Exogenous Application of Methyl Jasmonate Increases Emissions of Volatile Organic Compounds in Pyrenean Oak Trees, Quercus pyrenaica
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area and Species
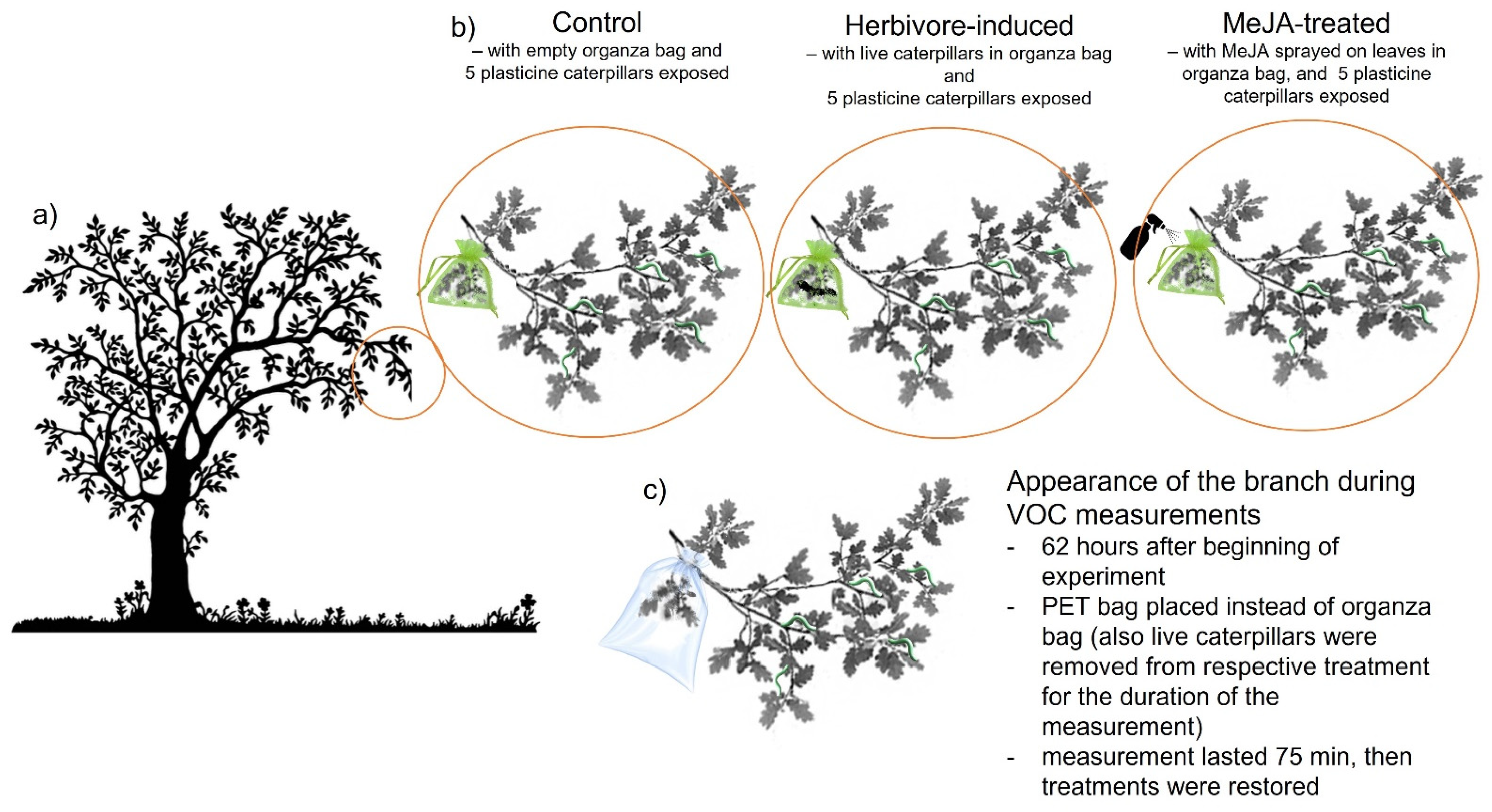
2.2. Experimental Design
2.3. Collection of Plant Volatiles
2.4. Analysis of Plant Volatiles
- (1)
- Cannabis terpene Mix B (CRM40937 Supleco, 2000 μg/mL of each component): Limonene (cyclohexene, 1-methyl-4-(1-methylethenyl)-), C10H16, CAS 138-86-3; β-pinene (bicyclo[3.1.1]heptane-6,6- trimethyl, 2-methylene), C10H16, CAS 127-91-3; β-Caryophyllene (trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene), C15H24, CAS 87-44-5; Phytol (3,7,11,15-Tetramethyl-2-hexadecen-1-ol), C20H39O, CAS 7541-49-3; Geraniol (trans-3,7-Dimethyl-2,6-octadien-1-ol), C10H18O, CAS 106-24-1; (1S)-(-)-Camphor ((1S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one), C10H16O, CAS 464-48-2; Terpinolene (p-Menth-1,4(8)-diene), C10H16, CAS 586-62-9; β-Eudesmol ((2R,4aR,8aS)-Decahydro-8-methylene-α,α,4a-trimethyl-2-naphthylmethanol), CAS 473-15-4; (+)-Borneol (endo-(1R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol), C10H18O, CAS 464-43-7; cis-Nerolidol (3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol), C15H26O, CAS 7212-44-4; α-Terpineol (2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol), C10H18O, CAS 98-55-5; (1S)-(+)-3-Carene ((1S)-3,7,7-Trimethylbicyclo[4.1.0]hept-3-ene), C10H16, CAS 498-15-7; Linalool ((±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene), C10H18O, CAS 76-70-6; p-Cymene (1-Isopropyl-4-methylbenzene), C10H14, CAS 99-87-6.
- (2)
- Cannabis terpene Mix A (CRM40755 Supleco, 2000 μg/mL of each component): α-Pinene (2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene), C10H16, CAS 80-56-8; Camphene (3-methylidenebicyclo[2.2.1]heptane), C10H16, CAS 79-92-5; β-Myrcene (7-Methyl-3-methylideneocta-1,6-diene), C10H16, CAS 12-35-3; 3-Carene (3,7,7-Trimethylbicyclo[4.1.0]hept-3-ene), C10H16, CAS 13466-78-9; D-Limonene (1-Methyl-4-(prop-1-en-2-yl)cyclohex-1-ene), C10H16, CAS 5989-27-5.
- (3)
- Single chemicals (Sigma-Aldrich): Caryophyllene oxide, CAS 1139-30-6; Ocimene (3,7-Dimethyl-1,3,6-octatrien), C10H16, CAS 13877-91-3; cis-3-hexenyl Acetate, C8H14O2, CAS 3681-71-8; Methyl Jasmonate (Methyl 3-oxo-2-(2-pentenyl) cyclopentaneacetate), C13H20O3, CAS 39924-52-2. Emissions were presented qualitatively.
2.5. Statistical Analyses
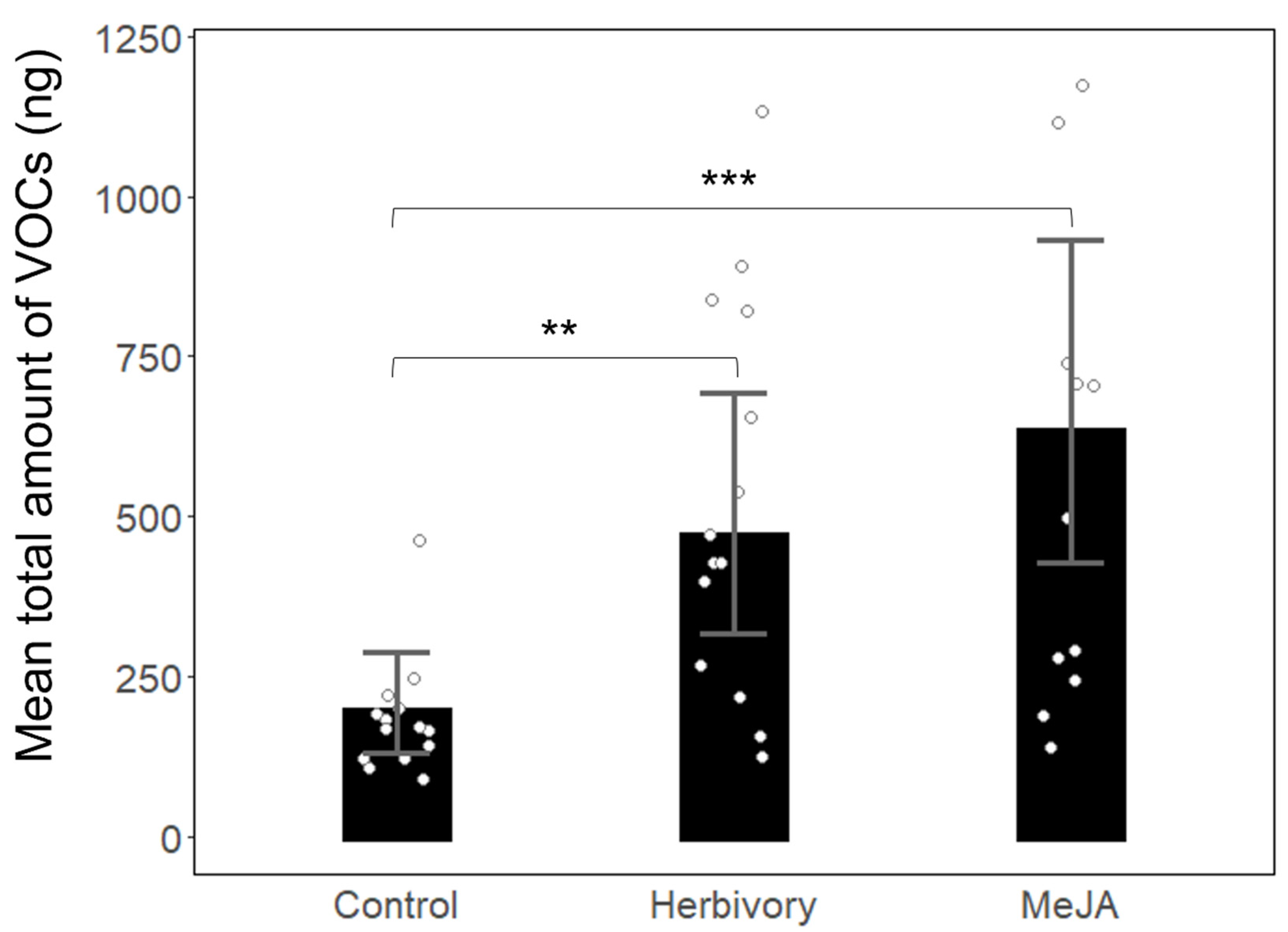
