Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming
Abstract
Simple Summary
Abstract
1. Introduction
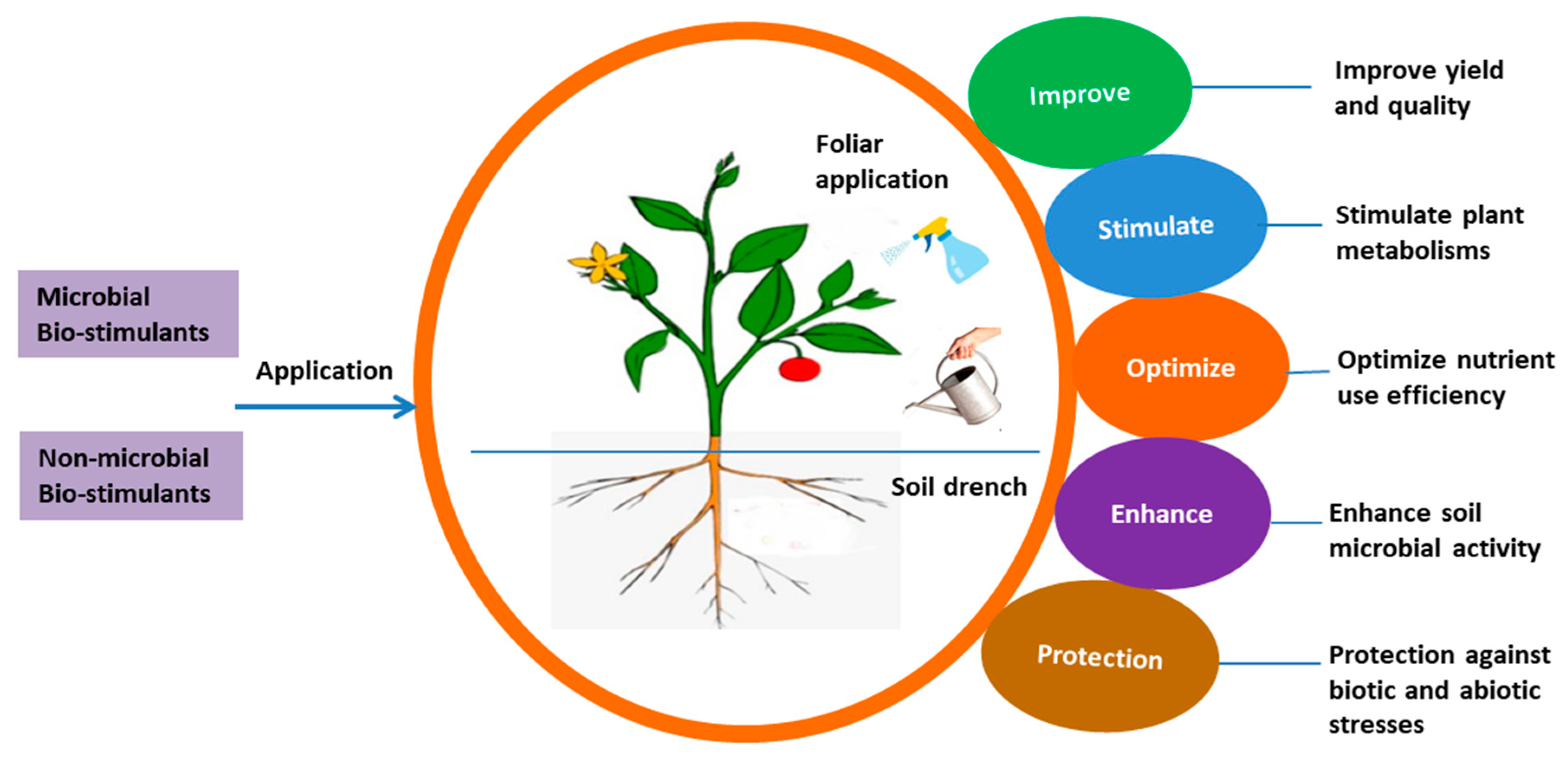
2. Microbial and Non-Microbial Biostimulants: Action/Mechanisms and Biostimulatory Effects on Plants
2.1. Non-Microbial Plant Biostimulants
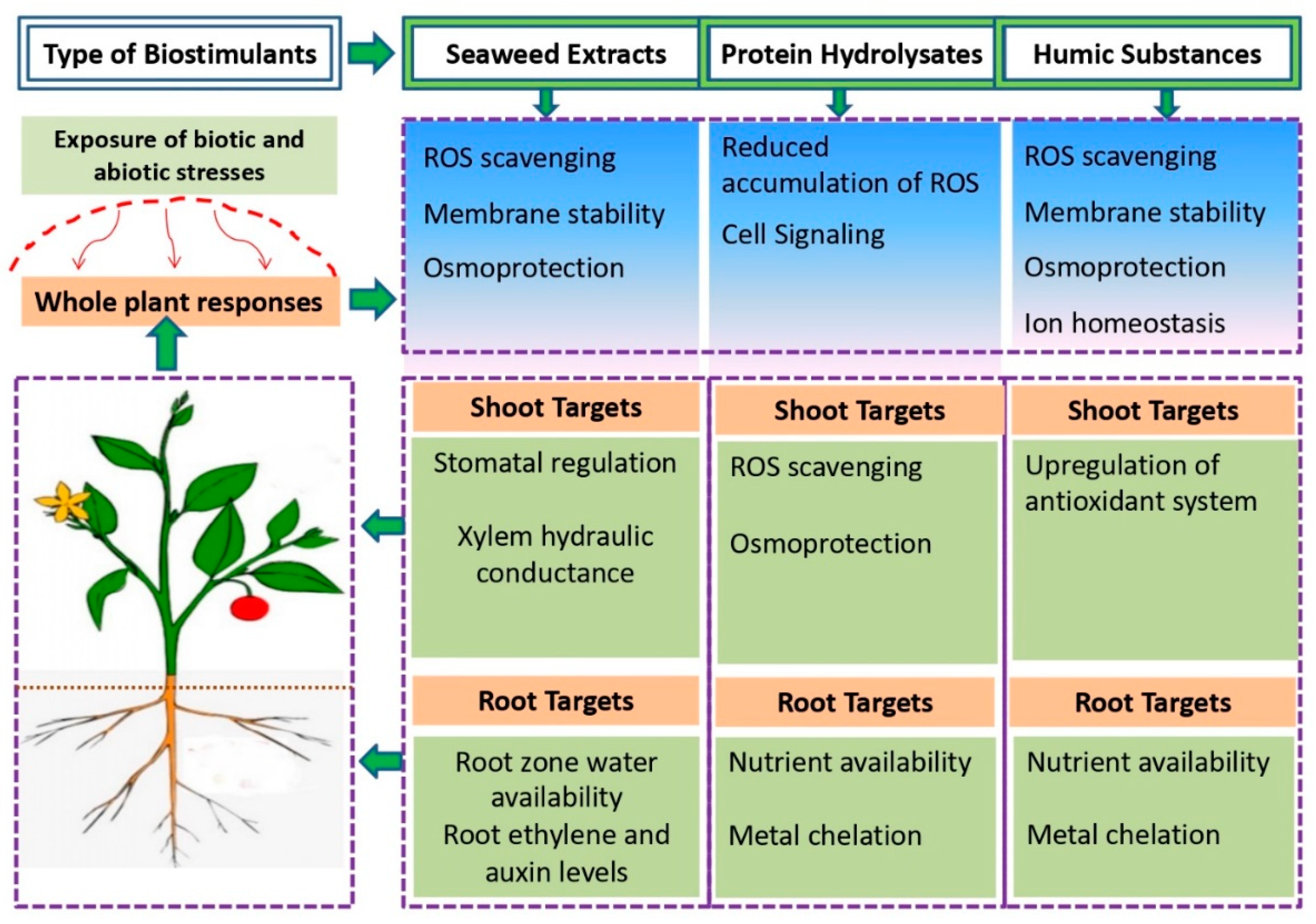
2.1.1. Humic Substances
2.1.2. Protein Hydrolysates
2.1.3. Seaweed Extracts
2.1.4. Bioconversion Compost-Derived Biostimulants
2.2. Microbial Plant Biostimulants
2.2.1. Fungal-Based Microbial Biostimulants
2.2.2. Bacterial-Based Microbial Biostimulants
3. Implications of Biostimulants for Enhancing Plant Nutrition in Organic Farming
3.1. Soil Nutrient Availability
3.2. Plant Nutrient Uptake
3.3. Plant Nutrient Assimilation
4. Implications of Biostimulants for Enhancing Crop Physiology, Productivity, and Quality
5. Implications of Biostimulants in Alleviating Stress in Crop Plants
6. Exploiting Synergistic Biostimulatory Interactions among Biostimulants
7. Ecological Considerations for Harnessing the Beneficial Functions of Biostimulants: Moving from Lab towards Successful Field Application
8. Concluding Remarks and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrlich, P.R.; Harte, J. Opinion: To feed the world in 2050 will require a global revolution. Proc. Natl. Acad. Sci. USA 2015, 112, 14743–14744. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Security 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality–A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2020, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Seneviratne, G.; Kulasooriya, S.A. Reinstating soil microbial diversity in agroecosystems: The need of the hour for sustainability and health. Agric. Ecosyst. Environ. 2013, 164, 181–182. [Google Scholar] [CrossRef]
- Huang, R.; McGrath, S.P.; Hirsch, P.R.; Clark, I.M.; Storkey, J.; Wu, L.; Zhou, J.; Liang, Y. Plant–microbe networks in soil are weakened by century-long use of inorganic fertilizers. Microb. Biotechnol. 2019, 12, 1464–1475. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]
- Dorais, M.; Alsanius, B.W. Recent advances in organic horticulture technology and management. Sci. Hortic. 2016, 208, 1–2. [Google Scholar] [CrossRef]
- Parvin, S.; Sani, M.N.H.; Modak, S.; Shahriar, S.A.; Uddain, J. Efficacy of different organic manures on growth and yield performance of organically grown tomato. Asian J. Adv. Agric. Res. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Dorais, M. Organic production of vegetables: State of the art and challenges. Can. J. Plant Sci. 2007, 87, 1055–1066. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, T.; Rijk, B.; Van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Ponisio, L.C.; M’Gonigle, L.K.; Mace, K.C.; Palomino, J.; de Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B 2015, 282, 20141396. [Google Scholar] [CrossRef]
- Trewavas, A. Urban myths of organic farming. Nature 2001, 410, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2006, 18, 248–255. [Google Scholar] [CrossRef]
- Van Bueren, E.L.; Jones, S.S.; Tamm, L.; Murphy, K.M.; Myers, J.R.; Leifert, C.; Messmer, M.M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS-Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Orsini, F.; Maggio, A.; Rouphael, Y.; De Pascale, S. “Physiological quality” of organically grown vegetables. Sci. Hortic. 2016, 208, 131–139. [Google Scholar] [CrossRef]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Zetterberg, C.; Björnberg, K.E. Time for a new EU regulatory framework for GM crops? J. Agric Env. Ethics 2017, 30, 325–347. [Google Scholar] [CrossRef]
- Barbieri, G.; Colonna, E.; Rouphael, Y.; De Pascale, S. Effect of the farming system and postharvest frozen storage on quality attributes of two strawberry cultivars. Fruits 2015, 70, 351–360. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Abbott, L.K.; Macdonald, L.M.; Wong, M.T.F.; Webb, M.J.; Jenkins, S.N.; Farrell, M. Potential roles of biological amendments for profitable grain production-a review. Agric. Ecosyst. Environ. 2018, 256, 34–50. [Google Scholar] [CrossRef]
- Wong, W.S.; Zhong, H.T.; Cross, A.T.; Yong, J.W.H. Plant biostimulants in vermicomposts: Characteristics and plausible mechanisms. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 155–180. [Google Scholar]
- de Vries, F.T.; Griffiths, R.I.; Knight, G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 39, 1620–1630. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant biostimulant regulatory framework: Prospects in Europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef] [PubMed]
- Critchley, A.T.; Critchley, J.S.; Norrie, J.; Gupta, S.; Van Staden, J. Perspectives on the global biostimulant market: Applications, volumes, and values, 2016 data and projections to 2022. In Biostimulants for Crops from Seed Germination to Plant Development; Gupta, S., van Staden, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 289–296. [Google Scholar]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R.; et al. Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Rajput, R.S.; Ram, R.M.; Vaishnav, A.; Singh, H.B. Microbe-based novel biostimulants for sustainable crop production. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Satyanarayana, T., Johri, B.N., Das, S.K., Eds.; Springer: Singapore, 2019; pp. 109–144. [Google Scholar]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Aguiar, N.O.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garcia-Mina, J.M. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root-and shoot-growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- García, A.C.; Santos, L.A.; de Souza, L.G.A.; Tavares, O.C.H.; Zonta, E.; Gomes, E.T.M.; Berbara, R.L.L. Vermicompost humic acids modulate the accumulation and metabolism of ROS in rice plants. J. Plant Physiol. 