoprotective, antiviral, anticancer, cardiovascular | [190,191,192,193] |
| Cedrus deodara | wikstromal, matairesinol, dibenzylbutyrolactol, cedrin, taxifolin, cedeodarin, dihydromyricetin, cedrinoside, deodardione, diosphenol, limonenecarboxylic acid, deodarin, sitosterol, deodarone, atlantone, α-himacholone, β-himacholone, α-pinene, β-pinene, myrcene, himachalene, cis-atlantone, α-atlantone | anti-inflammatory, analgesic, anti-spasmodic, immunomodulatory, anti-hyperglycemic, anticancer, antibacterial | [194] |
| Plantago lanceolata | pyrocatechol, picatechin, vanillin, verbascoside, taxifolin, luteolin 7-glucoside, hesperidin, hyperoside, apigenin 7-glucoside, pinoresinol, eriodictyol, quercetin, luteolin, kaempferol, apigenin, baicalein, plantaginin, aucubin, indicain, plantagonin | antiulcerative, antidiabetic, antidiarrhoeal, anti-inflammatory, anticancer, antinociceptive, antioxidant, anti-fatigue, antibacterial, antiviral | [195,196] |
| Picrorhiza kurroa | veronicoside, pikuroside, cucurbitacins, 4-hydroxy-3-methoxy acetophenone, apocyanin, drosin | hepatoprotective, anticholestatic, antioxidant, anti-inflammatory, anticancer, antiviral, hepatoprotective, anticonvulsant, nephroprotective | [197] |
| Rumex dentatus | rumejaposides E, cassialoin, emodin | astringent, antioxidant, antibacterial | [198] |
| Persicaria hydropiper | catechin, epicatechin, hyperin, isoquercitrin, isorhamnetin, kaempferol, quercetin, quercitrin, rhamnazin, rutin, 3-β-angeloyloxy-7-epifutronolide, 7-ketoisodrimenin, changweikangic acid A, dendocarbin L, fuegin, futronolide, polygonumate, winterin, confertifolin, isodrimeninol | antioxidant, antibacterial, antifungal, anthelminthic, cytotoxic, anti-inflammatory, antifertility, neuroprotective | [199] |
| Aconitum heterophyllum | aconitine, heterophylline A, heterophylline B, heteratisine, heterophyllisine, heterophyllidine, mesaconitine, 3 acetylaconitine, atidine, isoatisine, hetidine, hetsinone, benzoylheteratisine, aconitic acid, N-Diethyl-N-formyllyaconitine, O-methylaconitine, methyl-N-succinoylanthranilate, hypaconitine, benzoylaconine, benzoylmesaconine, benzoylhypaconine, 6-dehydroacetylsepaconitine, 13-hydroxylappaconitine | antidiarrheal, expectorant, diuretic, hepatoprotective, antipyretic, analgesic, antioxidant, alexipharmic, anodyne, anti-atrabilious, immunostimulant, febrifuge, anthelmintic, anti-cancerous, anti-emetic, anti-inflammatory, anti-flatulent, anti-periodic, anti-phlegmatic, anti-diabetic, antifungal, antimicrobial, antiviral and carminative | [200,201,202,203] |
| Prunus persica | lutein, β-cryptoxanthin, β-carotene, champagne, hexahydroxydiphenic acid, gallic acid, protocatechuic acid, guercetin, kaempferol, isorhamnetin, myricetin, cyanidin-3-O-glucoside, cyanidin-3-O-rutinoside, catechin, epicatechin | antioxidant, antimicrobial, antidiabetic, anti-inflammatory, cardioprotective, neuroprotective, anticancer | [204] |
| Galium aparine | gallic acid, caffeic acid, isoquercitrin, rutin, quercetin, luteolin, asperulosidic acid, deacetylasperulosidic acid, β-sitosterol, daucosterol, vanillin, γ-sitosterol | antioxidant, antimicrobial, anticancer, hepatoprotective, antifeedant | [205,206,207] |