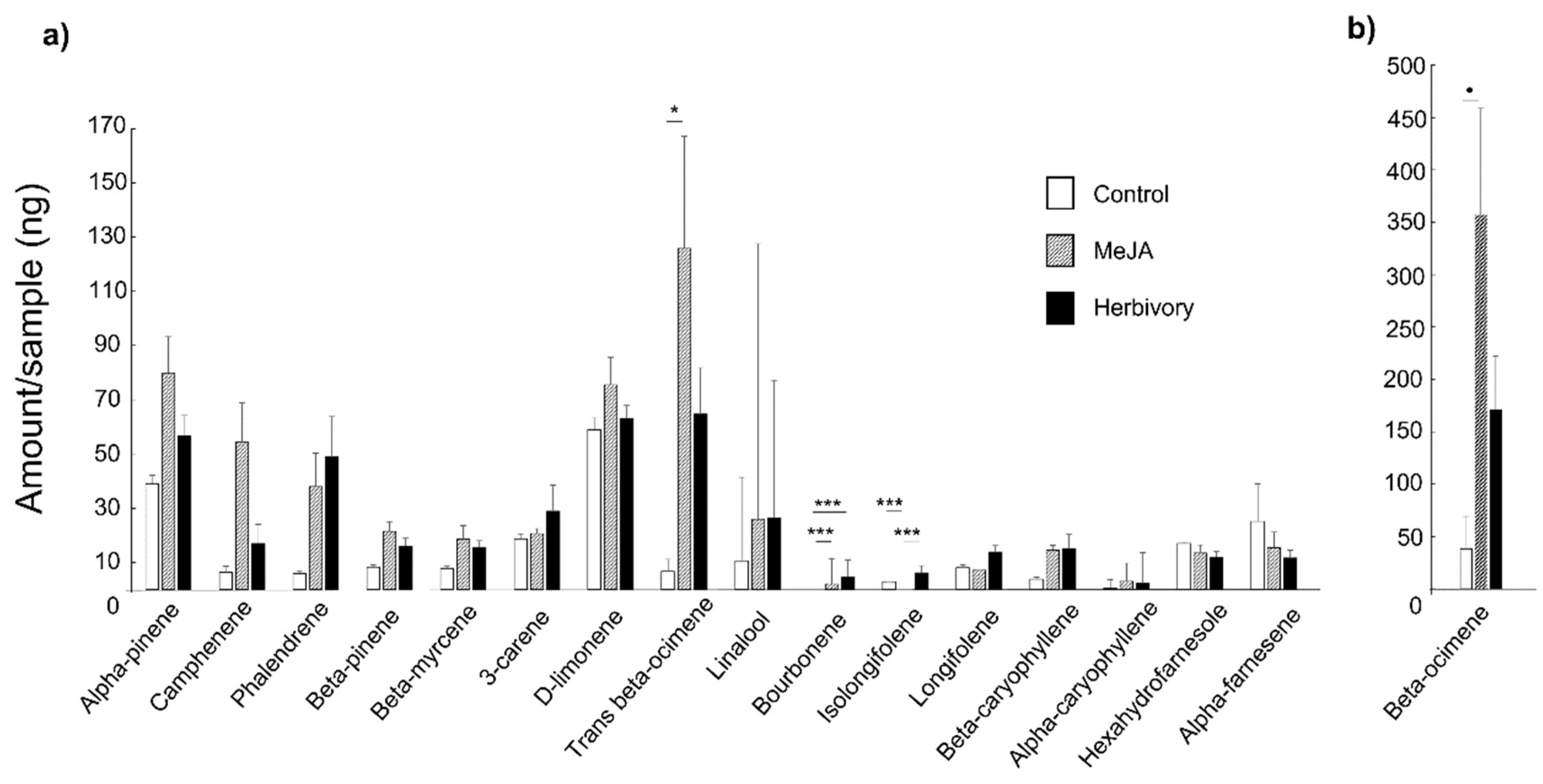
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heil, M. Herbivore-induced plant volatiles: Targets, perception and unanswered questions. New Phytol. 2014, 204, 297–306. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Xue, J.; Peng, G.; Wang, X. Effect of volatile emissions, especially alpha-pinene, from persimmon trees infested by Japanese wax scales or treated with methyl jasmonate on recruitment of ladybeetle predators. Environ. Entomol. 2009, 38, 1439–1445. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Loreto, F.; Reichstein, M. Physiological and physiochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Bonaventure, G.; VanDoorn, A.; Baldwin, I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011, 16, 294–299. [Google Scholar] [CrossRef]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Farag, M.A.; Paré, P.W.; Dicke, M. Jasmonate-deficient plants have reduced direct and indirect defenses against herbivores. Ecol. Lett. 2002, 5, 764–774. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Induced synthesis of plant volatiles. Nature 1997, 385, 30–31. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Phsyiol. 1999, 121, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Mattiacci, L.; Rocca, B.A.; Scascighini, N.; D’Alessandro, M.; Hern, A.; Dorn, S. Systemically induced plant volatiles emitted at the time of “danger”. J. Chem. Ecol. 2001, 27, 2233–2252. [Google Scholar] [CrossRef]
- Volf, M.; Weinhold, A.; Seifert, C.L.; Holicová, T.; Uthe, H.; Alander, E.; Richter, R.; Salminen, J.-P.; Wirth, C.; Van Dam, N.M. Branch-Localized Induction Promotes Efficacy of Volatile Defences and Herbivore Predation in Trees. J. Chem. Ecol. 2020, 47, 99–111. [Google Scholar] [CrossRef]
- Dicke, M.; Sabelis, M.W.; Takabayashi, J.; Bruin, J.; Posthumus, M.A. Plant strategies of manipulating predator-prey interactions through allelochemicals: Prospects for application in pest control. J. Chem. Ecol. 1990, 16, 3091–3118. [Google Scholar] [CrossRef] [Green Version]
- Turlings, T.C.; Humlington, J.H.; Lewis, W.J. Exploitation of herbivore induced plant odors by host-seeking parasite wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef] [Green Version]
- Turlings, T.C.J.; Tumlinson, J.H. Systemic release of chemical signals by herbivore-injured corn. Proc. Natl. Acad. Sci. USA 1992, 89, 8399–8402. [Google Scholar] [CrossRef] [Green Version]