2016, 192, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Olivares, F.L.; Aguiar, N.O.; Rosa, R.C.C.; Canellas, L.P. Substrate biofortification in combination with foliar sprays of plant growth promoting bacteria and humic substances boosts production of organic tomatoes. Sci. Hortic. 2015, 183, 100–108. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Kant, C.; Turan, M. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological responses to Megafol® treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish by-product use as biostimulants: An overview of the current state of the art, including relevant legislation and regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Scientia. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Seaweed carbohydrates. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 57–96. [Google Scholar]
- Stirk, W.A.; Rengasamy, K.R.R.; Kulkarni, M.G.; van Staden, J. Plant biostimulants from seaweeds. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 33–56. [Google Scholar]
- MacKinnon, S.L.; Hiltz, D.; Ugarte, R.; Craft, C.A. Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J. Appl. Phycol. 2010, 22, 489–494. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in Arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Kuwada, K.; Wamocho, L.S.; Utamura, M.; Matsushita, I.; Ishii, T. Effect of red and green algal extracts on hyphal growth of arbuscular mycorrhizal fungi, and on mycorrhizal development and growth of papaya and passion fruit. Agron. J. 2006, 98, 1340–1344. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, X.; Sun, Y.; Zhao, J.; Awasthi, M.K.; Liu, T.; Zhang, Z. Improvement of the composition and humification of different animal manures by black soldier fly bioconversion. J. Clean. Prod. 2021, 278, 123397. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Solarte, Z. Vermiculture in greenhouse plants, field crop production, and hydroponics. In The Oxford Encyclopaedia of Agriculture and the Environment; Hazlet, R.W., Ed.; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motyka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: A phytohormonal approach. Plant Soil 2020, 446, 145–162. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, S.N.; Teo, C.H.; Yew, Y.R.; Ge, L.; Chen, X.; Yong, J.W.H. Analysis of phytohormones in vermicompost using a novel combinative sample preparation strategy of ultrasound-assisted extraction and solid-phase extraction coupled with liquid chromatography–tandem mass spectrometry. Talanta 2015, 139, 189–197. [Google Scholar] [CrossRef]
- Zhang, K.; Letham, D.S.; John, P.C.L. Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta 1996, 200, 2–12. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, I.B.; Kieber, J.J. Molecular mechanisms of cytokinin action. Curr. Opin. Plant Biol. 1999, 2, 359–364. [Google Scholar] [CrossRef]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Yong, J.W.H. The importance of phytohormones and microbes in biofertilizers: A critical review. In Bacterial Metabolites in Sustainable Agroecosystem; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 105–158. [Google Scholar]
- Zhang, H.; Tan, S.N.; Wong, W.S.; Ng, C.Y.L.; Teo, C.H.; Ge, L.; Yong, J.W.H. Mass spectrometric evidence for the occurrence of plant growth promoting cytokinins in vermicompost tea. Biol. Fertil. Soils 2014, 50, 401–403. [Google Scholar] [CrossRef]
- Ge, L.; Yong, J.W.H.; Tan, S.N.; Yang, X.H.; Ong, E.S. Analysis of positional isomers of hydroxylated aromatic cytokinins by micellar electrokinetic chromatography. Electrophoresis 2005, 26, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yong, J.W.H.; Tan, S.N.; Hua, L.; Ong, E.S. Analyses of gibberellins in coconut water by partial filling-micellar electrokinetic chromatography-mass spectrometry with reversal of electroosmotic flow. Electrophoresis 2008, 29, 2126–2134. [Google Scholar] [CrossRef] [PubMed]
- Darmawan, R.; Dewi, V.G.P.; Rizaldi, M.A.; Juliastuti, S.R.; Gunawan, S.; Aparamarta, H.W.; Wiguno, A. Production of liquid bio-fertilizer from old coconut water and molasses using consortium microbes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012007. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Aremu, A.O.; Stirk, W.A.; Kulkarni, M.G.; Tarkowská, D.; Turečková, V.; Gruz, J.; Van Staden, J. Evidence of phytohormones and phenolic acids variability in garden-waste-derived vermicompost leachate, a well-known plant growth stimulant. Plant Growth Regul. 2015, 75, 483–492. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, X.; Luo, L.; Ni, Y.; Yao, L.; Ni, W. Biostimulants in bioconversion compost of organic waste: A novel booster in sustainable agriculture. J. Clean. Prod. 2021, 319, 128704. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphung, T.; Takabe, T.; Cha-um, S. Exogenous foliar application of glycine betaine to alleviate water deficit tolerance in two Indica rice genotypes under greenhouse conditions. Agronomy 2019, 9, 138. [Google Scholar] [CrossRef]
- Pozo, M.J.; López-Ráez, J.A.; Azcón-Aguilar, C.; García-Garrido, J.M. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytol. 2015, 205, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Kumar, A.; Khan, M.L. Role of biostimulants for enhancing abiotic stress tolerance in Fabaceae plants. In The Plant Family Fabaceae; Springer: Singapore, 2020; pp. 223–236. [Google Scholar]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant–fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Zhou, J.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Graham, J.H. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant Soil 2013, 363, 411–419. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Gilbert, L. Interplant signalling through hyphal networks. New Phytol. 2015, 205, 1448–1453. [Google Scholar] [CrossRef]
