Genistein Restricts the Epithelial Mesenchymal Transformation (EMT) and Stemness of Hepatocellular Carcinoma via Upregulating miR-1275 to Inhibit the EIF5A2/PI3K/Akt Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Cell Lines and Culture
2.3. Genistein Treatment
2.4. Mimic and Plasmid Transfection
2.5. Lentivirus Construction and Infection
2.6. Cell Viability Assay
2.7. miRNAs Transcriptome Sequencing
2.8. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Cell Counting Kit-8 (CCK-8) Test
2.10. 5-Ethynyl-2′-deoxyuridine (EdU) Test
2.11. Scratch-Healing Test
2.12. Migration Test
2.13. Spheroid Formation Test
2.14. 3D Spheroid Invasion Test
2.15. Comet Test
2.16. Flow Cytometry Analysis
2.17. Western Blot (WB) Analysis
2.18. Xenografts of HCC In Vivo
2.19. Lung Metastasis Model of HCC In Vivo
2.20. Dual Luciferase Reporter Test
2.21. Online Public Database and Bioinformatics Analysis
2.22. Statistical Analysis
3. Results
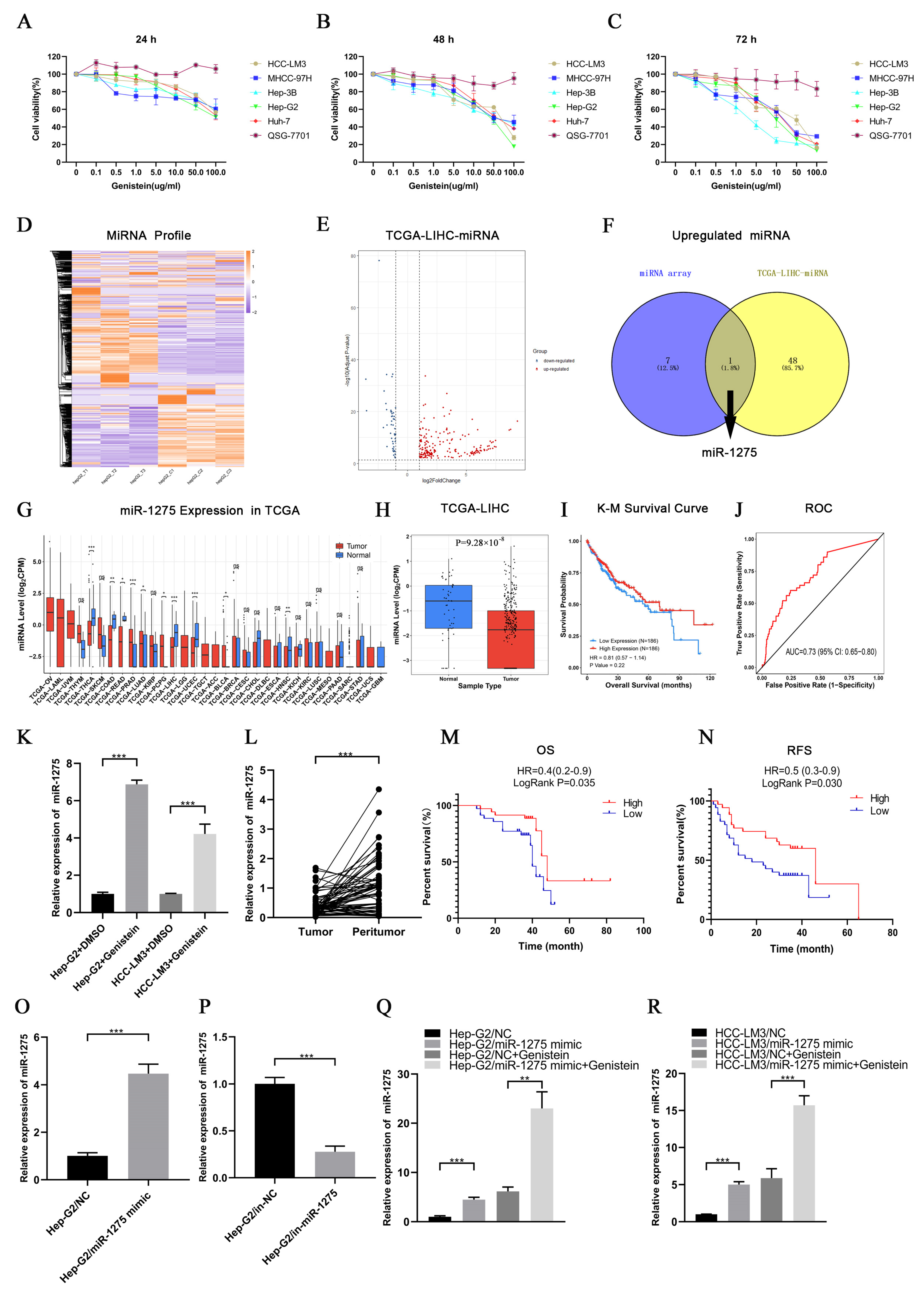
3.1. Genistein Inhibited the Viability of HCC Cells and Upregulated miR-1275 in HCC Cells
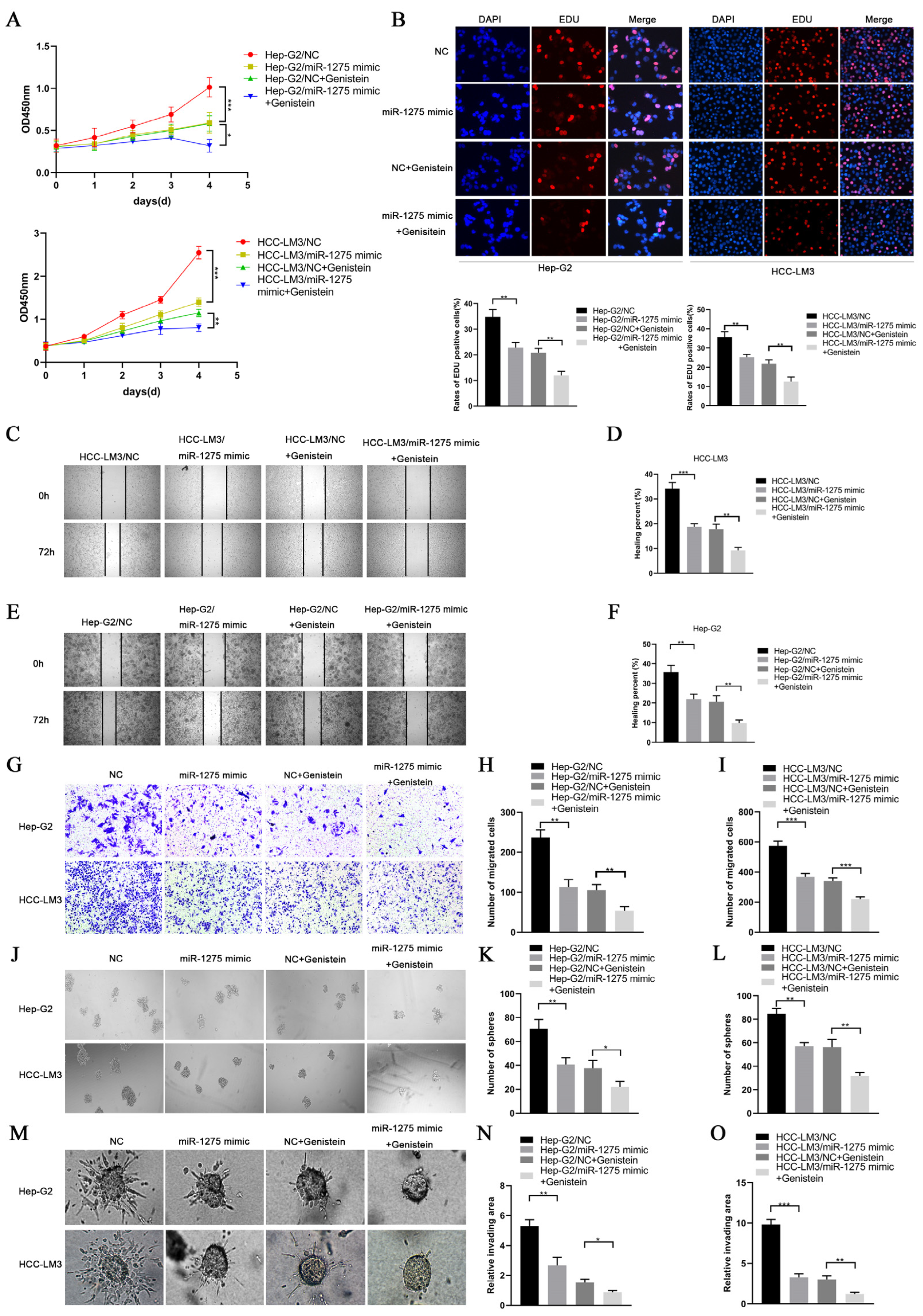
3.2. Genistein Inhibited the EMT and Stemness of HCC Cells by Upregulating miR-1275 In Vitro
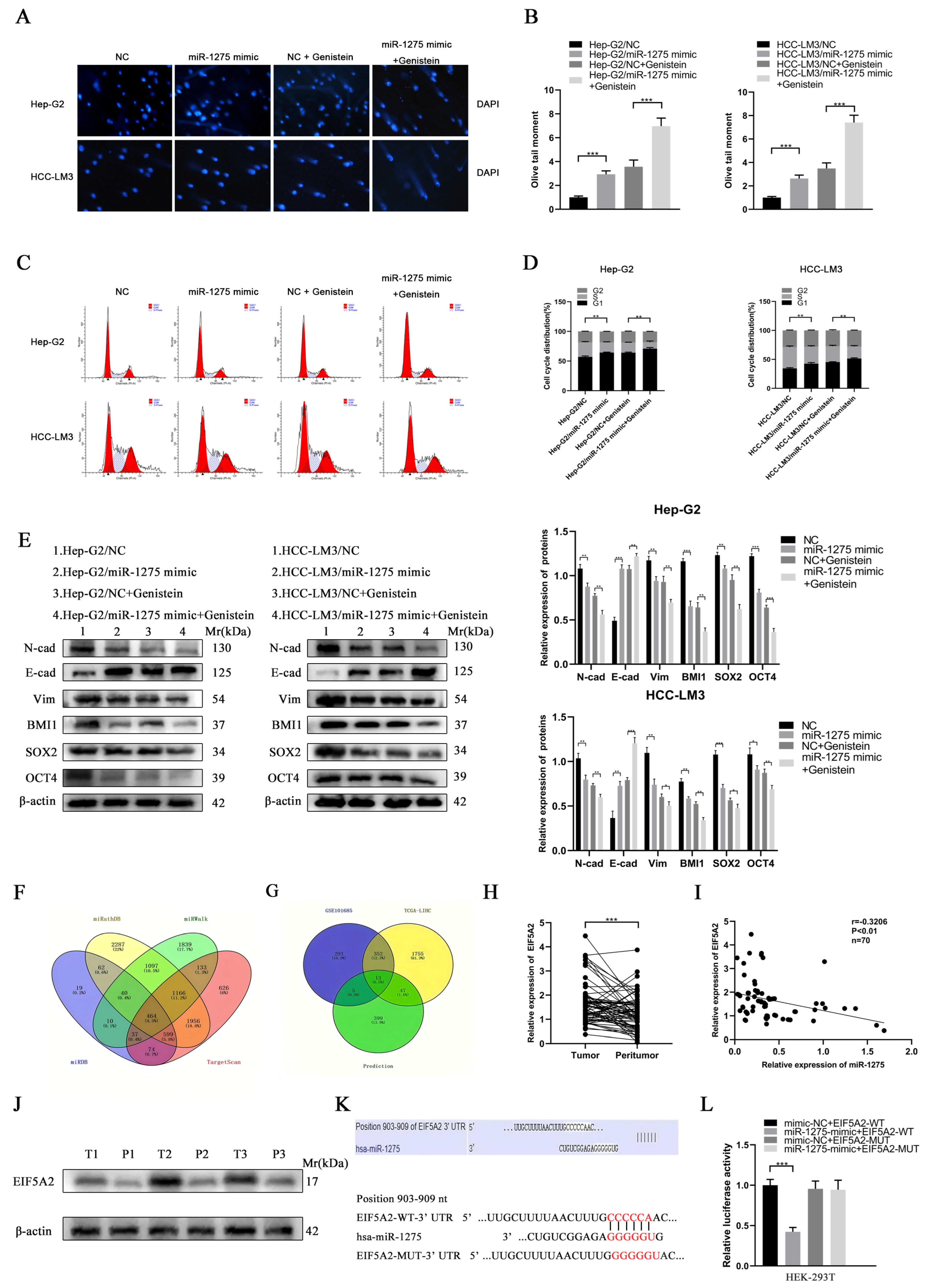
3.3. EIF5A2 Was the Target Gene of miR-1275 in HCC Cells
3.4. MiR-1275 Upregulated by Genistein Suppressed the Progression and Metastasis of HCC by Inhibiting EIF5A2 In Vivo
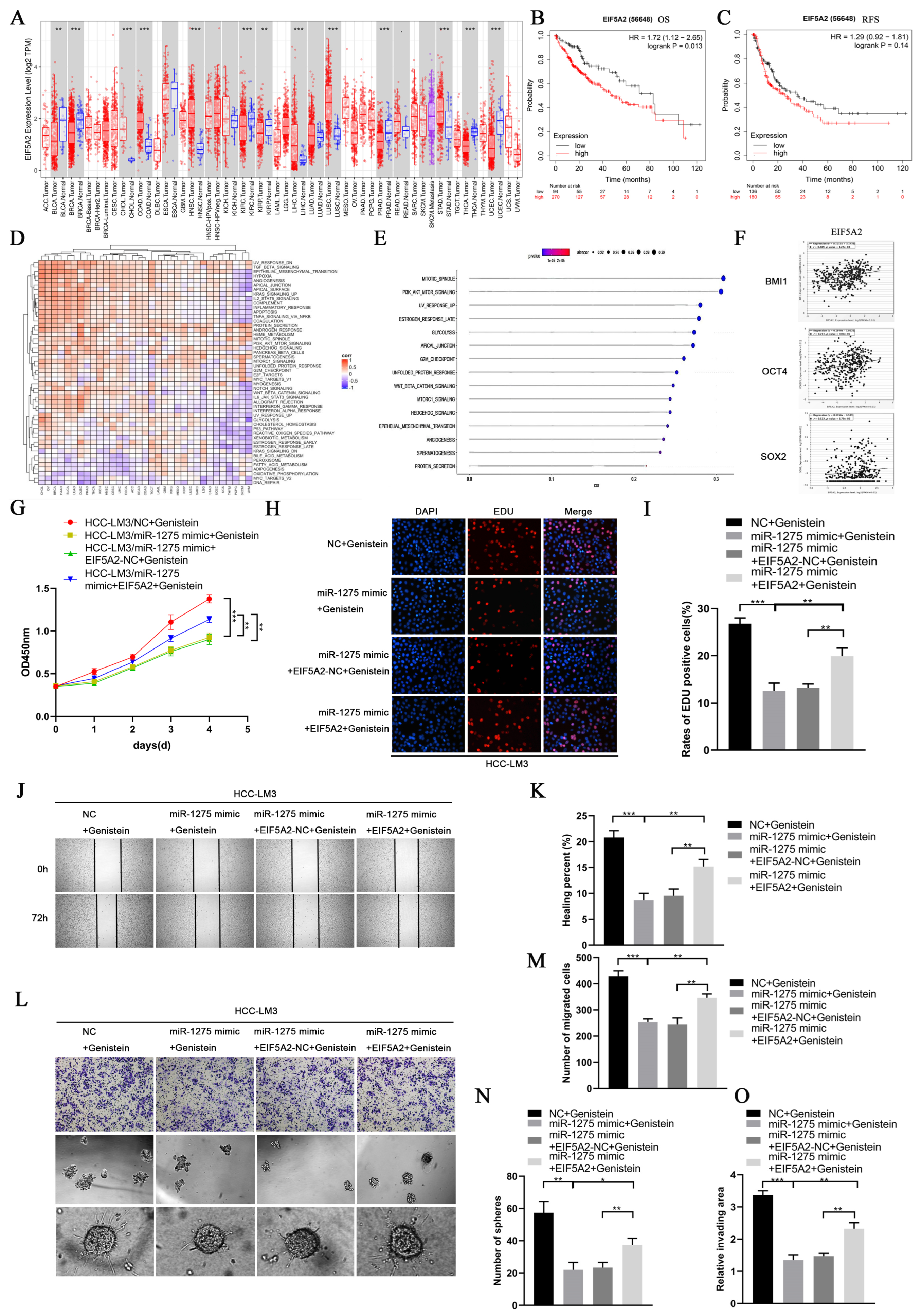
3.5. MiR-1275 Upregulated by Genistein Attenuated the EMT and Stemness of HCC Cells by Inhibiting the EIF5A2/PI3K/Akt Signaling Pathway
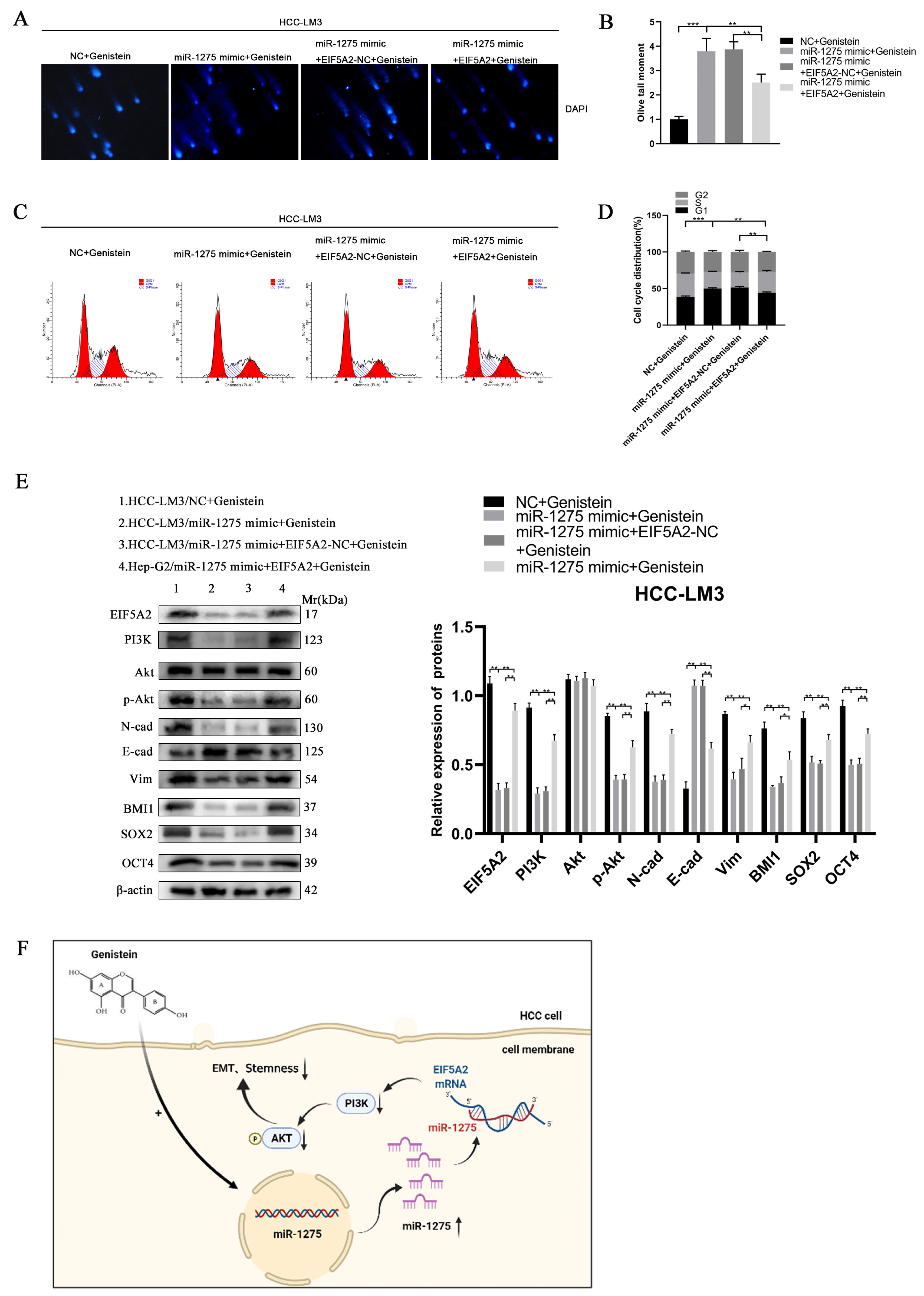