| Salix alba | anthocyanins, p-hydroxybenzoic, gallic acid, Gentisic acid, sisymbrifolin | antibacterial, antifungal, antioxidant | [208] |
| Aesculus indica | quercetin, mandelic acid | Immunomodulatory | [209,210] |
| Dodonaea viscosa | aliarin, pinocembrin, viscosol, sakuranetin, isokaempferide, dodoviscins A-J, isorhamnetin, quercetin, jegosapogenol, β-pinene, myrcene, limonene, p-cymene, citronellal, linalool, linalyl acetate, Υ-terpineol, geraniol, α-spinasterol, 4-hydroxy-3,5-diprenylbenzaldehyde, β-sitosterol, stearic acid, syringic acid, fraxetin, cleomiscosin A, cleomiscosin C, and β-sitosterol β -D-glucoside | Antidiabetic, antimicrobial, antioxidant, cytotoxic, antifertility, anti-inflammatory, analgesic, antiulcer, anti-spasmodic | [211] |
| Bergenia ciliata | bergenin, tannic acid, gallic acid, catechin, β-sitosterol, arbutin, afzelechin, quercetin 3-o-β-D xylopyranoside, quercetin 3-o-α-L-arbinofuranoxide, Linalool, | antidiabetic, anticancer, antiulcer, antibacterial, antimalarial, antifungal, antiviral | [212] |
| Verbascum thapsus | verbascoside, aucubin, harpagoside, ajugol, isocatalpol, methylcatalpol, 6-O-α-L-rhamnopyranosylcatalpol, saccatoside, harpagide, ningpogenin, 10-deoxyeucommiol, jioglutolideforsythoside B, alyssonoside, arenarioside, saikogenin A, thapsuine A, β-spinasterol, veratric acid | antioxidant, antimicrobial, antiviral, anticancer, antihyperlipidemic | [213] |
| Datura stramonium | atropine, scopoline, 3-(hydroxyacetoxy) tropan, 3-hydroxy-6-(2-methylbutyryloxy) tropan, aponorscopolamine, 7-hydroxyhyoscyamin, aponorscopolamine, aposcopolamine, hygrine, hyoscyamine, littorine, meteloidine, scopine scopolamine, tropinone, tropine | antimicrobial, analgesic, anti-asthmatic, anticancer, antioxidant | [214] |
| Urtica dioica | acetylcholine, histamine, and 5-hydroxytryptamine, formic acid, histamine, serotonin, carvacrol, carvone, isolectins | anti-inflammatory, analgesic, antiviral, hepatoprotective, anti-colitis, | [215] |
| Sambucus wightiana | - | Antimicrobial | [216] |
| Viburnum grandiflorum | luteolin 3′-O-b-d-xylopyranosyl(1!2)-O-b-d-glucopyranoside, quercetine | Antifungal | [217,218] |
| Phytochemical | PubChem CID | Chemical Formula | Biological Activities | Mechanism of Action | Reference(s) |
|---|---|---|---|---|---|
| Rutin | 5280805 | C27H30O16 | Modifies the cognitive and various behavioral symptoms of neurodegenerative diseases | Effects processing, aggregation and action of amyloid beta (Aβ); shift of the oxidant–antioxidant balance allied with neuronal cell loss | [221] |
| Antihyperglycemic | Decrease in carbohydrates’ absorption from the small intestine, inhibition of tissue gluconeogenesis, an increase of tissue glucose uptake, stimulation of insulin secretion from beta cells, and protecting Langerhans’ islet against degeneration | [222] | |||
| Quercetin | 5280343 | C15H10O7 | Anti-inflammatory | Inhibits LPS-induced mRNA levels of cytokines in colloid cells, such as tumor necrosis factor (TNF)-a and IL-1a | [223] |
| Anticancer | Suppression of many kinases involved in the growth of cancer cells, proliferation and metastasis | [224] | |||
| Lupeol | 259846 | C30H50O | Anti-inflammatory | Suppresses and alters the phagocytic activity of macrophages and T lymphocytes, and suppresses CD4+ T cell mediated cytokine generation | [225] |