- Simard, S.W.; Beiler, K.J.; Bingham, M.A.; Deslippe, J.R.; Philip, L.J.; Teste, F.P. Mycorrhizal networks: Mechanisms, ecology and modelling. Fungal Biol. Rev. 2012, 26, 39–60. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular mycorrhizas: A promising component of plant production systems provided favorable conditions for their growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef]
- Candido, V.; Campanelli, G.; D’Addabbo, T.; Castronuovo, D.; Perniola, M.; Camele, I. Growth and yield promoting effect of artificial mycorrhization on field tomato at different irrigation regimes. Sci. Hortic. 2015, 187, 35–43. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant growth promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Jog, R.; Nareshkumar, G.; Rajkumar, S. Enhancing soil health and plant growth promotion by actinomycetes. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 33–45. [Google Scholar]
- Swarnalakshmi, K.; Senthilkumar, M.; Ramakrishnan, B. Endophytic actinobacteria: Nitrogen fixation, phytohormone production, and antibiosis. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 123–145. [Google Scholar]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Das, P.; Singh, S.K.; Singh, P.; Zeyad, M.T.; Aamir, M.; Upadhyay, R.S. Actinomycetes as biostimulants and their application in agricultural practices. In Microbiome Stimulants for Crops; White, J., Kumar, A., Droby, S., Eds.; Woodhead Publishing: Duxford/Kidlington, UK, 2021; pp. 267–282. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef]
- Chen, Y.; Nobili, M.D.; Aviad, T. Stimulatory effects of humic substances on plant growth. Soil organic matter in sustainable agriculture. In Soil Organic Matter in Sustainable Agriculture; Magdoff, F., Weil, R.R., Eds.; CRC Press: New York, NY, USA, 2004; pp. 103–129. [Google Scholar]
- García-Mina, J.M.; Antolín, M.C.; Sanchez-Diaz, M. Metal-humic complexes and plant micronutrient uptake: A study based on different plant species cultivated in diverse soil types. Plant Soil 2004, 258, 57–68. [Google Scholar] [CrossRef]
- Farrell, M.; Prendergast-Miller, M.; Jones, D.L.; Hill, P.W.; Condron, L.M. Soil microbial organic nitrogen uptake is regulated by carbon availability. Soil Biol. Biochem. 2014, 77, 261–267. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Hepworth, G.; McClintock, D.; McClintock, R. Effect of seaweed extract application on wine grape yield in Australia. J. Appl. Phycol. 2021, 33, 1883–1891. [Google Scholar] [CrossRef]
- Hegazy, E.A.; Abdel-Rehim, H.; Diaa, D.A.; El-Barbary, A. Controlling of degradation effects in radiation processing of polymers. In Controlling of Degradation Effects in Radiation Processing of Polymers; International Atomic Energy Agency: Vienna, Austria, 2009; p. 67. [Google Scholar]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Hasan, M.; Uddain, J.; Subramaniam, S. Impact of application of Trichoderma and biochar on growth, productivity, and nutritional quality of tomato under reduced NPK fertilization. Ann. Agric. Sci. 2020, 65, 107–115. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Colla, G. Role of arbuscular mycorrhizal fungi in alleviating the adverse effects of acidity and aluminium toxicity in zucchini squash. Sci. Hortic. 2015, 188, 97–105. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Di Mattia, E.; Tullio, M.; Rea, E.; Colla, G. Enhancement of alkalinity tolerance in two cucumber genotypes inoculated with an arbuscular mycorrhizal biofertilizer containing Glomus intraradices. Biol. Fertil. Soils 2010, 46, 499–509. [Google Scholar] [CrossRef]
- Morrison, E.; Lagos, L.; Al-Agely, A.; Glaab, H.; Johnson, W.; Jorquera, M.A.; Ogram, A. Mycorrhizal inoculation increases genes associated with nitrification and improved nutrient retention in soil. Biol. Fertil. Soils 2017, 53, 275–279. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio./Technol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Elena, A.; Diane, L.; Eva, B.; Marta, F.; Roberto, B.; Zamarreño, A.M.; García-Mina, J.M. The root application of a purified leonardite humic acid modifies the transcriptional regulation of the main physiological root responses to Fe deficiency in Fe-sufficient cucumber plants. Plant Physiol. Biochem. 2009, 47, 215–223. [Google Scholar] [CrossRef]
- Pacholczak, A.; Nowakowska, K.; Pietkiewicz, S. Physiological aspects in propagation of Ninebark (Physocarpus opulifolius Maxim) by stem cuttings treated with auxin or biostimulator. Not. Bot. Horti Agrobo. 2016, 44, 85–91. [Google Scholar] [CrossRef][Green Version]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. Z. Pflanzenernähr. Bodenk. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- Rubin, R.L.; van Groenigen, K.J.; Hungate, B.A. Plant growth promoting rhizobacteria are more effective under drought: A meta-analysis. Plant Soil 2017, 416, 309–323. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. Z. Pflanzenernähr. Bodenk. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Ervin, E.H. Optimizing dosages of seaweed extract-based cytokinins and zeatin riboside for improving creeping bentgrass heat tolerance. Crop Sci. 2010, 50, 316–320. [Google Scholar] [CrossRef]