3.6. The Inhibitory Effect of miR-1275 on the EMT and Stemness of HCC Can Be Reversed by EIF5A2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Z.; Li, Y.; Li, Y.; Zhang, J.; Li, M.; Ji, L.; Tang, Y.; Zheng, Y.; Sheng, J.; Han, Q.; et al. Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, J.; Zhang, H.; Liao, Z.; Liu, F.; Su, C.; Wang, W.; Han, M.; Zhang, L.; Zhu, H.; et al. EIF4A3-induced circTOLLIP promotes the progression of hepatocellular carcinoma via the miR-516a-5p/PBX3/EMT pathway. J. Exp. Clin. Cancer Res. 2022, 41, 164. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, X.; Kwong, D.L.; Zhang, Y.; Verhoeft, K.; Gong, L.; Zhang, B.; Chen, J.; Yu, Q.; Luo, J.; et al. Targeting TROY-mediated P85a/AKT/TBX3 signaling attenuates tumor stemness and elevates treatment response in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2022, 41, 182. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Mao, Z.; Han, C.; Ding, Z.; Yuan, Q.; Zhang, G.; Li, C.; Wu, X.; Chen, J.; Guo, M.; et al. A novel epithelial-mesenchymal transition gene signature correlated with prognosis, and immune infiltration in hepatocellular carcinoma. Front. Pharmacol. 2022, 13, 863750. [Google Scholar] [CrossRef]
- Husain, A.; Chiu, Y.T.; Sze, K.M.; Ho, D.W.; Tsui, Y.M.; Suarez, E.M.S.; Zhang, V.X.; Chan, L.K.; Lee, E.; Lee, J.M.; et al. Ephrin-A3/EphA2 axis regulates cellular metabolic plasticity to enhance cancer stemness in hypoxic hepatocellular carcinoma. J. Hepatol. 2022, 77, 383–396. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Chen, X.; Cheng, J.; Zhang, H.; Shen, J.; Shan, J.; Xu, Y.; Yang, Z.; Lai, M.; et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology 2016, 64, 117–129. [Google Scholar] [CrossRef]
