Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Conditions and Experimental Design
2.2. Statistical Analysis
3. Results
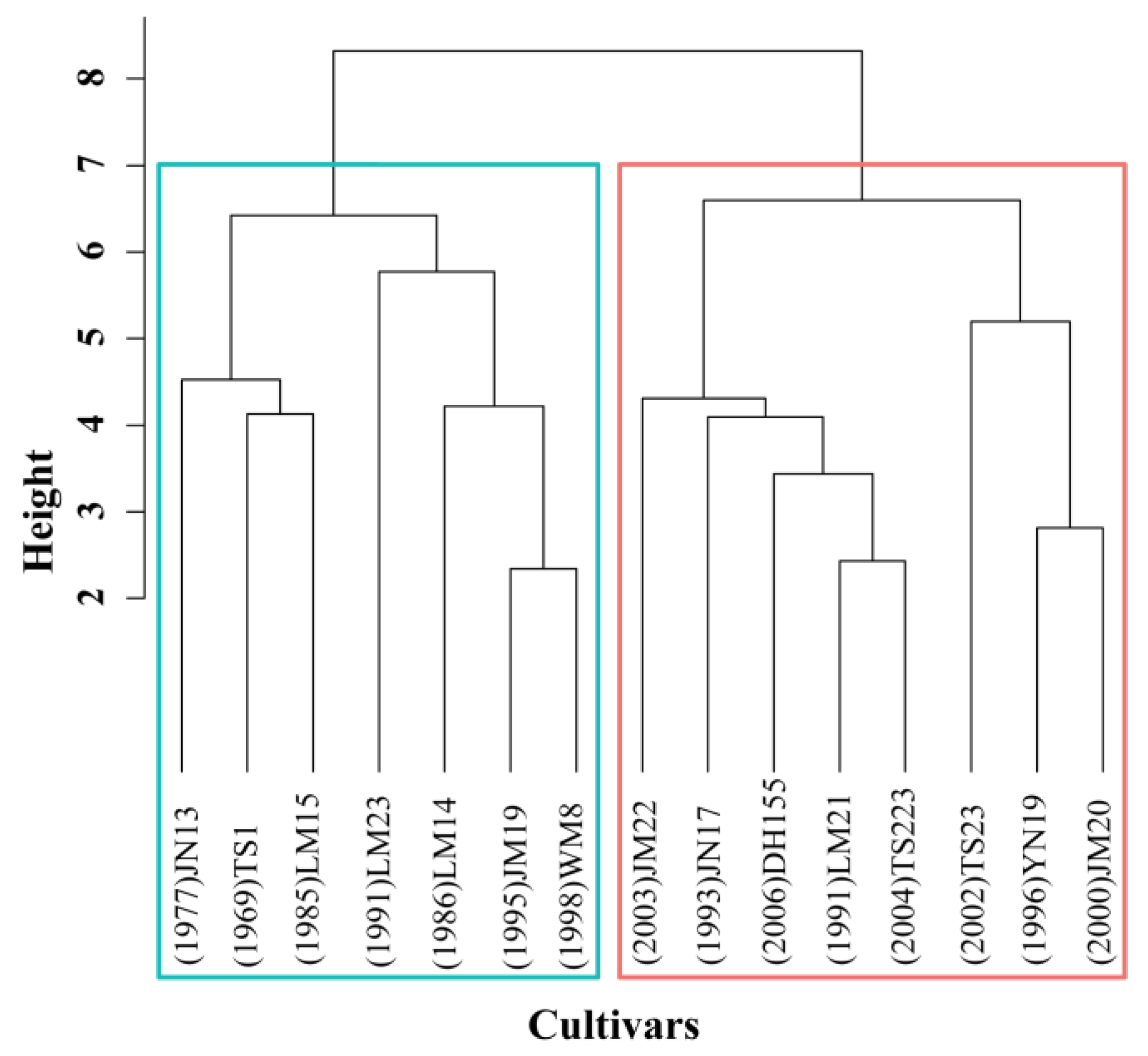
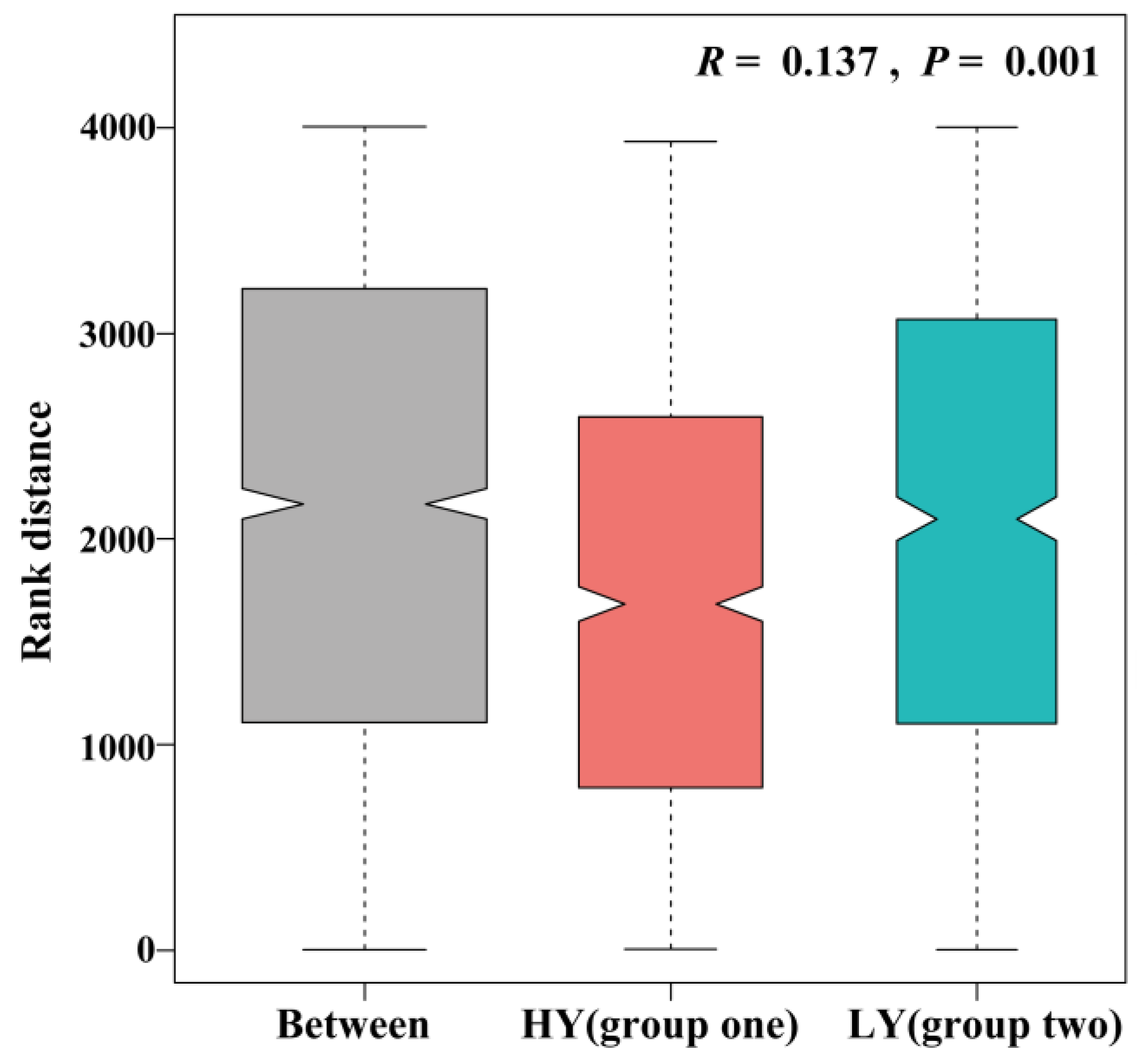
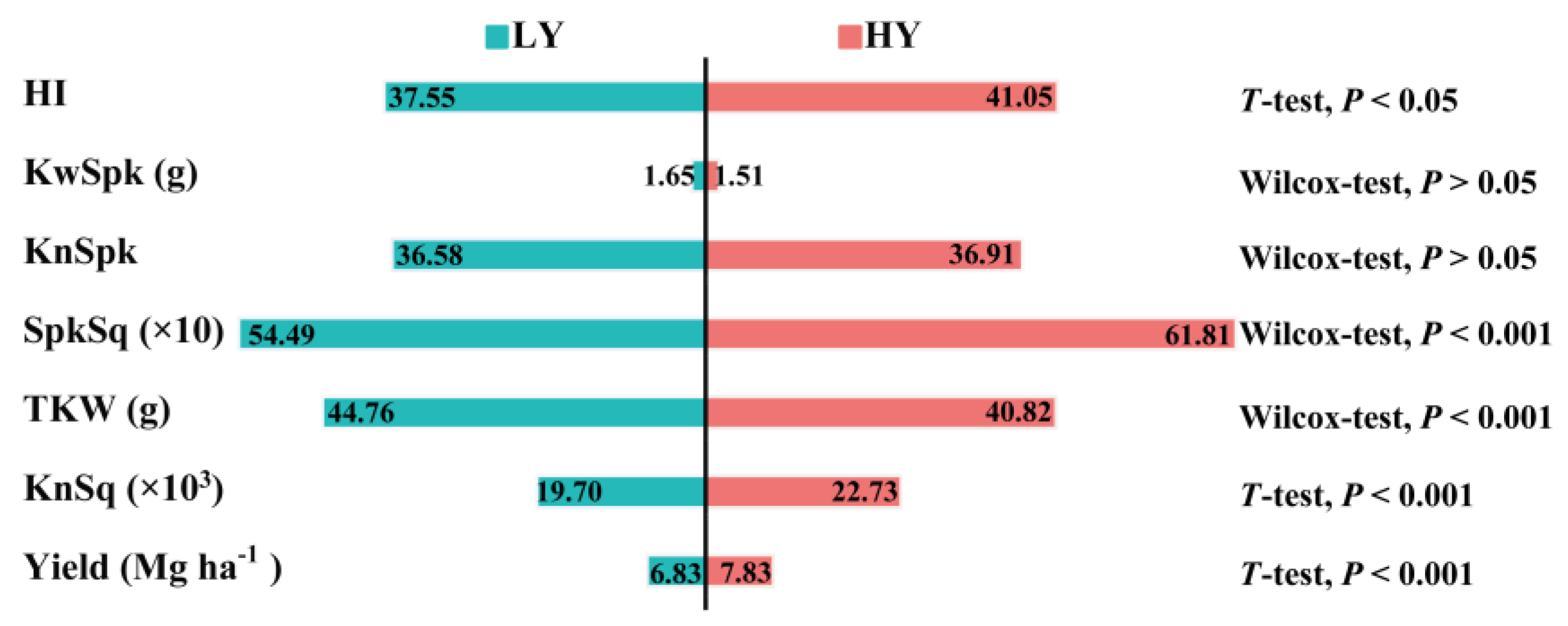