| Apigenin | 5280443 | C15H10O5 | Anxiolytic | Inhibits the binding of flunitrazepam to brain membranes without influencing the binding of muscimol to GABAA receptors. | [226] |
| Anti-inflammatory | Downregulates the expression of IL-1β and TNF-α in LPS-stimulated mouse macrophages and human monocytes | [227] | |||
| Calotropin | 16142 | C29H40O9 | Cytotoxic | Upregulated the expression of p27 leading to cell arrest by downregulating the G2/M regulatory proteins, cyclins A and B, and by upregulating the cdk inhibitor, p27 | [228] |
| α-hederin | 319412227 | C41H66O12 | Anticancer | Induces depolarization of mitochondrial membrane potential which released Apaf-1 and cytochrome c from the intermembrane space into the cytosol, where they promoted caspase-3 and caspase-9 activation | [229] |
| Luteolin | 5280445 | C15H10O6 | Antibacterial | Disrupts the integrity of the bacterial cell membrane and cell wall, resulting in the leakage of cell contents and damage to the barrier function of the cell wall and membrane | [230] |
| Rupesin | 134715087 | C15H22O5 | Antitumor | Inhibits the proliferation of Glioma Stem Cells (GSCs) | [231] |
| Kaempferol | 5280863 | C15H10O6 | Anticancer | Induces apoptosis, cell cycle arrest at the G2/M phase, downregulation of Epithelial–Mesenchymal Transition (EMT)-related markers, and phosphoinositide 3-kinase/protein kinase B signaling pathways | [232] |
| Rottlerin | 5281847 | C30H28O8 | Antitumor | Sensitizes MCF-7 breast cancer cells to TRIAL-mediated apoptosis by PKCδ-dependent inhibition of the transcription factor nuclear factor κB (NFκB), | [233] |
| Heterophylline | 251575 | C22H26N2O4 | Alzheimer’s disease | Inhibits muscle-contracting enzymes acetylcholineatrase and butyrlcholinestrase | [234] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, Z.A.; Farooq, A.; Sarwar, S.; Negi, V.S.; Shah, A.A.; Singh, B.; Siddiqui, S.; Pant, S.; Alghamdi, H.; Mustafa, M. Scientific Appraisal and Therapeutic Properties of Plants Utilized for Veterinary Care in Poonch District of Jammu and Kashmir, India. Biology 2022, 11, 1415. https://doi.org/10.3390/biology11101415
Wani ZA, Farooq A, Sarwar S, Negi VS, Shah AA, Singh B, Siddiqui S, Pant S, Alghamdi H, Mustafa M. Scientific Appraisal and Therapeutic Properties of Plants Utilized for Veterinary Care in Poonch District of Jammu and Kashmir, India. Biology. 2022; 11(10):1415. https://doi.org/10.3390/biology11101415
Chicago/Turabian StyleWani, Zishan Ahmad, Adil Farooq, Sobia Sarwar, Vikram S. Negi, Ali Asghar Shah, Bikarma Singh, Sazada Siddiqui, Shreekar Pant, Huda Alghamdi, and Mahmoud Mustafa. 2022. "Scientific Appraisal and Therapeutic Properties of Plants Utilized for Veterinary Care in Poonch District of Jammu and Kashmir, India" Biology 11, no. 10: 1415. https://doi.org/10.3390/biology11101415
APA StyleWani, Z. A., Farooq, A., Sarwar, S., Negi, V. S., Shah, A. A., Singh, B., Siddiqui, S., Pant, S., Alghamdi, H., & Mustafa, M. (2022). Scientific Appraisal and Therapeutic Properties of Plants Utilized for Veterinary Care in Poonch District of Jammu and Kashmir, India. Biology, 11(10), 1415. https://doi.org/10.3390/biology11101415