- Vet, L.E.M.; Dicke, M. Ecology of info chemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- van Loon, J.J.A.; de Boer, J.G.; Dicke, M. Parasitoid-plant mutualism: Parasitoid attack of herbivore increases plant reproduction. Entomol. Exp. Appl. 2000, 97, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Fritzsche Hoballah, M.E.; Turlings, T.C.J. Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol. Ecol. Res. 2001, 3, 553–565. [Google Scholar]
- Schuman, M.C.; Barthel, K.; Baldwin, I.T. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. ELife 2012, 1, e00007. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Mäntylä, E.; Klemola, T.; Haukioja, E. Attraction of willow warblers to sawfly-damaged mountain birches: Novel function of inducible plant defences? Ecol. Lett. 2004, 7, 915–918. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Mäntylä, E.; Klemola, T.; Sirkiä, P.; Laaksonen, T. Low light reflectance may explain the attraction of birds to defoliated trees. Behav. Ecol. 2008, 19, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Mäntylä, E.; Alessio, G.A.; Blande, J.D.; Heijari, J.; Holopainen, J.K.; Laaksonen, T.; Piirtola, P.; Klemola, T. From plants to birds: Higher avian predation rates in trees responding to insect herbivory. PLoS ONE 2008, 3, e2832. [Google Scholar] [CrossRef]
- Amo, L.; Jansen, J.J.; Dam, N.M.; Dicke, M.; Visser, M.E. Birds exploit herbivore–induced plant volatiles to locate herbivorous prey. Ecol. Lett. 2013, 16, 1348–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäntylä, E.; Kleier, S.; Kipper, S.; Hilker, M. The attraction of insectivorous tit species to herbivore-damaged Scots pines. J. Ornithol. 2017, 158, 479–491. [Google Scholar] [CrossRef]
- Mäntylä, E.; Kipper, S.; Hilker, M. Insectivorous birds can see and smell systemically herbivore-induced pines. Ecol. Evol. 2020, 10, 9358–9370. [Google Scholar] [CrossRef] [PubMed]
- Rubene, D.; Leidefors, M.; Ninkovic, V.; Eggers, S.; Low, M. Disentangling olfactory and visual information used by field foraging birds. Ecol. Evol. 2019, 9, 545–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.; Charlier, T.D.; Bonadonna, F.; Caro, S. Olfactory detection of trace amounts of plant volatiles is correlated with testosterone in a passerine bird. Horm. Behav. 2021, 136, 105045. [Google Scholar] [CrossRef]
