Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Chemical Characterization of Lemongrass and Its Major Bioactive Compounds
2.1. Composition of Lemongrass Products
2.2. Chemical Characterization and Metabolism of the Lemongrass and Its Main Compounds
3. Cardiovascular Activity of Lemongrass and Citral
3.1. Antioxidant and Anti-Inflammatory Activities of Citral and Lemongrass In Vitro
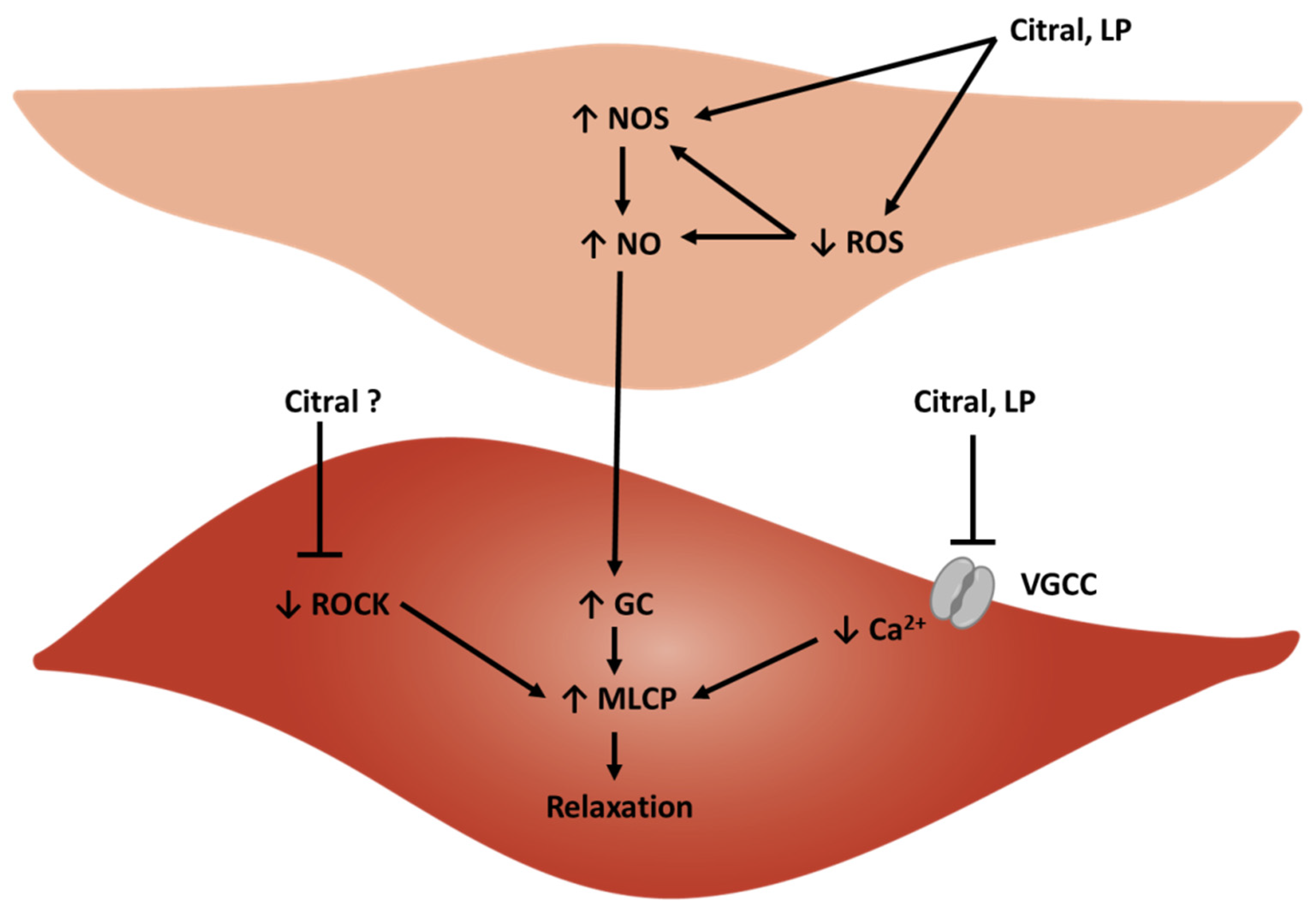
3.2. Vasorelaxant Activity of Citral Ex Vivo
3.3. Anti-Hypertensive Activity of Citral In Vivo
3.4. Cardiac Activity of Lemongrass Products Ex Vivo
3.5. Vasorelaxant Activity of Lemongrass Products Ex Vivo
3.6. Anti-Hypertensive Activities of Lemongrass In Vivo
3.6.1. Vasodilation and Cardiac Suppression Activity
| Authors | Animal Species/Strain | Lemongrass Product (Dose/Administration) | Main Results |
|---|---|---|---|
| Somparn et al. (2018) [68] | Healthy male Sprague-Dawley rats (N = 30, undisclosed age and weight) | Water extract of the whole plant (250, 500 and 1000 mg/kg/bw/day per os for 30 days) | Significant decrease in total cholesterol, LDL and atherogenic index. Significant increase in serum antioxidant activity and decrease in lipid peroxidation. |
| Arome et al. (2014) [105] | Healthy Swiss albino mice (N = 5, 18–30 g, both sexes, undisclosed age) | Water extract of roots (200, 400 and 600 mg/kg) | Significant reduction in anxiety behavior (reduced body temperature in stress-induced hyperemia model; increased time spent in the open arm in an elevated plus maze model; increased locomotion and decreased rearing and defecation in an open-field model). |
| Carbajal et al. (1989) [29] | Healthy Wistar rats (N = 5, 180–220 g, undisclosed age) under sodium pentobarbital anesthesia | Water extract of leaves (1, 2 and 3 mL/kg i.v.) | Significant decrease in blood pressure. |
| Singi et al. (2005) [102] | Healthy Wistar rats (N = 7, 400 g, undisclosed age) under sodium pentobarbital anesthesia | Water/ethanol extract of fresh leaves (aprox. 0.8 mg/kg) | Significant and short-lived decrease in mean blood pressure. |
| Water/ethanol extract of fresh lemongrass leaves and garlic bulbs (aprox. 0.8 mg/kg of each) | Significant and short-lived decrease in mean blood pressure | ||
| Moreira et al. (2010) [104] | Healthy male Wistar rats (200–300 g, undisclosed number and age) | Essential oil of fresh leaves (5–20 mg/kg i.v.) | Bradycardia fully opposed by atropine and partially by sodium thiopental, but not by L-NAME or indomethacin. Hypotension fully opposed by atropine but not by L-NAME or indomethacin. |
| Dzeufiet et al. (2014) [31] | Healthy (N = 6) and ethanol/sucrose-induced hypertensive (N = 6) Wistar rats (6–8 w.o., 150–160 g) under urethane anesthesia | Water extract of fresh leaf of avocado, fresh leaves and stems of lemongrass, citron and honey (50, 100 and 150 mg/kg, respectively) | Significant reduction in heart rate, systolic, diastolic and mean blood pressure in comparison with ethanol/sucrose-induced hypertensive animals. |
| Jutabha et al. (1995) [101] | Mongrel dogs (N = 5, 12–18 kg) anesthetized with sodium pentobarbital (25 mg/kg i.v.) | Leaves (1.25, 2.5, 5.0 and 10 g/kg administered orally) | Significant decrease in heart rate from 1.5 to 2.5 h after administration, probably due to baroreflex. Non-significant increase in blood pressure. |
| Tcheutchoua et al. (2022) [103] | Male Wistar rats (6–8 w.o., 150–160 g, undisclosed number) | Water extract of leaves and stems (200 mg/kg administered orally (1/day) for 7 weeks) | Significant decrease in systolic, diastolic and mean blood pressure. |
3.6.2. Central Nervous System-Depressing Activity
3.6.3. Diuretic Activity
3.7. Clinical Studies—Cardiovascular Activities in Humans
| Authors | Study Sample | Lemongrass Product | Administration Route | Main Cardiovascular Effects |
|---|---|---|---|---|
| Leite et al. (1986) [33] | Young and healthy subjects (N = 9, 18–35 y.o., undisclosed sex ratio) | Tea made from infusion of and powdered leaves | Oral, once | No difference in pulse rate after subjected to the Stroop test in comparison with controls. |
| Ekpenyong et al. (2016) [69] | Young healthy subjects (N = 105, both sexes) | Tea made from infusion of powdered leaves | Oral, once daily for 30 days | Mean and diastolic blood pressure, pulse pressure and heart rate decreased on day 10 and day. |
| Sobha (2014) [107] | Pre-hypertensive and hypertensive subjects (N = 60, both sexes) | Tea made from leaves decoction | Oral, 250 mL once a day for 14 days | Significant decrease in systolic blood pressure. |
| Goes et al. (2015) [46] | Young healthy subjects (N = 40 males, 18–30 y.o.) | Essential oil | Inhalation (3 deep breaths) of 3 or 6 drops in a paper | Significant decrease in anxiety levels. No significant change in heart rate. |