- Goñi, G.; Łangowski, L.; Feeney, E.; Quille, P.; O’Connell, S. Reducing nitrogen input in barley crops while maintaining yields using an engineered biostimulant derived from Ascophyllum nodosum to enhance nitrogen use efficiency. Front. Plant Sci. 2021, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Briand, X.; Meyer, C. The effect of marine bioactive substances (N PRO) and exogenous cytokinins on nitrate reductase activity in Arabidopsis thaliana. Physiol Plant 2003, 119, 489–493. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, Y.; Zaid, A.; Khan, M.M.A. Adaptive physiological responses of plants under abiotic stresses: Role of phytohormones. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I.; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 797–824. [Google Scholar]
- Hosseinzadeh, S.R.; Amiri, H.; Ismaili, A. Evaluation of photosynthesis, physiological, and biochemical responses of chickpea (Cicer arietinum L. cv. Pirouz) under water deficit stress and use of vermicompost fertilizer. J. Integr. Agric. 2019, 17, 2426–2437. [Google Scholar] [CrossRef]
- Ali, Q.; Shehzad, F.; Waseem, M.; Shahid, S.; Hussain, A.I.; Haider, M.Z.; Habib, N.; Hussain, S.M.; Javed, M.T.; Perveen, R. Plant-based biostimulants and plant stress responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I.; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 625–661. [Google Scholar]
- Briglia, N.; Petrozza, A.; Hoeberichts, F.A.; Verhoef, N.; Povero, G. Investigating the impact of biostimulants on the row crops corn and soybean using high-efficiency phenotyping and next generation sequencing. Agronomy 2019, 9, 761. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen use and uptake efficiency and crop performance of baby spinach (Spinacia oleracea L.) and lamb’s lettuce (Valerianella locusta L.) grown under variable sub-optimal N regimes combined with plant-based biostimulant application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Effect on yield, total phenolic, total flavonoid and total isothiocyanate content of two broccoli cultivars (Brassica oleraceae var Italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). AFSci 2014, 23, 28–37. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Islam, M.N.; Uddain, J.; Chowdhury, M.S.N.; Subramaniam, S. Synergistic effect of microbial and nonmicrobial biostimulants on growth, yield, and nutritional quality of organic tomato. Crop Sci. 2020, 60, 2102–2114. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Khan, W.; Singh, S.; Karthikeyan, N.; Imran, M.; Rai, J.P.; Sarma, B.K.; Manna, M.C.; Chaurasia, R.; et al. Earthworm grazed-Trichoderma harzianum biofortified spent mushroom substrates modulate accumulation of natural antioxidants and bio-fortification of mineral nutrients in tomato. Front. Plant Sci. 2018, 9, 1017. [Google Scholar] [CrossRef]
- Pichereaux, C.; Laurent, E.-A.; Gargaros, A.; Viudes, S.; Durieu, C.; Lamaze, T.; Grieu, P.; Burlet-Schiltz, O. Analysis of durum wheat proteome changes under marine and fungal biostimulant treatments using large-scale quantitative proteomics: A useful dataset of durum wheat proteins. J. Proteom. 2019, 200, 28–39. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar]
- Dookie, M.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Flowering gene regulation in tomato plants treated with brown seaweed extracts. Sci. Hortic. 2021, 276, 109715. [Google Scholar] [CrossRef]
- Vasantharaja, R.; Abraham, L.S.; Inbakandan, D.; Thirugnanasambandam, R.; Senthilvelan, T.; Jabeen, S.K.A.; Prakash, P. Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp). Biocatal. Agric. Biotechnol. 2019, 17, 589–594. [Google Scholar] [CrossRef]
- Mattner, S.W.; Wite, D.; Riches, D.A.; Porter, I.J.; Arioli, T. The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol. Agric. Hortic. 2013, 29, 258–270. [Google Scholar] [CrossRef]
- Flores, P.; Pedreño, M.A.; Almagro, L.; Hernández, V.; Fenoll, J.; Hellín, P. Increasing nutritional value of broccoli with seaweed extract and trilinolein. J. Food Compos. Anal. 2021, 98, 103834. [Google Scholar] [CrossRef]
- Ahmed, Y.M.; Shalaby, E.A. Effect of different seaweed extracts and compost on vegetative growth, yield and fruit quality of cucumber. J. Hortic. Sci. Ornam. Plants 2012, 4, 235–240. [Google Scholar]
- Manna, D.; Sarkar, A.; Maity, T.K. Impact of biozyme on growth, yield and quality of chilli (Capsicum annuum L.). J. Crop Weed 2012, 8, 40–43. [Google Scholar]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. New Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Di Zinno, J.; Montanini, B.; Manfredi, M.; Marengo, E.; Fornasier, F.; Ferrarini, A.; Careri, M.; Visioli, G. Biostimulants applied to maize seeds modulate the enzymatic activity and metaproteome of the rhizosphere. Appl. Soil Ecol. 2020, 148, 103480. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Zamboni, A.; Sega, D.; Varanini, Z.; Pinton, R. Water-extractable humic substances speed up transcriptional response of maize roots to nitrate. Environ. Exp. Bot. 2018, 147, 167–178. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N.; Aranjuelo, I.; Garmendia, I. Nutritional quality and yield of onion as affected by different application methods and doses of humic substances. J. Food Compos. Anal. 2016, 51, 37–44. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria × Ananassa duch) cv. Camarosa. J. Plant Nutr. 2016, 39, 1821–1829. [Google Scholar] [CrossRef]
- Machiani, M.A.; Rezaei-Chiyaneh, E.; Javanmard, A.; Maggi, F.; Morshedloo, M.R. Evaluation of common bean (Phaseolus vulgaris L.) seed yield and quali-quantitative production of the essential oils from fennel (Foeniculum vulgare Mill.) and dragonhead (Dracocephalum moldavica L.) in intercropping system under humic acid application. J. Clean. Prod. 2019, 235, 112–122. [Google Scholar] [CrossRef]