- Mäntylä, E.; Kleier, S.; Lindstedt, C.; Kipper, S.; Hilker, M. Insectivorous birds are attracted by plant traits induced by insect egg deposition. J. Chem. Ecol. 2018, 44, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Mrazova, A.; Sam, K. Application of methyl jasmonate to grey willow (Salix cinerea) attracts insectivorous birds in nature. Arthropod Plant Interact. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Mrazova, A.; Sam, K.; Amo, L. What do we know about birds’ use of plant volatile cues in tritrophic interactions? Curr. Opin. Insect Sci. 2019, 32, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hopke, J.; Donath, J.; Blechert, S.; Boland, W. Herbivore-induced volatiles: The emission of acyclic homoterpenes from leaves of Phaseolus lunatus and Zea mays can be triggered by a betaglucosidase and jasmonic acid. FEBS Lett. 1994, 352, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Degenhardt, D.C.; Lincoln, D.E. Volatile emissions from an odorous plant in response to herbivory and methyl jasmonate exposure. J. Chem. Ecol. 2006, 32, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Mäntylä, E.; Blande, J.D.; Klemola, T. Does application of methyl jasmonate to birch mimic herbivory and attract insectivorous birds in nature? Arthropod Plant Interact. 2014, 8, 143–153. [Google Scholar] [CrossRef]
- Saavedra, I.; Amo, L. Are birds attracted to methyl-jasmonate-treated trees? Behaviour 2018, 155, 945–967. [Google Scholar] [CrossRef]
- Mrazova, A.; Sam, K. Exogenous application of methyl jasmonate to Ficus hahliana attracts predators of insects along an altitudinal gradient in Papua New Guinea. J. Trop. Ecol. 2019, 35, 157–164. [Google Scholar] [CrossRef]
- Betts, M.M. The food of Titmice in Oak Woodland. J. Anim. Ecol. 1955, 24, 282–323. [Google Scholar] [CrossRef]
- Mols, C.M.M.; Visser, M.E. Great tits can reduce caterpillar damage in apple orchards. J. Appl. Ecol. 2002, 39, 888–899. [Google Scholar] [CrossRef]
- Posa, M.R.C.; Sodhi, N.S.; Koh, L.P. Predation on artificial nests and caterpillar models across a disturbance gradient in Subic Bay, Philippines. J. Trop. Ecol. 2007, 23, 27–33. [Google Scholar] [CrossRef]
- Richards, L.A.; Coley, P.D. Seasonal and habitat differences affect the impact of food and predation on herbivores: A comparison between gaps and understory of a tropical forest. Oikos 2007, 116, 31–40. [Google Scholar] [CrossRef]