| Kamkaen et al. (2015) [51] | Healthy subjects (N = 8 males and 21 females, 18–82 y.o., mean age 50.48 y.o.) | Essential oil | Inhalation of essential oil applied to the skin during massage | Significant decrease in diastolic blood pressure in all groups. Comparison of effect was not performed against control groups. |
| Siahaan et al. (2014) [111] | Young and healthy but stress-prone female subjects (N = 20, undisclosed age) | 3% suspension of ylang-ylang, lemongrass, and patchouli essential oil mixture | Inhalation during massage therapy | Significant decrease in mean blood pressure, larger than control subjects. |
3.8. Additional Presumed Mechanisms of Action
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wierzejska, E.; Giernaś, B.; Lipiak, A.; Karasiewicz, M.; Cofta, M.; Staszewski, R. A global perspective on the costs of hypertension: A systematic review. Arch. Med. Sci. 2020, 16, 1078–1091. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2007, 25, 1105–1187. [Google Scholar] [CrossRef]
- Catalysing Ancient Wisdom and Modern Science for the Health of People and the Planet. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 5 August 2022).
- Gopal, N.M.; Tejaswini, J.; Mantry, S.; Kumar, S.A. International standards of medicinal plants. Int. J. Innov. Pharm. Sci. Res. 2014, 2, 2498–2532. [Google Scholar]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Bishop, F.L.; Yardley, L.; Lewith, G.T. A systematic review of beliefs involved in the use of complementary and alternative medicine. J. Health Psychol. 2007, 12, 851–867. [Google Scholar] [CrossRef]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 2008, 15188733. [Google Scholar] [CrossRef]
- Bertea, C.M.; Maffei, M.E. The Genus Cymbopogon Botany, Including Anatomy, Physiology, Biochemistry, and Molecular Biology. In Essential Oil Bearing Grasses The Genus Cymbopogon; Akhila, A., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef]
- Machraoui, M.; Kthiri, Z.; Ben Jabeur, M.; Hamada, W. Ethnobotanical and phytopharmacological notes on Cymbopogon citratus (DC.) Stapf. J. New Sci. 2018, 55, 3642–3652. [Google Scholar] [CrossRef]
- Ravinder, K.; Pawan, K.; Gaurav, S.; Paramjot, K.; Gagan, S.; Appramdeep, K. Pharmacognostical Investigation of Cymbopogon citratus (DC) Stapf. Sch. Res. Libr. 2010, 2, 181–189. [Google Scholar]
- Gaba, J.; Bhardwaj, G.; Sharma, A. Lemongrass. In Antioxidants in Vegetables and Nuts-Properties and Health Benefits; Springer: Berlin/Heidleberg, Germany, 2020; pp. 75–103. ISBN 9789811574702. [Google Scholar]
- Ganjewala, D. Cymbopogon essential oils: Chemical compositions and bioactivities. Int. J. Essent. Oil Ther. 2009, 3, 56–65. [Google Scholar]
- Vanisha, S. Nambiar and Hema Matela Potential Functions of Lemon Grass (Cymbopogon citratus) in Health and Disease. Int. J. Pharm. Biol. Arch. 2016, 3, 1035–1043. [Google Scholar]
- Amirdivani, S.; Baba, A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Skaria, B.P.; Joy, P.P.; Mathew, G.; Mathew, S.; Joseph, A. Lemongrass. Handb. Herbs Spices Second Ed. 2012, 2, 348–370. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef]
- Kamoga, O.L.M.; Kirabira, J.B.; Byaruhanga, J.K. The Potential of Cymbopogon nardus in the Production of Pulp for Paper Industry. In Proceedings of the International Conference on Computing, Mechanical and Electronics Engineering (ICCMEE’2015), Singapore, 9–10 July 2015; pp. 21–28. [Google Scholar] [CrossRef]
- Nur Firdaus, M.Y.; Osman, H.; Metselaar, H.S.C.; Rozyanty, A.R. A simple method for the production of pure crystalline silica from lemon grass. BioResources 2015, 11, 1270–1279. [Google Scholar] [CrossRef]
- Bekele, L.D.; Zhang, W.; Liu, Y.; Duns, G.J.; Yu, C.; Jin, L.; Li, X.; Jia, Q.; Chen, J. Preparation and Characterization of Lemongrass Fiber (Cymbopogon species) for Reinforcing Application in Thermoplastic Composites. Biosources 2017, 12, 5664–5681. [Google Scholar] [CrossRef]
- Babarinde, A.; Ogundipe, K.; Sangosanya, K.T.; Akintola, B.D.; Hassan, A.O.E. Comparative study on the biosorption of Pb(II), Cd(II) and Zn(II) using Lemon grass (Cymbopogon citratus): Kinetics, isotherms and thermodynamics. Chem. Int. 2016, 2, 89–102. [Google Scholar]
- Alfa, I.M.; Dahunsi, S.O.; Iorhemen, O.T.; Okafor, C.C.; Ajayi, S.A. Comparative evaluation of biogas production from Poultry droppings, Cow dung and Lemon grass. Bioresour. Technol. 2014, 157, 270–277. [Google Scholar] [CrossRef]
- Wannissorn, B.; Jarikasem, S.; Soontorntanasart, T. Antifungal activity of lemon grass oil and lemon grass oil cream. Phyther. Res. 1996, 10, 551–554. [Google Scholar] [CrossRef]
- Pedroso, R.B.; Ueda-Nakamura, T.; Dias Filho, B.P.; Cortez, D.A.G.; Cortez, L.E.R.; Morgado-Díaz, J.A.; Nakamura, C.V. Biological activities of essential oil obtained from Cymbopogon citratus on Crithidia deanei. Acta Protozool. 2006, 45, 231–240. [Google Scholar]