- Dehsheikh, A.B.; Sourestani, M.M.; Zolfaghari, M.; Enayatizamir, N. Changes in soil microbial activity, essential oil quantity, and quality of Thai basil as response to biofertilizers and humic acid. J. Clean. Prod. 2020, 256, 120439. [Google Scholar] [CrossRef]
- Roomi, S.; Masi, A.; Conselvan, G.B.; Trevisan, S.; Quaggiotti, S.; Pivato, M.; Arrigoni, G.; Yasmin, T.; Carletti, P. Protein profiling of Arabidopsis roots treated with humic substances: Insights into the metabolic and interactome networks. Front. Plant Sci. 2018, 9, 1812. [Google Scholar] [CrossRef] [PubMed]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Rodríguez-Morgado, B.; Paneque, P.; Parrado, J. Effects of foliar fertilization of a biostimulant obtained from chicken feathers on maize yield. Eur. J. Agron. 2018, 96, 54–59. [Google Scholar] [CrossRef]
- Ertani, A.; Nardi, S.; Francioso, O.; Sanchez-Cortes, S.; Foggia, M.D.; Schiavon, M. Effects of two protein hydrolysates obtained from chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) plants. Front. Plant Sci. 2019, 10, 954. [Google Scholar] [CrossRef]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome-wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef]
- González-González, M.F.; Ocampo-Alvarez, H.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Casarrubias-Castillo, K.; Becerril-Espinosa, A.; Castañeda-Nava, J.J.; Hernández-Herrera, R.M. Physiological, ecological, and biochemical implications in tomato plants of two plant biostimulants: Arbuscular mycorrhizal fungi and seaweed extract. Front. Plant Sci. 2020, 11, 999. [Google Scholar] [CrossRef]
- Song, Z.; Bi, Y.; Zhang, J.; Gong, Y.; Yang, H. Arbuscular mycorrhizal fungi promote the growth of plants in the mining associated clay. Sci. Rep. 2020, 10, 2663. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Kalam, S.; Basu, A.; Podile, A.R. Functional and molecular characterization of plant growth promoting Bacillus isolates from tomato rhizosphere. Heliyon 2020, 6, e04734. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Singh, P.C.; Chaudhry, V.; Shirke, P.A.; Chakrabarty, D.; Farooqui, A.; Nautiyal, C.S.; Sane, A.P.; Sane, V.A. PGPR-induced OsASR6 improves plant growth and yield by altering root auxin sensitivity and the xylem structure in transgenic Arabidopsis thaliana. J. Plant Physiol. 2019, 240, 153010. [Google Scholar] [CrossRef] [PubMed]
- Dal Cortivo, C.; Barion, G.; Visioli, G.; Mattarozzi, M.; Mosca, G.; Vamerali, T. Increased root growth and nitrogen accumulation in common wheat following PGPR inoculation: Assessment of plant-microbe interactions by ESEM. Agric. Ecosyst. Environ. 2017, 247, 396–408. [Google Scholar] [CrossRef]
- Ureche, M.A.L.; Pérez-Rodriguez, M.M.; Ortiz, R.; Monasterio, R.P.; Cohen, A.C. Rhizobacteria improve the germination and modify the phenolic compound profile of pepper (Capsicum annum L.). Rhizosphere 2021, 18, 100334. [Google Scholar] [CrossRef]
- Ejaz, S.; Batool, S.; Anjum, M.A.; Naz, S.; Qayyum, M.F.; Naqqash, T.; Shah, K.H.; Ali, S. Effects of inoculation of root associative Azospirillum and Agrobacterium strains on growth, yield and quality of pea (Pisum sativum L.) grown under different nitrogen and phosphorus regimes. Sci. Hortic. 2020, 270, 109401. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Chaudhary, D.; Narula, N.; Sindhu, S.S.; Behl, R.K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 2013, 19, 515–519. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef]
- Selvakumar, G.; Kundu, S.; Joshi, P.; Nazim, S.; Gupta, A.D.; Mishra, P.K.; Gupta, H.S. Characterization of a cold-tolerant plant growth-promoting bacterium Pantoea dispersa 1A isolated from a sub-alpine soil in the north western Indian Himalayas. World J. Microbiol. Biotechnol. 2008, 24, 955–960. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; Dhondt-Cordelier, S.; Baillieul, F.; Clément, C.; Barka, E.A. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. MPMI 2012, 25, 241–249. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashraf, M. Microbial ACC-deaminase: Prospects and applications for inducing salt tolerance in plants. Crit. Rev. Plant Sci. 2010, 29, 360–393. [Google Scholar] [CrossRef]
- Bradáčová, K.; Weber, N.F.; Morad-Talab, N.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem. Biol. Technol. Agric. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Höfte, M. Rhizobacteria-induced systemic resistance. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2009; Volume 51, pp. 223–281. [Google Scholar]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of Next-Gen agricultural challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Coltelli, P.; Gualtieri, P. Paramylon treatment improves quality profile and drought resistance in Solanum lycopersicum L. cv. Micro-Tom. Agronomy 2019, 9, 394. [Google Scholar] [CrossRef]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal effects of an enzymatically hydrolyzed animal protein-based biostimulant (Pepton) in water-stressed tomato plants. Front. Plant Sci. 2019, 10, 758. [Google Scholar] [CrossRef]
- do Rosário Rosa, V.; Farias dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Rayirath, P.; Benkel, B.; Hodges, D.M.; Allan-Wojtas, P.; MacKinnon, S.; Critchley, A.T.; Prithiviraj, B. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 2009, 230, 135–147. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Khatri, K.; Rathore, M.S.; Pandey, S.P. Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol. Biochem. 2019, 136, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, M.N.; Shukla, P.S.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 2019, 38, 463–478. [Google Scholar] [CrossRef]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef]