- Pelullo, M.; Zema, S.; Nardozza, F.; Checquolo, S.; Screpanti, I.; Bellavia, D. Wnt, Notch, and TGF-beta pathways impinge on hedgehog signaling complexity: An open window on cancer. Front. Genet. 2019, 10, 711. [Google Scholar] [CrossRef]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef]
- Wei, T.T.; Chandy, M.; Nishiga, M.; Zhang, A.; Kumar, K.K.; Thomas, D.; Manhas, A.; Rhee, S.; Justesen, J.M.; Chen, I.Y.; et al. Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell 2022, 185, 1676–1693.e1623. [Google Scholar] [CrossRef]
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; Bramer, W.M.; Ahmadizar, F.; Chowdhury, R.; Danser, A.J.; Roks, A.J.; Voortman, T.; Franco, O.H.; et al. Associations between phytoestrogens, glucose homeostasis, and risk of diabetes in women: A systematic review and meta-analysis. Adv. Nutr. 2018, 9, 726–740. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Wang, Q.; Yang, S.; Cao, Y.; Hao, J.N.; Niu, D.; Chen, Y.; Han, B.; Jia, X.; et al. A “valve-closing” starvation strategy for amplification of tumor-specific chemotherapy. Adv. Sci. 2022, 9, 2104671. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dai, W.; Zhang, Q.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br. J. Cancer 2017, 117, 1518–1528. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borras, C.; Vina, J. The multimodal action of genistein in Alzheimer’s and other age-related diseases. Free Radic. Biol. Med. 2022, 183, 127–137. [Google Scholar] [CrossRef]
- Youssef, M.M.; Tolba, M.F.; Badawy, N.N.; Liu, A.W.; El-Ahwany, E.; Khalifa, A.E.; Zada, S.; Abdel-Naim, A.B. Novel combination of sorafenib and biochanin-A synergistically enhances the anti-proliferative and pro-apoptotic effects on hepatocellular carcinoma cells. Sci. Rep. 2016, 6, 30717. [Google Scholar] [CrossRef]