- Francisco, V.; Figueirinha, A.; Neves, B.M.; García-Rodríguez, C.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011, 133, 818–827. [Google Scholar] [CrossRef]
- Khadri, A.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Neffati, M.; Smiti, S.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC-mass spectrometry and 13C NMR. Food Chem. 2008, 109, 630–637. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Remadevi, R.; Naebe, M. Lemongrass (Cymbopogon): A review on its structure, properties, applications and recent developments. Cellulose 2018, 25, 5455–5477. [Google Scholar] [CrossRef]
- Darias, V.; Bravo, L.; Rabanal, R.; Mateo, C.S.; Luis, R.M.G.; Pérez, A.M.H. New contribution to the ethnopharmacological study of the canary islands. J. Ethnopharmacol. 1989, 25, 77–92. [Google Scholar] [CrossRef]
- Carbajal, D.; Casaco, A.; Arruzazabala, L.; Gonzalez, R.; Tolon, Z. OF CYMBOPOGON or Cymbopogon Plant muterials Blood pressure measurement Measurement of diuretic activity Anti-inflammatory effect. J. Ethnopharmacol. 1989, 25, 103–107. [Google Scholar] [CrossRef]
- Gómez, Y.M.; García, C.J.; Javier, A.; González, D. Caña santa para el tratamiento de ancianos con hipertensión arterial / Lemongrass for treating aged persons with hypertension MsC. Medisan 2010, 14, 1061–1067. [Google Scholar]
- Dzeufiet, P.D.D.; Mogueo, A.; Bilanda, D.C.; Aboubakar, B.F.O.; Tédong, L.; Dimo, T.; Kamtchouing, P. Antihypertensive potential of the aqueous extract which combine leaf of Persea americana Mill. (Lauraceae), stems and leaf of Cymbopogon citratus (D.C) Stapf. (Poaceae), fruits of Citrus medical L. (Rutaceae) as well as honey in ethanol and sucrose experi. BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Locksley, H.; Fayez, M.; Radwan, A.; Chari, V.; Cordell, G.; Wagner, H. Constituents of Local Plants. Planta Med. 1982, 45, 20–22. [Google Scholar] [CrossRef]
- Leite, J.; Seabra, M.; Maluf, E.; Assolant, K.; Suchecki, D.; Tufik, S.; Klepacz, S.; Calil, H.; Carlini, E. Pharmacology of lemongrass. III. Assessment of eventual toxic, hypnotic and anxiolytic effects on humans. J. Ethnopharmacol. 1986, 17, 75–83. [Google Scholar] [CrossRef]
- Formigoni, M.L.O.S.; Lodder, H.M.; Filho, O.G.; Ferreira, T.M.S.; Carlini, E.A. Pharmacology of lemongrass (Cymbopogon citratus Stapf). II. Effects of daily two month administration in male and female rats and in offspring exposed “in utero”. J. Ethnopharmacol. 1986, 17, 65–74. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Herbs Used for the Treatment of Hypertension and their Mechanism of Action. Curr. Hypertens. Rep. 2017, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Lo, C. “Lemongrass” and its applications for the treatment of hypertension. Infect. Dis. Herb. Med. 2021, 2, 172. [Google Scholar] [CrossRef]
- Olorunnisola, S.K.; Asiyanbi, H.T.; Hammed, A.M.; Simsek, S. Biological properties of lemongrass: An overview. Int. Food Res. J. 2014, 21, 455–462. [Google Scholar]
- Kamaruddin, Z.H.; Jumaidin, R.; Selamat, M.Z.; Ilyas, R.A. Characteristics and Properties of Lemongrass (Cymbopogan Citratus): A Comprehensive Review. J. Nat. Fibers 2021, 1–18. [Google Scholar] [CrossRef]
- Wifek, M.; Saeed, A.; Rehman, R.; Nisar, S. Lemongrass: A review on its botany, properties, applications and active components Evaluation of the effects of Zinc on the chemical composition and biological activity of basil essential oil by using Raman spectroscopy View project Lemongrass: A review. Int. J. Chem. Biochem. Sci. 2016, 9, 79–84. [Google Scholar]
- Karami, S.; Yargholi, A.; Lamardi, S.N.S.; Soleymani, S.; Shirbeigi, L.; Rahimi, R. A review of ethnopharmacology, phytochemistry and pharmacology of cymbopogon species. Res. J. Pharmacogn. 2021, 8, 83–112. [Google Scholar] [CrossRef]
- Manvitha, B.B.K. Review on pharmacological activity of Cymbopogon citratus. Int. J. Herb. Med. 2014, 1, 5–7. [Google Scholar] [CrossRef]
- Carlson, L.H.C.; Machado, R.A.F.; Spricigo, C.B.; Pereira, L.K.; Bolzan, A. Extraction of lemongrass essential oil with dense carbon dioxide. J. Supercrit. Fluids 2001, 21, 33–39. [Google Scholar] [CrossRef]
- Majewska, E.; Kozlowska, M.; Gruczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation—A review. Pol. J. Food Nutr. Sci. 2019, 69, 327–341. [Google Scholar] [CrossRef]
- Aftab, K.; Ali, M.D.; Aijaz, P.; Beena, N.; Gulzar, H.J.; Sheikh, K.; Sofia, Q.; Abbas, S.T. Determination of different trace and essential element in lemon grass samples by X-ray fluorescence spectroscopy technique. Int. Food Res. J. 2011, 18, 265–270. [Google Scholar]
- Goes, T.C.; Ursulino, F.R.C.; Almeida-Souza, T.H.; Alves, P.B.; Teixeira-Silva, F. Effect of lemongrass aroma on experimental anxiety in humans. J. Altern. Complement. Med. 2015, 21, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Cannon, J.B. Lemongrass productivity, oil content, and composition as a function of nitrogen, sulfur, and harvest time. Agron. J. 2011, 103, 805–812. [Google Scholar] [CrossRef]