- Kaluzewicz, A.; Bosiacki, M.; Spizewski, T. Influence of biostimulants on the content of macro-and micronutrients in broccoli plants exposed to drought stress. J. Elem. 2018, 23, 287–297. [Google Scholar]
- Yang, M.; Lei, Z.; Xu, T.; McLaughlin, N.B.; Liu, J. Effect of water-soluble humic acid applied to potato foliage on plant growth, photosynthesis characteristics and fresh tuber yield under different water deficits. Sci. Rep. 2020, 10, 7854. [Google Scholar]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Bahtiyar, M.; Kucukoduk, M. The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol. Environ. Saf. 2018, 155, 66–75. [Google Scholar] [CrossRef]
- Saidimoradi, D.; Ghaderi, N.; Javadi, T. Salinity stress mitigation by humic acid application in strawberry (Fragaria x ananassa Duch.). Sci. Hortic. 2019, 256, 108594. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalaji, H.M.; Valizadeh, G.R.; Behrozyar, E.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.-S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- and seaweed Extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Dehkordi, R.A.; Roghani, S.R.; Mafakheri, S.; Asghari, B. Effect of biostimulants on morpho-physiological traits of various ecotypes of fenugreek (Trigonella foenum-graecum L.) under water deficit stress. Sci. Hortic. 2021, 283, 110077. [Google Scholar] [CrossRef]
- Eroğlu, Ç.G.; Cabral, C.; Ravnskov, S.; Bak Topbjerg, H.; Wollenweber, B. Arbuscular mycorrhiza influences carbon-use efficiency and grain yield of wheat grown under pre- and post-anthesis salinity stress. Plant Biol. J. 2020, 22, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A.M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Abayomi Sobowale, S.P.; Olubode, A.; Mudathir, R.; Adebayo, R.; Adeoye, S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead and zinc contaminated soils. Rhizosphere 2021, 18, 100325. [Google Scholar] [CrossRef]
- Rahimi, S.; Talebi, M.; Baninasab, B.; Gholami, M.; Zarei, M.; Shariatmadari, H. The role of plant growth-promoting rhizobacteria (PGPR) in improving iron acquisition by altering physiological and molecular responses in quince seedlings. Plant Physiol. Biochem. 2020, 155, 406–415. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Ali, H.M.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef]
- Brunetti, C.; Saleem, A.R.; Della Rocca, G.; Emiliani, G.; De Carlo, A.; Balestrini, R.; Khalid, A.; Mahmood, T.; Centritto, M. Effects of plant growth-promoting rhizobacteria strains producing ACC deaminase on photosynthesis, isoprene emission, ethylene formation and growth of Mucuna pruriens (L.) DC. in response to water deficit. J. Biotechnol. 2021, 331, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Babar, M.; Rasul, S.; Aslam, K.; Abbas, R.; Manzoor, I.; Hanif, M.K.; Naqqash, T. Mining of halo-tolerant plant growth promoting rhizobacteria and their impact on wheat (Triticum aestivum L.) under saline conditions. J. King Saud Univ-Sci. 2021, 33, 101372. [Google Scholar] [CrossRef]
- Rabiei, Z.; Hosseini, S.J.; Pirdashti, H.; Hazrati, S. Physiological and biochemical traits in coriander affected by plant growth-promoting rhizobacteria under salt stress. Heliyon 2020, 6, e05321. [Google Scholar] [CrossRef]
- Yasmeen, T.; Ahmad, A.; Arif, M.S.; Mubin, M.; Rehman, K.; Shahzad, S.M.; Iqbal, S.; Rizwan, M.; Ali, S.; Alyemeni, M.N.; et al. Biofilm forming rhizobacteria enhance growth and salt tolerance in sunflower plants by stimulating antioxidant enzymes activity. Plant Physiol. Biochem. 2020, 156, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Sadeghi, A.; Safaie, N. Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Sci. Hortic. 2020, 265, 109206. [Google Scholar] [CrossRef]
- Baldotto, L.E.B.; Baldotto, M.A.; Canellas, L.P.; Bressan-Smith, R.; Olivares, F.L. Growth promotion of pineapple “vitória” by humic acids and Burkholderia spp. during acclimatization. Rev. Bras. Ciênc. Solo 2010, 34, 1593–1600. [Google Scholar] [CrossRef]
- Bettoni, M.M.; Mogor, Á.F.; Pauletti, V.; Goicoechea, N. Growth and metabolism of onion seedlings as affected by the application of humic substances, mycorrhizal inoculation and elevated CO2. Sci. Hortic. 2014, 180, 227–235. [Google Scholar] [CrossRef]
- Nikbakht, A.; Pessarakli, M.; Daneshvar-Hakimi-Maibodi, N.; Kafi, M. Perennial ryegrass growth responses to mycorrhizal infection and humic acid treatments. Agron. J. 2014, 106, 585–595. [Google Scholar] [CrossRef]
- Prakash, P.; Medhi, S.; Saikia, S.; Narendrakumar, G.; Thirugnanasambandam, T.; Abraham, L.S. Production, formulation and application of seaweed liquid fertilizer using humic acid on growth of Arachis hypogaea. Biosci. Biotechnol. Res. Asia 2014, 11, 1515–1519. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of combined applications of Trichoderma virens and a biopolymer-based biostimulant on lettuce agronomical, physiological, and qualitative properties under variable N regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef]
- Anli, M.; Kaoua, M.E.; ait-el-Mokhtar, M.; Boutasknit, A.; ben-Laouane, R.; Toubali, S.; Baslam, M.; Lyamlouli, K.; Hafidi, M.; Meddich, A. Seaweed extract application and arbuscular mycorrhizal fungal inoculation: A tool for promoting growth and development of date palm (Phoenix dactylifera L.) cv “Boufgous”. S. Afr. J. Bot. 2020, 132, 15–21. [Google Scholar] [CrossRef]
- Djouhou, F.M.C.; Nwaga, D.; Fokou, E. Comparative effect of arbuscular mycorrhizal fungi and biostimulants on the antioxidant and nutritional potential of Moringa oleifera. Nutr. Food Sci. Int. J. 2019, 9, 555757. [Google Scholar]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. yield and quality as influenced by cropping season, protein hydrolysates, and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Ngoroyemoto, N.; Kulkarni, M.G.; Stirk, W.A.; Gupta, S.; Finnie, J.F.; van Staden, J. Interactions between microorganisms and a seaweed-derived biostimulant on the growth and biochemical composition of Amaranthus hybridus L. Nat. Prod. Commun. 2020, 15, 1–11. [Google Scholar]