- Remmel, T.; Davison, J.; Tammaru, T. Quantifying predation on folivorous insect larvae: The perspective of life-history evolution. Biol. J. Linnean. Soc. 2011, 104, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Tvardikova, K.; Novotny, V. Predation on exposed and leaf-rolling artificial caterpillars in tropical forests of Papua New Guinea. J. Trop. Ecol. 2012, 28, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Sam, K.; Koane, B.; Novotny, V. Herbivore damage increases avian and ant predation of caterpillars on trees along a complete elevational forest gradient in Papua New Guinea. Ecography 2015, 38, 293–300. [Google Scholar] [CrossRef]
- Saavedra, I.; Amo, L. Insectivorous birds eavesdrop on the pheromones of their prey. PLoS ONE 2018, 13, e0190415. [Google Scholar] [CrossRef] [Green Version]
- Amo, L.; Saavedra, I. Attraction to smelly food in birds: Insectivorous birds discriminate between the pheromones of their prey and those of non-prey insects. Biology 2021, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 December 2021).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Length, R.V.; Buerkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. emmeans: Estimated Marginal Means, aka Least-Squares Means. 2020. R package Version 1.4.8. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 December 2021).
- Sam, K.; Kovarova, E.; Freiberga, I.; Uthe, H.; Weinhold, A.; Jorge, R.G.; Sreekar, R. Great tits (Parus major) flexibly learn that herbivore-induced plant volatiles indicate prey location: An experimental evidence with two tree species. Ecol. Evol. 2021, 11, 10917–10925. [Google Scholar] [CrossRef]
- Koski, T.M.; Laaksonen, T.; Mäntylä, E.; Ruuskanen, S.; Li, T.; Girón-Calva, P.S.; Huttunen, L.; Blande, J.D.; Holopainen, J.K.; Klemola, T. Do Insectivorous Birds use Volatile Organic Compounds from Plants as Olfactory Foraging Cues? Three Experimental Tests. Ethology 2015, 121, 1131–1144. [Google Scholar] [CrossRef]
- Bonello, P.; Blodgett, J.T. Pinus nigra–Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol. Mol. Plant Pathol. 2003, 63, 249–261. [Google Scholar] [CrossRef]
- Eyles, A.; Bonello, P.; Ganley, R.; Mohammed, C. Induced resistance to pests and pathogens in trees. New Phytol. 2010, 185, 893–908. [Google Scholar] [CrossRef]
- Neuvonen, S.; Haukioja, E.; Molarius, A. Delayed inducible resistance against a leaf-chewing insect in four deciduous tree species. Oecologia 1987, 74, 363–369. [Google Scholar] [CrossRef]
- Piggott, N.; Ekramoddoullah, A.K.; Liu, J.-J.; Yu, X. Gene cloning of a thaumatin-like (PR-5) protein of western white pine (Pinus monticola D. Don) and expression studies of members of the PR-5 group. Physiol. Mol. Plant Pathol. 2004, 64, 1–8. [Google Scholar] [CrossRef]