- Chisowa, E.H.; Hall, D.R.; Farman, D.I. Volatile constituents of the essential oil of Cymbopogon citratus Stapf grown in Zambia. Flavour Fragr. J. 1998, 13, 29–30. [Google Scholar] [CrossRef]
- Kasali, A.A.; Oyedeji, A.O.; Ashilokun, A.O. Volatile leaf oil constituents of Cymbopogon citratus (DC) Stapf. Flavour Fragr. J. 2001, 16, 377–378. [Google Scholar] [CrossRef]
- Dutta, D.; Kumar, P.; Nath, A.; Verma, N.; Gangwar, B. Qualities of lemongrass (Cymbopogan citratus) essential oil at different drying conditions. Int. J. Agric. Environ. Biotechnol. 2014, 7, 903–909. [Google Scholar] [CrossRef]
- Kamkaen, N.; Ruangrungsi, N.; Patalung, N.N.; Watthanachaiyingcharoen, R. Physiological and Psychological Effects of Lemongrass and Sweet Almond Massage Oil. J. Health Res. 2015, 29, 85–91. [Google Scholar]
- Barbosa, L.C.A.; Pereira, U.A.; Martinazzo, A.P.; Maltha, C.R.Á.; Teixeira, R.R.; Melo, E.D.C. Evaluation of the chemical composition of Brazilian commercial Cymbopogon citratus (D.C.) stapf samples. Molecules 2008, 13, 1864–1874. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E.; Daniel, N.E. Phytochemical Constituents, Therapeutic Applications and Toxicological Profile of Cymbopogon citratus Stapf (DC) Leaf Extract. J. Pharmacogn. Phytochem. 2014, 3, 133–141. [Google Scholar]
- Mansour; Fikry, R.M.; Saad, M.M.; Mohamed, A.M. Chemical composition, antioxidant and antimicrobial activity of (Cymbopogon citratus) essential oil cultivated in Madinah Monawara, Saudi Arabia and its comparison to the Egyptian chemotype. Int. J. Food Nutr. Sci. 2014, 4, 33. [Google Scholar]
- Farias, P.K.S.; Lopes Silva, J.C.R.; de Souza, C.N.; da Fonseca, F.S.A.; Brandi, I.V.; Martins, E.R.; Azevedo, A.M.; de Almeida, A.C. Antioxidant activity of essential oils from condiment plants and their effect on lactic cultures and pathogenic bacteria. Cienc. Rural 2019, 49, 1–12. [Google Scholar] [CrossRef]
- Moutassem, D.; Belabid, L.; Bellik, Y.; Ziouche, S.; Baali, F. Efficacy of essential oils of various aromatic plants in the biocontrol of Fusarium wilt and inducing systemic resistance in chickpea seedlings. Plant Prot. Sci. 2019, 55, 202–217. [Google Scholar] [CrossRef]
- Matasyoh, J.C.; Wagara, I.N.; Nakavuma, J.L.; Kiburai, A.M. Chemical composition of Cymbopogon citratus essential oil and its effect on mycotoxigenic Aspergillus species. Afr. J. Food Sci. 2011, 5, 138–142. [Google Scholar]
- Mbili, N.C.; Opara, U.L.; Lennox, C.L.; Vries, F.A. Citrus and lemongrass essential oils inhibit Botrytis cinerea on “golden delicious”, “pink lady” and “granny Smith” apples. J. Plant Dis. Prot. 2017, 124, 499–511. [Google Scholar] [CrossRef]
- Abegaz, B.; Yohannes, P.G.; Dieter, R.K. Constituents of the essential oil of ethiopian Cymbopogon citratus staff. J. Nat. Prod. 1983, 46, 424–426. [Google Scholar] [CrossRef]
- Crawford, M.; Hanson, S.W.; Koker, M.E.S. The structure of cymbopogone, a novel triterpenoid from lemongrass. Tetrahedron Lett. 1975, 16, 3099–3102. [Google Scholar] [CrossRef]
- Hanson, S.W.; Crawford, M.; Koker, M.E.S.; Menezes, F.A. Cymbopogonol, a new triterpenoid from Cymbopogon citratus. Phytochemistry 1976, 15, 1074–1075. [Google Scholar] [CrossRef]
- Shaikh, M.N.; Suryawanshi, Y.C.; Mokat, D.N. Volatile Profiling and Essential Oil Yield of Cymbopogon citratus (DC.) Stapf Treated with Rhizosphere Fungi and Some Important Fertilizers. J. Essent. Oil-Bearing Plants 2019, 22, 477–483. [Google Scholar] [CrossRef]
- Verma, R.K.; Verma, R.S.; Chauhan, A.; Bisht, A. Evaluation of essential oil yield and chemical composition of eight lemongrass (Cymbopogon spp.) cultivars under Himalayan region. J. Essent. Oil Res. 2015, 27, 197–203. [Google Scholar] [CrossRef]
- Tajidin, N.; Ahmad, S.; Rosenani, A.; Azimah, H.; Munirah, M. Chemical composition and citral content in lemongrass (Cymbopogon citratus) essential oil at three maturity stages. Afr. J. Biotechnol. 2012, 11, 2685–2693. [Google Scholar] [CrossRef]
- Coelho, M.; Rocha, C.; Cunha, L.M.; Cardoso, L.; Alves, L.; Lima, R.C.; Pereira, M.J.; Campos, F.M.; Pintado, M. Influence of harvesting factors on sensory attributes and phenolic and aroma compounds composition of Cymbopogon citratus leaves infusions. Food Res. Int. 2016, 89, 1029–1037. [Google Scholar] [CrossRef]
- Hanaa, A.R.M.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef]
- Asaolu, M.; Oyeyemi, O.; Olanlokun, J. Chemical Compositions, Phytochemical Constituents and in vitro Biological Activity of Various Extracts of Cymbopogon citratus. Pak. J. Nutr. 2009, 8, 1920–1922. [Google Scholar] [CrossRef]