- Fang, J.H.; Zhang, Z.J.; Shang, L.R.; Luo, Y.W.; Lin, Y.F.; Yuan, Y.; Zhuang, S.M. Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology 2018, 68, 1459–1475. [Google Scholar] [CrossRef]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef]
- Huang, X.Y.; Huang, Z.L.; Huang, J.; Xu, B.; Huang, X.Y.; Xu, Y.H.; Zhou, J.; Tang, Z.Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20. [Google Scholar] [CrossRef]
- Hu, Q.; Li, Y.; Chen, H.; Liao, H.; He, Y.; Zheng, Q. CCDC88A post-transcriptionally regulates VEGF via miR-101 and subsequently regulates hepatocellular carcinoma. Front. Immunol. 2022, 13, 859331. [Google Scholar] [CrossRef]
- Tey, S.K.; Wong, S.W.K.; Chan, J.Y.T.; Mao, X.; Ng, T.H.; Yeung, C.L.S.; Leung, Z.; Fung, H.L.; Tang, A.H.N.; Wong, D.K.H.; et al. Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. J. Hepatol. 2022, 76, 883–895. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, Y.; Liu, S.; Wang, C.; Zhang, S.; Zhang, T.; Yang, X. EIF5A2 enhances stemness of epithelial ovarian cancer cells via a E2F1/KLF4 axis. Stem Cell Res. Ther. 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, W.; Dong, P.; Watari, H.; Guo, Y.; Pfeffer, L.M.; Tigyi, G.; Yue, J. EIF5A2 controls ovarian tumor growth and metastasis by promoting epithelial to mesenchymal transition via the TGFbeta pathway. Cell Biosci. 2021, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, L.; Zhao, G.R. Systems metabolic engineering of escherichia coli coculture for de novo production of genistein. ACS Synth. Biol. 2022, 11, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.M.; Lee, G.S.; An, B.S.; Jeung, E.B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, Y.; Cao, M.; Fan, W.; Cui, Y.; Cui, Y.; Zhan, Y.; Gu, R.; Tian, F.; Zhang, S.; et al. A novel EHD1/CD44/Hippo/SP1 positive feedback loop potentiates stemness and metastasis in lung adenocarcinoma. Clin. Transl. Med. 2022, 12, e836. [Google Scholar] [CrossRef]
- Gaza, A.; Fritz, V.; Malek, L.; Wormser, L.; Treiber, N.; Danner, J.; Kremer, A.E.; Thasler, W.E.; Siebler, J.; Meister, G.; et al. Identification of novel targets of miR-622 in hepatocellular carcinoma reveals common regulation of cooperating genes and outlines the oncogenic role of zinc finger CCHC-type containing 11. Neoplasia 2021, 23, 502–514. [Google Scholar] [CrossRef]
- Gu, Y.; Ji, F.; Liu, N.; Zhao, Y.; Wei, X.; Hu, S.; Jia, W.; Wang, X.W.; Budhu, A.; Ji, J.; et al. Loss of miR-192-5p initiates a hyperglycolysis and stemness positive feedback in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 268. [Google Scholar] [CrossRef]
- Shu, G.; Su, H.; Wang, Z.; Lai, S.; Wang, Y.; Liu, X.; Dai, L.; Bi, Y.; Chen, W.; Huang, W.; et al. LINC00680 enhances hepatocellular carcinoma stemness behavior and chemoresistance by sponging miR-568 to upregulate AKT3. J. Exp. Clin. Cancer Res. 2021, 40, 45. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.J.; Tan, Y.X.; Ren, H.; Qi, Z.T. Identification of deregulated miRNAs and their targets in hepatitis B virus-associated hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 5442–5453. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y.; Fang, C.M. Unveiling the tumour-regulatory roles of miR-1275 in cancer. Pathol. Res. Pract. 2022, 230, 153745. [Google Scholar] [CrossRef]