- Rubert-Nason, K.F.; Couture, J.J.; Major, I.T.; Constabel, C.P.; Lindroth, R.L. Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J. Chem. Ecol. 2015, 41, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Lämke, J.S.; Unsicker, S.B. Phytochemical variation in treetops: Causes and consequences for tree-insect herbivore interactions. Oecologia 2018, 187, 377–388. [Google Scholar] [CrossRef]
- Dicke, M.; Gols, R.; Ludeking, D.; Posthumus, M.A. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J. Chem. Ecol. 1999, 25, 1907–1922. [Google Scholar] [CrossRef]
- Hare, J.D. Variation in herbivore and methyl jasmonate-induced volatiles among genetic lines of Datura wrightii. J. Chem. Ecol. 2007, 33, 2028–2043. [Google Scholar] [CrossRef]
- Strapasson, P.; Pinto-Zevallos, D.M.; Paudel, S.; Rajotte, E.G.; Felton, G.W.; Zarbin, P.H. Enhancing plant resistance at the seed stage: Low concentrations of methyl jasmonate reduce the performance of the leaf miner Tuta absoluta but do not alter the behavior of its predator Chrysoperla externa. J. Chem. Ecol. 2014, 40, 1090–1098. [Google Scholar] [CrossRef]
- Dicke, M. Specificity of herbivore-induced plant defences. In Insect-Plant Interactions and Induced Plant Defence; Chadwick, D.J., Goode, J., Eds.; Wiley & Sons: Chicester, UK, 1999; pp. 43–59. [Google Scholar]
- Ozawa, R.; Arimura, G.; Takabayashi, J.; Shimoda, T.; Nishioka, T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000, 41, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaler, J.S. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 1999, 399, 686–688. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Paré, P.W.; Henneberry, T.J. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 2001, 27, 679–695. [Google Scholar] [CrossRef]
- Smart, L.E.; Martin, J.L.; Limpalaër, M.; Bruce, T.J.; Pickett, J.A. Responses of herbivore and predatory mites to tomato plants exposed to jasmonic acid seed treatment. J. Chem. Ecol. 2013, 39, 1297–1300. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular Interactions Between Plants and Insect Herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Madilao, L.L.; Ralph, S.; Bohlmann, J. Insect-induced conifer defense. White pine weevil and Methyl Jasmonate induce traumatic resinosis, de novo formed volatile emissions, and accumulation of terpenoid synthase and putative octadecanoid pathway transcripts in Sitka spruce. Plant Physiol. 