- Somparn, N.; Saenthaweeuk, S.; Naowaboot, J.; Thaeomor, A.; Kukongviriyapan, V. Effect of lemongrass water extract supplementation on atherogenic index and antioxidant status in rats. Acta Pharm. 2018, 68, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, C.; Osim, E.; America, S. Changes in blood pressure indices in normotensive adults after the consumption of lemongrass tea. J. Coast. Life Med. 2016, 4, 802–808. [Google Scholar] [CrossRef]
- Soares, M.O.; Alves, R.C.; Pires, P.C.; Oliveira, M.B.P.P.; Vinha, A.F. Angolan Cymbopogon citratus used for therapeutic benefits: Nutritional composition and influence of solvents in phytochemicals content and antioxidant activity of leaf extracts. Food Chem. Toxicol. 2013, 60, 413–418. [Google Scholar] [CrossRef]
- Gazola, R.; MacHado, D.; Ruggiero, C.; Singi, G.; Alexandre, M.M. Lippia alba, Melissa officinalis and Cymbopogon citratus: Effects of the aqueous extracts on the isolated hearts of rats. Pharmacol. Res. 2004, 50, 477–480. [Google Scholar] [CrossRef]
- Campos, J.; Schmeda-Hirschmann, G.; Leiva, E.; Guzmán, L.; Orrego, R.; Fernández, P.; González, M.; Radojkovic, C.; Zuñiga, F.A.; Lamperti, L.; et al. Lemon grass (Cymbopogon citratus (D.C) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chem. 2014, 151, 175–181. [Google Scholar] [CrossRef]
- Simões, D.M.; Malheiros, J.; Antunes, P.E.; Figueirinha, A.; Cotrim, M.D.; Fonseca, D.A. Vascular activity of infusion and fractions of Cymbopogon citratus (DC) Stapf. in human arteries. J. Ethnopharmacol. 2020, 258, 6–11. [Google Scholar] [CrossRef]
- Stotz, S.C.; Vriens, J.; Martyn, D.; Clardy, J.; Clapham, D.E. Citral sensing by TRANSient receptor potential channels in dorsal root ganglion neurons. PLoS ONE 2008, 3, e2082. [Google Scholar] [CrossRef]
- Diliberto, J.J.; Usha, G.; Birnbaum, L.S. Disposition of citral in male Fischer rats. Drug Metab. Dispos. 1988, 16, 721–727. [Google Scholar] [PubMed]
- Baldwin, J.R.; Witiak, D.T.; Feller, D.R. Studies on the biotransformation and excretion of 14C-clofibrate in the rat. Fed. Proc. 1977, 36, 537–540. [Google Scholar]
- Diliberto, J.J.; Srinivas, P.; Overstreet, D.; Usha, G.; Burka, L.T.; Birnbaum, L.S. Metabolism of citral, an α,β-unsaturated aldehyde, in male F344 rats. Drug Metab. Dispos. 1990, 18, 866–875. [Google Scholar] [PubMed]
- Gaworski, C.; Vollmuth, T.; York, R.; Heck, J.; Arany, C. Developmental toxicity evaluation of inhaled citral in Sprague-Dawley rats. Food Chem. Toxicol. 1992, 30, 269–275. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyamoto, M.; Murakami, A.; Ohigashi, H.; Osawa, T.; Uchida, K. A phase II detoxification enzyme inducer from lemongrass: Identification of citral and involvement of electrophilic reaction in the enzyme induction. Biochem. Biophys. Res. Commun. 2003, 302, 593–600. [Google Scholar] [CrossRef]
- Li, C.C.; Yu, H.F.; Chang, C.H.; Liu, Y.T.; Yao, H.T. Effects of lemongrass oil and citral on hepatic drug-metabolizing enzymes, oxidative stress, and acetaminophen toxicity in rats. J. Food Drug Anal. 2018, 26, 432–438. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, H.; Liu, J.; Fang, C.; Miao, R. Effects of Citral on Lipopolysaccharide-Induced Inflammation in Human Umbilical Vein Endothelial Cells. Inflammation 2016, 39, 663–671. [Google Scholar] [CrossRef]
- Bachiega, T.F.; Sforcin, J.M. Lemongrass and citral effect on cytokines production by murine macrophages. J. Ethnopharmacol. 2011, 137, 909–913. [Google Scholar] [CrossRef]
- Katsukawa, M.; Nakata, R.; Takizawa, Y.; Hori, K.; Takahashi, S.; Inoue, H. Citral, a component of lemongrass oil, activates PPAR α and γ and suppresses COX-2 expression. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1214–1220. [Google Scholar] [CrossRef]
- Safaeian, L.; Sajjadi, S.E.; Montazeri, H.; Ohadi, F.; Javanmard, S. Citral protects human endothelial cells against hydrogen peroxide-induced oxidative stress. Turk. J. Pharm. Sci. 2020, 17, 549–554. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Amaral, J.T.; Fernandes, A.; Sousa, J.P.B.; Bastos, J.K. Lemongrass effects on IL-1β and IL-6 production by macrophages. Nat. Prod. Res. 2009, 23, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Mediesse, F.K.; Boudjeko, T.; Hasitha, A.; Gangadhar, M.; Mbacham, W.F.; Yogeeswari, P. Inhibition of lipopolysaccharide (LPS)-induced neuroinflammatory response by polysaccharide fractions of Khaya grandifoliola (C.D.C.) stem bark, Cryptolepis sanguinolenta (Lindl.) Schltr and Cymbopogon citratus Stapf leaves in raw 264.7 macrophages and U8. BMC Complement. Altern. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.C.; Sim, S.M.; Ismail, R. Effect of Cymbopogon citratus and citral on vascular smooth muscle of the isolated thoracic rat aorta. Evidence-Based Complement. Altern. Med. 2012, 2012, 539475. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Marques, A.M.; Sudo, R.T.; Kaplan, M.A.C.; Zapata-Sudo, G. Vasodilator activity of the essential oil from aerial parts of Pectis brevipedunculata and its main constituent citral in rat aorta. Molecules 2013, 18, 3072–3085. [Google Scholar] [CrossRef] [PubMed]