- Zhang, Z.; He, G.; Lv, Y.; Liu, Y.; Niu, Z.; Feng, Q.; Hu, R.; Xu, J. HERC3 regulates epithelial-mesenchymal transition by directly ubiquitination degradation EIF5A2 and inhibits metastasis of colorectal cancer. Cell Death Dis. 2022, 13, 74. [Google Scholar] [CrossRef]
- Bai, H.Y.; Liao, Y.J.; Cai, M.Y.; Ma, N.F.; Zhang, Q.; Chen, J.W.; Zhang, J.X.; Wang, F.W.; Wang, C.Y.; Chen, W.H.; et al. Eukaryotic initiation factor 5A2 contributes to the maintenance of CD133(+) hepatocellular carcinoma cells via the c-Myc/microRNA-29b axis. Stem Cells 2018, 36, 180–191. [Google Scholar] [CrossRef]
- Zhou, J.N.; Zhang, B.; Wang, H.Y.; Wang, D.X.; Zhang, M.M.; Zhang, M.; Wang, X.K.; Fan, S.Y.; Xu, Y.C.; Zeng, Q.; et al. A functional screening identifies a new organic selenium compound targeting cancer stem cells: Role of c-Myc transcription activity inhibition in liver cancer. Adv. Sci. 2022, 9, 2201166. [Google Scholar] [CrossRef]
- Hassan, A.A.; Artemenko, M.; Tang, M.K.S.; Shi, Z.; Chen, L.Y.; Lai, H.C.; Yang, Z.; Shum, H.C.; Wong, A.S.T. Ascitic fluid shear stress in concert with hepatocyte growth factor drive stemness and chemoresistance of ovarian cancer cells via the c-Met-PI3K/Akt-miR-199a-3p signaling pathway. Cell Death Dis. 2022, 13, 537. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Lan, Y.M.; Lee, P.S.; Lin, Q.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Piceatannol prevents colon cancer progression via dual-targeting to M2-polarized tumor-associated macrophages and the TGF-beta1 positive feedback signaling pathway. Mol. Nutr. Food Res. 2022, 66, 2200248. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Tsui, Y.M.; Shi, C.; Wang, Y.; Zhang, X.; Yan, Q.; Chen, M.; Jiang, C.; Yuan, Y.F.; et al. RALYL increases hepatocellular carcinoma stemness by sustaining the mRNA stability of TGF-beta2. Nat. Commun. 