- Torun, H.; Toprak, B. Arbuscular mycorrhizal fungi and K-humate combined as biostimulants: Changes in antioxidant defense system and radical scavenging capacity in Elaeagnus angustifolia. J. Soil Sci. Plant Nutr. 2020, 20, 2379–2393. [Google Scholar] [CrossRef]
- Allaga, H.; Bóka, B.; Poór, P.; Nagy, V.D.; Szűcs, A.; Stankovics, I.; Takó, M.; Manczinger, L.; Vágvölgyi, C.; Kredics, L.; et al. A composite bioinoculant based on the combined application of beneficial bacteria and fungi. Agronomy 2020, 10, 220. [Google Scholar] [CrossRef]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-plant-microbe interactions in stressed agriculture management: A review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- French, E.; Kaplan, I.; Iyer-Pascuzzi, A.; Nakatsu, C.H.; Enders, L. Emerging strategies for precision microbiome management in diverse agroecosystems. Nat. Plants 2021, 7, 256–267. [Google Scholar] [CrossRef]
- Mohanram, S.; Kumar, P. Rhizosphere microbiome: Revisiting the synergy of plant-microbe interactions. Ann. Microbiol. 2019, 69, 307–320. [Google Scholar] [CrossRef]
- Copeland, J.K.; Yuan, L.; Layeghifard, M.; Wang, P.W.; Guttman, D.S. Seasonal community succession of the phyllosphere microbiome. MPMI 2015, 28, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Bouskill, N.J.; Wood, T.E.; Baran, R.; Ye, Z.; Bowen, B.P.; Lim, H.; Zhou, J.; Nostrand, J.D.V.; Nico, P.; Northen, T.R.; et al. Belowground response to drought in a tropical forest soil. I. Changes in microbial functional potential and metabolism. Front. Microbiol. 2016, 7, 525. [Google Scholar] [CrossRef]
- Wong, W.S.; Morald, T.K.; Whiteley, A.S.; Nevill, P.G.; Trengove, R.D.; Yong, J.W.H.; Dixon, K.W.; Valliere, J.M.; Stevens, J.C.; Veneklaas, E.J. Microbial inoculation to improve plant performance in mine waste substrates—A test using pigeon pea (Cajanus cajan). Land Degrad. Dev. 2022, 33. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Letham, D.S.; Wong, S.C.; Farquhar, G.D. Rhizobium-induced elevation in xylem cytokinin delivery in pigeonpea induces changes in shoot development and leaf physiology. Funct. Plant Biol. 2014, 41, 1323–1335. [Google Scholar] [CrossRef] [PubMed]

| BSs Applied | Crop | Effect on Crop Growth, Yield and Quality | Reference |
|---|---|---|---|
| SWEs (Ascophyllum nodosum) | Wheat | Increased in grain yield and protein quantity | [140] |
| SWEs (E. maxima, A. nodosum, Sargassum sp.) | Tomato | Increased mineral (Fe, Zn) content, enhanced germination, plant height, chlorophyll content, yield Expression of 6 flowering genes, increased flower bud and fruits | [141,142,143] |
| SWEs (Sargassum swartzii) | Cowpea | Increased phenolic and flavonoid content | [144] |
| SWEs (A. nodosum, Laminaria ochroleuca) | Broccoli | Increased antioxidants, flavonoids, and phenolic Enhanced both glucosinolates and phenolic compounds | [145,146] |
| SWEs (E. intestinelis) | Cucumber | Increased mineral (Fe, Mn, Zn) content of fruits, yield | [147] |
| SWEs (A. nodosum) | Pepper | Increased growth (height), chlorophyll content, yield | [148] |
| SWEs (Ecklonia maxima) | Spinach | Increased leaf number, chlorophyll, carotenoids, proteins, phytohormones, and phenolic acid | [149] |
| SWEs (commercial mixture) | Maize | Enhanced carbohydrate, organic substance and phosphorus metabolism, increased PGPR in rhizosphere | [150] |
| SWEs (A. nodosum) | Strawberry | Increased 10% marketable yield | [151] |
| SWEs (Ecklonia maxima) | Common bean | Increased yield and anthocyanins content in the seeds Increased synthesis of phenolics, flavonoid, anthocyanins and antioxidant activities | [152,153] |
| HSs | Maize | Increased leaf biomass, chlorophyll and carotene content Increased growth, grain yield and water use efficiency Faster induction of a higher capacity to take up nitrate | [154,155] |
| HSs | Onion | Increased yield, carbohydrate, protein and mineral contents in bulb | [156,157] |
| HSs | Strawberry | Increased growth, nutritional and chemical composition | [158] |
| HSs | Common bean | Increased seed yield and mineral content | [159] |
| HSs | Thai basil | Increased leaf nitrogen content | [160] |
| HSs | Arabidopsis | Enzyme activation of the glycolytic pathway and up-regulation of ribosomal protein | [161] |
| PHs | Tomato | Increased photosynthesis, antioxidant activities, total soluble solids, mineral composition Regulated the expression of genes involved in nitrate, ammonium and amino acid transporters as well as the key genes involved in N metabolism | [162] |
| PHs | Maize | Increased macro-and micro-nutrients in leaves, protein content in grain and yield Increased growth and accumulation of N-compounds (proteins, chlorophylls and phenols) Increased root growth and accumulation of K, Zn, Cu, and Mn in roots | [163,164,165] |
| AMF | Tomato | Increased foliar and root growth and protein content | [166] |
| AMF | Maize | Increased biomass and yield through biological improvement of soil properties | [167] |
| Trichoderma-based BSs | Lettuce, Rocket | Increased growth, yield and nutritional quality | [38,138,168] |
| PGPR (Bacillus spp.) | Tomato | Increased growth and yield | [169] |
| PGPR (Bacillus amyloliquefaciens) | Arabidopsis | Increased photosynthesis, biomass and seed yield | [170] |
| PGPR (consortia) | Wheat | Increased root growth and nitrogen accumulation | [171] |
| PGPR (Cellulosimicrobium and Pseudomonas) | Pepper | Increased phenolic compounds | [172] |