- Moreira, F. Cardiovascular Effects of the Citral, Major Monoterpene of the Essential oil of Cymbopogon citratus, in Rats. Ph.D. Thesis, Núcleo de Pós-Graduação em Medicina, Doutorado em Ciências da Saúde—NPGME/UFS, Universidade Federal de Sergipe, São Cristóvão, Brazil, 2013. [Google Scholar]
- Wistar Kyoto (WKY) Rat|Charles River. Available online: https://www.criver.com/products-services/find-model/wistar-kyoto-wky-rat?region=3616 (accessed on 1 August 2020).
- Höferl, M.; Krist, S.; Buchbauer, G. Chirality influences the effects of linalool on physiological parameters of stress. Planta Med. 2006, 72, 1188–1192. [Google Scholar] [CrossRef]
- Yayama, K.; Sasahara, T.; Ohba, H.; Funasaka, A.; Okamoto, H. Orthovanadate-induced vasocontraction is mediated by the activation of Rho-kinase through Src-dependent transactivation of epidermal growth factor receptor. Pharmacol. Res. Perspect. 2014, 2, e00039. [Google Scholar] [CrossRef]
- Ribeiro-Filho, H.V.; De Souza Silva, C.M.; De Siqueira, R.J.; Lahlou, S.; Dos Santos, A.A.; Magalhães, P.J.C. Biphasic cardiovascular and respiratory effects induced by β-citronellol. Eur. J. Pharmacol. 2016, 775, 96–105. [Google Scholar] [CrossRef]
- Bastos, J.F.A.; Moreira, Í.J.A.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; Santos, M.R.V. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 331–337. [Google Scholar] [CrossRef]
- Andrade, F.C.; Mota, M.M.; Barreto, A.S.; Sousa, D.P.; Quintans-Junior, L.J.; Santos, M.R.V. Antihypertensive therapeutic potential of citronellal. Lat. Am. J. Pharm. 2012, 31, 767–771. [Google Scholar]
- Kundu, S.; Shabir, H.; Basir, S.F.; Khan, L.A. Inhibition of As(III) and Hg(II) caused aortic hypercontraction by eugenol, linalool and carvone. J. Smooth Muscle Res. 2014, 50, 93–102. [Google Scholar] [CrossRef]
- Kang, P.; Seol, G.H. Linalool elicits vasorelaxation of mouse aortae through activation of guanylyl cyclase and K+ channels. J. Pharm. Pharmacol. 2015, 67, 714–719. [Google Scholar] [CrossRef]
- Runnie, I.; Salleh, M.N.; Mohamed, S.; Head, R.J.; Abeywardena, M.Y. Vasorelaxation induced by common edible tropical plant extracts in isolated rat aorta and mesenteric vascular bed. J. Ethnopharmacol. 2004, 92, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.; Runnie, I.; Nizar, M.; Suhaila, M.; Head, R. Polyphenol-enriched extract of oil palm fronds (Elaeis guineensis) promotes vascular relaxation via endothelium-dependent mechanisms. Asia Pac. J. Clin. Nutr. 2002, 11 (Suppl. 7), S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Galán Martínez, L. Effect of a tincture of Cymbopogon citratus leaves on vascular smooth muscle of rats. Int. J. Pharma Sci. Res. 2020, 11, 97–101. [Google Scholar]
- Jutabha, P.; Chomdej, B. Effect of Cymbopogon citratus Stapf. on renal functions in dogs. Chula Med. J. 1995, 39, 425–435. [Google Scholar]
- Singi, G.; Damasceno, D.D.; Silva, G.A. Artigo Efeitos agudos dos extratos hidroalcoólicos do alho. Rev. Bras. Farmacogn. 2005, 15, 94–97. [Google Scholar] [CrossRef]
- Tcheutchoua, Y.C.; Bilanda, D.C.; Dzeufiet, P.D.D.; Djunie Neali, O.C.; Owona, P.E.; Bidingha, R.À.G.; Ngapout, R.F.; Mbolang, L.N.; Noubom, M.; Dimo, T.; et al. Preventive Potential of the Aqueous Extract of the Mixture of Bidens pilosa (Asteraceae) and Cymbopogon citratus (Poaceae) Aerial Parts on Hypertension Induced by a Chronic Salt and Alcohol Consumption on the Rats. Evidence-Based Complement. Altern. Med. 2022, 2022, 1980622. [Google Scholar] [CrossRef]
- Moreira, F.V.; Bastos, J.F.A.; Blank, A.F.; Alves, P.B.; Santos, M.R.V. Chemical composition and cardiovascular effects induced by the essential oil of Cymbopogon citratus DC. Stapf, Poaceae, in rats. Rev. Bras. Farmacogn. 2010, 20, 904–909. [Google Scholar] [CrossRef]
- Arome, D.; Enegide, C.; Ameh, S. Pharmacological evaluation of anxiolytic property of aqueous root extract of Cymbopogon citratus in mice. Chron. Young Sci. 2014, 5, 33. [Google Scholar] [CrossRef]
- Ekpenyong, C.E. Lemongrass tea consumption and changes in Acid-Base Balance and Electrolyte homeostasis. Arch. Food Nutr. Sci. 2018, 2, 41–51. [Google Scholar]
- Sobha, R. The Effect of Lemongrass Decoction in Reduction of Blood Pressure Among Individuals With Hypertension in Selected Community Area, Kerala. Ph.D. Thesis, Thanthai Roever College of Nursing, Perambalur, India, 2014. [Google Scholar]
- Patin, R.; Kanlayavattanakul, M.; Lourith, N. Aromatherapy and Essential Oils in Thai Spa Business. Isan J. Pharm. Sci. 2010, 5, 160–166. [Google Scholar]
- Sriraksa, N.; Kaewwongse, M.; Phachonpai, W.; Hawiset, T. Effects of Lemongrass (Cymbopogon citratus) Essential Oil Inhalation on Cognitive Performance and Mood in Healthy Women. Thai Pharm. Health Sci. J. 2018, 13, 80–88. [Google Scholar]