2005, 137, 369–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Lewis, W.J.; Pare, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Takabayashi, J.; Dicke, M.; Posthumus, M.A. Variation in composition of predator attracting allelochemicals emitted by herbivore-infested plants: Relative influence of plant and herbivore. Chemoecology 1991, 2, 1–6. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Schultz, J.C. Differential activity of peroxidase isozymes in response to wounding, gypsy moth, and plant hormones in northern red oak (Quercus rubra L.). J. Chem. Ecol. 2004, 30, 1363–1379. [Google Scholar] [CrossRef]
- Turlings, T.C.F.; Wäckers, F. Recruitment of predators and parasitoids by herbivore-injured plants. Adv. Insect Chem. Ecol. 2004, 2, 21–75. [Google Scholar]
- Geervliet, J.B.F.; Ariens, S.J.; Dicke, M.; Vet, L.E.M. Long-distance assessment of patch profitability through volatile infochemicals by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae). Biol. Control 1998, 11, 113–121. [Google Scholar] [CrossRef]
- Shiojiri, K.; Takabayashi, J.; Yano, S.; Takafuji, A. Infochemically mediated tritrophic interaction webs on cabbage plants. Popul. Ecol. 2001, 43, 23–29. [Google Scholar] [CrossRef]
- Girling, R.D.; Stewart-Jones, A.; Dherbecourt, J.; Staley, J.T.; Wright, D.J.; Poppy, G.M. Parasitoids select plants more heavily infested with their caterpillar hosts: A new approach to aid interpretation of plant headspace volatiles. Proc. Biol. Sci. 2011, 278, 2646–2653. [Google Scholar] [CrossRef] [Green Version]
- Scascighini, N.; Mattiacci, L.; D’Alessandro, M.; Hern, A.; Rott, A.S.; Dorn, S. New insights in analysing parasitoid attracting synomones: Early volatile emission and use of stir bar sorptive extraction. Chemoecology 2005, 15, 97–104. [Google Scholar] [CrossRef]
- Kigathi, R.N.; Unsicker, S.B.; Reichelt, M.; Kesselmeier, J.; Gershenzon, J.; Weisser, W.W. Emission of volatile organic compounds after herbivory from Trifolium pratense (L.) under laboratory and field conditions. J. Chem. Ecol. 2009, 35, 1335–1348. [Google Scholar] [CrossRef] [Green Version]
- Shiojiri, K.; Ozawa, R.; Kugimiya, S.; Uefune, M.; van Wijk, M.; Sabelis, M.W.; Takabayashi, J. Herbivore-specific, density-dependent induction of plant volatiles: Honesty or “cry wolf” signals? PLoS ONE 2010, 5, e12161. [Google Scholar] [CrossRef]