2021, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, T.; Zhou, B.; Wei, J.; Fang, Y.; Lu, J.; Guo, L.; Chen, W.; Liu, Z.P.; Luo, J. Mg(II)-Catechin nanoparticles delivering siRNA targeting EIF5A2 inhibit bladder cancer cell growth in vitro and in vivo. Biomaterials 2016, 81, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Chen, Y.; Shahryari, V.; Liu, J.; Zaman, M.S.; Hirata, H.; Yamamura, S.; Ueno, K.; et al. Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer. Cancer Res. 2010, 70, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Zhang, Y.X.; Tang, L.H.; Yang, X.J.; Cui, W.M.; Han, C.C.; Ji, W.Y. MicroRNA-1469, a p53-responsive microRNA promotes Genistein induced apoptosis by targeting Mcl1 in human laryngeal cancer cells. Biomed. Pharmacother. 2018, 106, 665–671. [Google Scholar] [CrossRef]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological | Number | Low (n = 35) | High (n = 35) | p Value |
|---|---|---|---|---|
| Parameters | miR-1275 | miR-1275 | ||
| Age (years) | 70 | 1.000 | ||
| ≤60 | 52 | 26 | 26 | |
| >60 | 18 | 9 | 9 | |
| Gender | 70 | 0.480 | ||
| Female | 10 | 4 | 6 | |
| Male | 60 | 31 | 29 | |
| Liver cirrhosis | 70 | 0.255 | ||
| Yes | 54 | 25 | 29 | |
| No | 16 | 10 | 6 | |
| HBsAg | 70 | 0.255 | ||
| Positive | 54 | 25 | 29 | |
| Negative | 16 | 10 | 6 | |
| Virus titer (copies/mL) | 70 | 0.434 | ||
| ≤100 | 49 | 23 | 26 | |
| >100 | 21 | 12 | 9 | |
| AFP (ng/mL) | 70 | 0.334 | ||
| ≤20 | 30 | 17 | 13 | |
| >20 | 40 | 18 | 22 | |
| DCP (ng/mL) | 70 | 0.803 | ||
| ≤40 | 45 | 22 | 23 | |
| >40 | 25 | 13 | 12 | |
| Tumor size (cm) | 70 | 0.008 * | ||