- Shimono, K.; Oka, H.; Suzuki, M.; Senda, K.; Komai, S. Aromatic Antihypertensive Agent, and Method for Lowering Blood Pressure in Mammals. U.S. Patent 2010/0216891 A1, 26 August 2010. [Google Scholar]
- Siahaan, R.; Rahardjo, T.B.; Ranti, A. Effectiveness of Indonesian Essential Oil Mixture of Lemongrass, Cananga, and Patchouli in Relaxation through Inhalation: A Clinical Test on Healthy Woman with High Potential for Stress. Makara J. Health Res. 2014, 18, 143–151. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. An updated review on efficacy and benefits of sweet almond, evening primrose and jojoba oils in skin care applications. Int. J. Cosmet. Sci. 2022, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef]
- Vincent, F.; Acevedo, A.; Nguyen, M.T.; Dourado, M.; DeFalco, J.; Gustafson, A.; Spiro, P.; Emerling, D.E.; Kelly, M.G.; Duncton, M.A.J. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009, 389, 490–494. [Google Scholar] [CrossRef]
- Rhiouani, H.; Settaf, A.; Lyoussi, B.; Cherrah, Y.; Lacaille-Dubois, M.A.; Hassar, M. Effects of saponins from Herniaria glabra on blood pressure and renal function in spontaneously hypertensive rats. Therapie 1999, 54, 735–739. [Google Scholar]
- Jouad, H.; Haloui, M.; Rhiouani, H.; Hilaly, J.E.; Eddouks, M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef]
- Chen, M.; Long, Z.; Wang, Y.; Liu, J.; Pian, H.; Wang, L.; Chen, Z. Protective effects of saponin on a hypertension target organ in spontaneously hypertensive rats. Exp. Ther. Med. 2013, 5, 429–432. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jirovetz, L. Aromatherapy—use of fragrances and essential oils as medicaments. Flavour Fragr. J. 1994, 9, 217–222. [Google Scholar] [CrossRef]
- Xiao, L.; Harrison, D.G. Inflammation in Hypertension. Can. J. Cardiol. 2020, 36, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.L.; Wohlford, G.F., IV; Abbate, A. Inflammation and Hypertension Causal or Not? Can. J. Cardiol. 2020, 36, 635–647. [Google Scholar] [CrossRef]


| Authors | Geographical Origin | Part Used | Compounds (Percentage) |
|---|---|---|---|
| Goes et al. (2015) [46] | Brazil | Undisclosed | Geranial (41.84), neral (31.49), geranyl acetate (9.04), geraniol (6.00), 6-metil-5-hepten-2-one (1.73), (E)-caryophyllene (1.68), canfene (1.37), (E)-isocitral (1.34), γ-cadinene (1.13), linalool (1.00), (Z)-isocitral (0.56), δ-cadinene (0.43), limonene (0.27), (Z)-β-ocimene (0.26), α-pinene (0.24), borneol (0.19) and triciclene (0.18) |
| Zheljazkov et al. (2011) [47] | USA | Dried aerial parts | Geranial (25–53), neral (20–45), caryophyllene oxide (1.3–7.2), t-caryophyllene (0.3–2.2) |
| Chisowa et al. (1998) [48] | Zambia | Dried leaves | Geranial (39.0), neral (29.4), β-myrcene (18.0), geraniol (1.7), linalool (1.3), 1,8-cineole (1.0), 6-methyl-hept-5-en-2-one (0.8), undecan-2-one (0.5), (Z)-β-ocimene (0.4), citronellol (0.3), (E)-β-ocimene (0.3), α-terpineol (0.3), limonene (0.2), tridecan-2-one (0.2), α-pinene (trace), verbenol (trace) |
| Kasali et al. (2001) [49] | Nigeria | Fresh leaves | Geranial (33.7), neral (26.5), β-myrcene (25.3), neomenthol (3.3), linalyl acetate (2.3), (Z)-β-ocimene (1.0), nerol (0.8), (E)-β-ocimene (0.7), linalool (0.6), p-cymene (0.5), β-caryophyllene (0.3), citronellal (0.3), tetrahydrolinalool (0.3), fenchone (0.2), geraniol (0.2), myrcenol (0.2), β-patchoulene (0.2), camphor (0.1), 2,6-dimethyloctane (0.1), β-elemene (0.1), sabinol (0.1), trans-allo-ocimene (0.1) |
| Dutta et al. (2014) [50] | India | Fresh leaves | Neral (42.15), geranial (35.12), β-myrcene (12.39), citronellal (1.56), carveol (0.84), geraniol (0.75), limonene (0.38), caryophyllene (0.35), geranyl acetate (0.26), nerol (0.12) |
| Kamkaen et al. (2015) [51] | Thailand | Undisclosed | Geranial (44.6), neral (33.7), β-myrcene (5.2), selina-6-en-4-ol (1.4), Z-β-ocimene (0.7) |
| Authors | Origin of Lemongrass | Type of Extract | Compounds |
|---|---|---|---|
| Asaolu et al. (2009) [67] | Nigeria | Water and ethanol extracts of powdered leaves | Alkaloids, saponins, tannins, anthraquinones, steroids, phenols and flavonoids |
| Ekpenyong et al. (2016) [69] | Nigeria | Water extract of powdered leaves | High levels of saponins, moderate levels of tannins, flavonoids and phenols and relatively low levels of anthraquinones, alkaloids and deoxy-sugars |
| Soares et. (2013) [70] | Angola | Water, methanol and ethanol extracts of powdered, shade-dried leaves | Aqueous—tannins, flavonoids and terpenoids Ethanolic—tannins, flavonoids and terpenoids Methanolic—tannins, flavonoids, alkaloids, steroids and terpenoids |
| Gazola et al. (2004) [71] | Brazil | Water extract of powdered dried leaves | Alkaloids, tannins and flavonoids |
| Campos et al. (2014) [72] | Chile | Water/methanol extract of air-dried leaves and stems | Chlorogenic acid, isoorientin, swertiajaponin, 6-C-pentosyl-8-C-hexosyl apigenin and luteolin C-rhamnosyl rhamnoside |