| PGPR (Azospirillum and Agrobacterium) | Pea | Increased nutrient uptake, vegetative growth, chlorophyll content and antioxidant capacity | [173] |
| BSs Applied | Type of Stress | Crop | Effect on Stress Tolerance and Crop Performance | Reference |
|---|---|---|---|---|
| SWEs (Euglena gracilis) | Drought/water stress | Tomato | Increased antioxidants (carotenoids, vitamins and phenolic acids) and soluble carbohydrates (glucose, fructose, and sucrose) in fruits;Increase endogenous indole-3-acetic acid (auxin), trans-zeatin (cytokinin), and jasmonic acid | [191,192] |
| SWEs (A. nodosum) | Drought | Soybean | Reduced Reactive Oxygen Species (ROS), increased antioxidant enzymes activity, stomatal conductance, higher energy efficiency | [193] |
| SWEs (Commercial) | Cold | Arabidopsis | Increased superoxide dismutase activity in the root and leaf tissue | [194] |
| SWEs (Gracilaria dura) | Drought | Wheat | Increased abscisic acid content and expression of stress-protective genes | [195] |
| SWEs (A. nodosum) | Drought | Spinach | Increased leaf-water relations, growth and yield | [196] |
| SWEs (A. nodosum) | Drought | Arabidopsis | Enhanced stomatal conductance and water use efficiency; regulation of stress-responsive genes | [197,198] |
| SWEs (A. nodosum) | Heat | Tomato | Gene transcription of protective heat shock proteins and increased flowering and fruit number | [199] |
| SWEs (A. nodosum) | Drought | Broccoli | Increased N, P, K, Mg, Cu and Mn contents | [200] |
| HSs | Drought | Potato | Increased growth, photosynthetic capacity and fresh tuber yield | [201] |
| HSs | Heavy metal stress (Cd) | Wheat | Increased activation of superoxide dismutase (SOD), catalase (CAT) and NADPH-oxidase (NOX) enzymes and ascorbate, glutathione | [202] |
| HSs | Salt | Strawberry | Enhanced leaf water content, membrane stability, chlorophyll content and increased biomass and yield | [203] |
| HSs | Drought | Rapeseed | Improved plants net photosynthesis via increasing the rate of gas exchange and electron transport flux | [204] |
| PHs | Salt | Common bean | Increased leaf photosynthetic pigments contents, membrane stability, relative water content | [205] |
| PHs | Drought | Grapevine | Reduced water loss, enhanced yield and quality | [206] |
| PHs (legume derived) | Mineral nutritional Stress (N) | Baby lettuce | Increased fresh weight, antioxidant capacity and total ascorbic acid content | [207] |
| PHs (legume derived) | Mineral nutritional Stress (N) | Baby rocket | Increased lipophilic antioxidant activity and total ascorbic acid content | [208] |
| PHs (legume derived) | Mineral nutritional Stress (N) | Baby spinach | Increased lipophilic and hydrophilic antioxidant activities, higher leaf chlorophylls and lower nitrate content | [136] |
| Trichoderma based BSs | Mineral nutritional stress (N) | Rocket | Improved root N uptake; increased ascorbic acid, K and Ca contents | [38] |
| AMF | Drought | Fenugreek | Increased root fresh weight, fresh plant weight and seed yield | [209] |
| AMF | Salt | Wheat | Increased photosynthesis and stomatal conductance, lower intrinsic water use efficiency and grain yield | [210] |
| AMF | Salt | Sweet basil | Increased chlorophyll content, water use efficiency and yield | [211] |
| AMF | Drought | Maize | Increased photosynthesis, proline, sugars and free amino acids; up-regulation of the antioxidant defense system | [212] |
| AMF | Heavy metal stress | Soybean | Retained heavy metals in roots and reduced translocation of Cu, Pb and Zn and improved overall growth and seed yield | [213] |
| PGPR (Pseudomonas fluorescens and Microccucuce yunnanensis) | Mineral nutritional stress (Fe) | Quince | Enhanced the expression of the genes related to Fe homeostasis, increased root, shoot biomass and chlorophyll content | [214] |
| PGPR (Cupriavidus necator and Pseudomonas fluorescens) | Water stress | Maize | Increased N and P use efficiency and biomass | [215] |
| PGPR (Pseudomonas aeruginosa and Burkholderia gladioli) | Heavy metal stress (Cd) | Tomato | Alleviated Cd toxicity and enhanced phenolic compounds, organic acids and osmoprotectants | [216] |
| PGPR (Enterobacter HS9 and Bacillus G9) | Water Stress | Velvet bean | Improved total biomass, water use efficiency and carbon assimilation | [217] |
| PGPR (Alcaligenes faecalis) | Salt | Wheat | Improved ionic balance, increased accumulation of osmolyte, photosynthetic pigments and improved photosystem II efficiency | [218] |
| PGPR (Azospirillum brasiliense and Azotobacter chroococcum) | Salt | Coriander | Increased chlorophyll content, fresh weight and yield | [219] |
| PGPR (Bacillus licheniformis and Pseudomonas plecoglossicida) | Salt | Sunflower | Increased fresh and dry biomass, yield, enhanced up-regulation of catalase (CAT), superoxide dismutase (SOD) and guaiacol peroxidase (GPX) antioxidant enzymes | [220] |
| PGPR (Streptomyces spp.) | Drought | Tomato | Increased leaf RWC, proline, MDA, H2O2 and total sugar content and yield | [221] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sani, M.N.H.; Yong, J.W.H. Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology 2022, 11, 41. https://doi.org/10.3390/biology11010041
Sani MNH, Yong JWH. Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology. 2022; 11(1):41. https://doi.org/10.3390/biology11010041
Chicago/Turabian StyleSani, Md. Nasir Hossain, and Jean W. H. Yong. 2022. "Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming" Biology 11, no. 1: 41. https://doi.org/10.3390/biology11010041
APA StyleSani, M. N. H., & Yong, J. W. H. (2022). Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology, 11(1), 41. https://doi.org/10.3390/biology11010041