| ≤5 | 37 | 13 | 24 | |
| >5 | 33 | 22 | 11 | |
| Tumor multiplicity | 70 | 0.495 | ||
| Single | 60 | 29 | 31 | |
| Multiple | 10 | 6 | 4 | |
| Microvascular invasion | 70 | 0.597 | ||
| Yes | 20 | 11 | 9 | |
| No | 50 | 24 | 26 | |
| Edmondson grade | 70 | 0.584 | ||
| I–II | 52 | 27 | 25 | |
| III–IV | 18 | 8 | 10 | |
| TNM stage | 70 | 0.075 | ||
| I–II | 47 | 20 | 27 | |
| III–IV | 23 | 15 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Jiang, W.; Kong, X.; Zhou, X.; Zhu, D.; Kong, L. Genistein Restricts the Epithelial Mesenchymal Transformation (EMT) and Stemness of Hepatocellular Carcinoma via Upregulating miR-1275 to Inhibit the EIF5A2/PI3K/Akt Pathway. Biology 2022, 11, 1383. https://doi.org/10.3390/biology11101383
Yang X, Jiang W, Kong X, Zhou X, Zhu D, Kong L. Genistein Restricts the Epithelial Mesenchymal Transformation (EMT) and Stemness of Hepatocellular Carcinoma via Upregulating miR-1275 to Inhibit the EIF5A2/PI3K/Akt Pathway. Biology. 2022; 11(10):1383. https://doi.org/10.3390/biology11101383
Chicago/Turabian StyleYang, Xiao, Wangjie Jiang, Xiangxu Kong, Xiao Zhou, Deming Zhu, and Lianbao Kong. 2022. "Genistein Restricts the Epithelial Mesenchymal Transformation (EMT) and Stemness of Hepatocellular Carcinoma via Upregulating miR-1275 to Inhibit the EIF5A2/PI3K/Akt Pathway" Biology 11, no. 10: 1383. https://doi.org/10.3390/biology11101383
APA StyleYang, X., Jiang, W., Kong, X., Zhou, X., Zhu, D., & Kong, L. (2022). Genistein Restricts the Epithelial Mesenchymal Transformation (EMT) and Stemness of Hepatocellular Carcinoma via Upregulating miR-1275 to Inhibit the EIF5A2/PI3K/Akt Pathway. Biology, 11(10), 1383. https://doi.org/10.3390/biology11101383