| Simões et al. (2020) [73] | Portugal | Water extract and fractions of dried leaves | Caffeic acid derivatives, p-coumaric acid derivatives, luteolin derivatives, apigenin derivatives and proanthocyanidins |
| Coelho et al. (2016) [65] | Portugal | Water extract of the whole dried plant | Phenolics (hydroxycinnamic acids—caffeic, p-coumaric, ferulic, chlorogenic and rosmarinic, flavonoid—quercitrin) |
| Somparn et al. (2018) [68] | Thailand | Water extract of the whole plant | Gallic acid, catechin, tannic acid, rutin, isoquercetin, hydroquinone, eriodictyol, quercetin |
| Authors | Animal Species/Strain | Dose | Main Results |
|---|---|---|---|
| Devi et al. (2012) [87] | Thoracic aorta of SHRs (250–300 g; undisclosed age) | 0.00624-6.24 mM | Attenuation of PE-, CaCl2- and KCl-mediated contraction in intact and endothelium-denuded vessels. |
| Thoracic aorta of WKYRs (250–300 g; undisclosed age) | Failure to attenuate PE-precontracted vessels. | ||
| Pereira et al. (2013) [88] | Thoracic aorta of WKYRs (15–17 w.o., undisclosed weight) | 10−4–6 mM | Attenuation of PE-, CaCl2- and KCl-mediated contraction. |
| Moreira (2013) [89] | Superior mesenteric artery of male Wistar rats (200–300 g, undisclosed age) | 10−5–10−2 mM | Attenuation of PE-, CaCl2, KCl- and sodium orthovanadate-mediated contraction. Vasorelaxation inhibited by endothelium denudation. |
| Male Wistar rats (200–300 g; undisclosed age) conscious or under thiopental anesthesia | 1, 5, 10 and 20 mg/kg (i.v.) | Hypotension and bradycardia in conscious animals. L-NAME attenuated hypotension and atropine abolished bradycardia; hexamethonium and thiopental abolished both responses. |
| Authors | Animal Species/Strain | Lemongrass Product (Concentration) | Main Results |
|---|---|---|---|
| Gazola et al. (2004) [71] | Isolated hearts from healthy male adult rats (undisclosed strain) (N = 7, ≈400 g) | Water extract of leaves (0.077, 0.77, 7.7 and 77 mg/mL) | Significant decrease in heart rate for all doses lasting 5 s; for the 38 mg dose only, the effect lasted for 15 s. |
| Runnie et al. (2004) [98] | Descending thoracic aorta from WKYRs (4 m.o.) | Methanol extract of powdered stalks (2.5–37.0 μg/mL for aorta assay; 100–10,000 μg/mL for mesenteric assay) | Vasorelaxation of NE-precontrated vessels, significantly decreased by incubation with NOLA but not by endothelium denudation. |
| Superior mesenteric artery from WKYRs (4 m.o., undisclosed weight) | Vasorelaxation of PE-precontracted vessels, significantly decreased by incubation with NOLA and indomethacin. | ||
| Abeywardena et al. (2002) [99] | Descending thoracic aorta from WKYRs (4 m.o., undisclosed weight) | Methanol extract of powdered stalks (2.5–5 mg) | Vasorelaxation of PE-precontracted vessels, significantly decreased by incubation with NOLA or by endothelium denudation. |
| Superior mesenteric artery from WKYRs (4 m.o., undisclosed weight) | Vasorelaxation of PE-precontracted vessels, unaffected by incubation with NOLA and indomethacin. | ||
| Devi et al. (2012) [87] | Thoracic aorta of WKYRs (250–300 g, undisclosed age) | Methanol extract of leaves, stems, and roots (1, 3, 10, 30, and 100 mg/mL) | Vasorelaxation of PE-precontracted vessels by all extracts. |
| Thoracic aorta of SHRs (250–300 g, undisclosed age) | Vasorelaxation of PE-precontracted vessels by leaf and root extracts, the former being abolished by L-NAME or indomethacin. Root extract-mediated vasorelaxation was potentiated by indomethacin. | ||
| Martínez et al. (2020) [100] | Aorta of male adult Wistar rats (7–8 w.o.) | 20% tincture of leaves (1, 3, 10, 30 and 100 mg/mL) | Vasorelaxation of endothelium-denuded PE- and KCl-precontracted vessels. |
| Campos et al. (2014) [72] | Human umbilical veins | Water/methanol extract of leaves and stems (10−10–10−6 M) | Inhibition of U46619-mediated vasoconstriction. |
| Simões et al. (2020) [73] | Human internal thoracic arteries | Infusion of leaves and fractions (0.002–0.2 mg/mL) | Infusion causes vasorelaxation, inhibited by indomethacin. Tannin fraction elicits the larger vasodilation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.; Bárbara, R. Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review. Biology 2022, 11, 1382. https://doi.org/10.3390/biology11101382
Silva H, Bárbara R. Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review. Biology. 2022; 11(10):1382. https://doi.org/10.3390/biology11101382
Chicago/Turabian StyleSilva, Henrique, and Rita Bárbara. 2022. "Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review" Biology 11, no. 10: 1382. https://doi.org/10.3390/biology11101382
APA StyleSilva, H., & Bárbara, R. (2022). Exploring the Anti-Hypertensive Potential of Lemongrass—A Comprehensive Review. Biology, 11(10), 1382. https://doi.org/10.3390/biology11101382


_Kwok.png)