- De Boer, J.G.; Posthumus, M.A.; Dicke, M. Identification of volatiles that are used in discrimination between plants infested with prey or non prey herbivores by a predatory mite. J. Chem. Ecol. 2004, 30, 2215–2230. [Google Scholar] [CrossRef] [PubMed]
- Van Den Boom, C.E.M.; Van Beek, T.A.; Posthumus, M.A.; De Groot, E.; Dicke, M. Qualitative and quantitative variation between volatile profiles induced by Tetranychus urticae feeding on different plants of various families. J. Chem. Ecol. 2004, 30, 69–89. [Google Scholar] [CrossRef]
- Bruinsma, M.; Posthumus, M.A.; Mumm, R.; Mueller, M.J.; van Loon, J.J.A.; Dicke, M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: Effects of time and dose, and comparison with induction by herbivores. J. Exp. Bot. 2009, 60, 2575–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gols, R.; Veenemans, C.; Potting, R.P.J.; Smid, H.M.; Dicke, M.; Harvey, J.A.; Bukovinszky, T. Variation in the specificity of plant volatiles and their use by a specialist and a generalist parasitoid. Anim. Behav. 2012, 83, 1231–1242. [Google Scholar] [CrossRef]
- Steidle, J.; van Loon, J. Dietary specialization and infochemical use in carnivorous arthropods: Testing a concept. Entomol. Exp. Appl. 2003, 108, 133–148. [Google Scholar] [CrossRef]
- Amo, L.; Dicke, M.; Visser, M.E. Are naïve birds attracted to herbivore-induced plant defences? Behaviour 2016, 153, 353–366. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amo, L.; Mrazova, A.; Saavedra, I.; Sam, K. Exogenous Application of Methyl Jasmonate Increases Emissions of Volatile Organic Compounds in Pyrenean Oak Trees, Quercus pyrenaica. Biology 2022, 11, 84. https://doi.org/10.3390/biology11010084
Amo L, Mrazova A, Saavedra I, Sam K. Exogenous Application of Methyl Jasmonate Increases Emissions of Volatile Organic Compounds in Pyrenean Oak Trees, Quercus pyrenaica. Biology. 2022; 11(1):84. https://doi.org/10.3390/biology11010084
Chicago/Turabian StyleAmo, Luisa, Anna Mrazova, Irene Saavedra, and Katerina Sam. 2022. "Exogenous Application of Methyl Jasmonate Increases Emissions of Volatile Organic Compounds in Pyrenean Oak Trees, Quercus pyrenaica" Biology 11, no. 1: 84. https://doi.org/10.3390/biology11010084
APA StyleAmo, L., Mrazova, A., Saavedra, I., & Sam, K. (2022). Exogenous Application of Methyl Jasmonate Increases Emissions of Volatile Organic Compounds in Pyrenean Oak Trees, Quercus pyrenaica. Biology, 11(1), 84. https://doi.org/10.3390/biology11010084