3.1. HY Group Has Larger Sink than LY Group
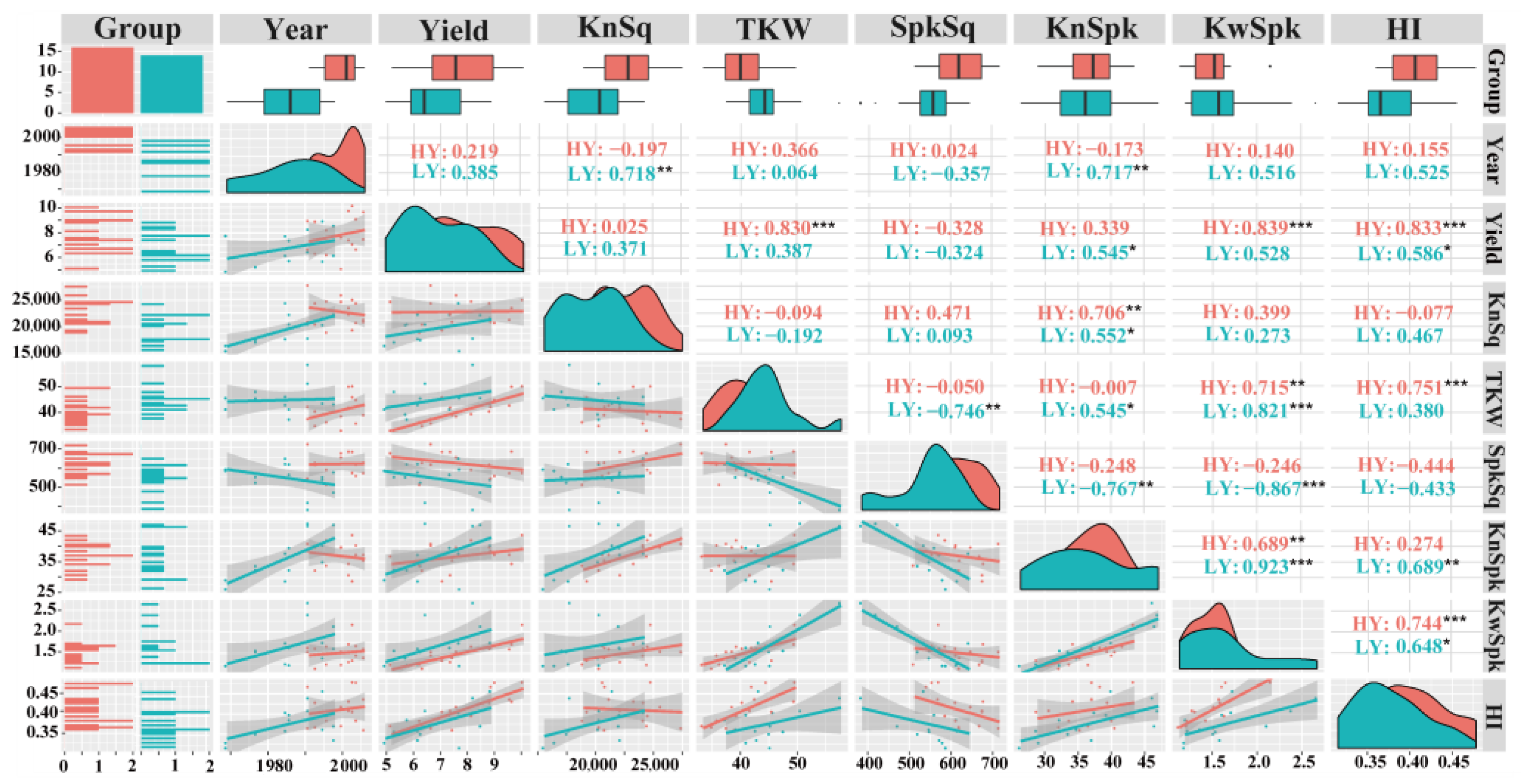
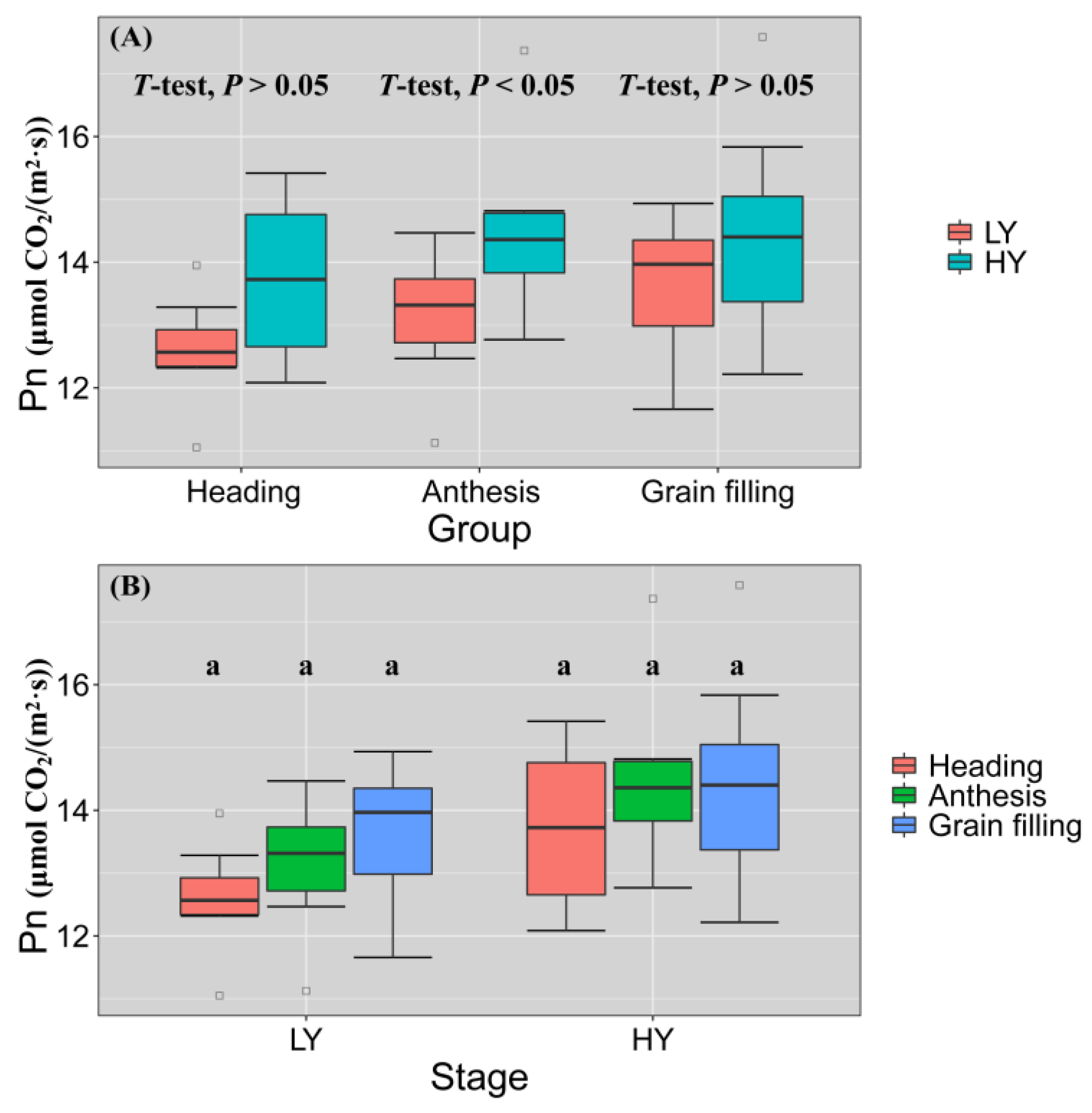
3.2. TKW, Pn and WSC Have Substantial Effects on HY Group Yield
3.3. HY Group Had Elevated Pn and Prolonged Active Photographic Duration
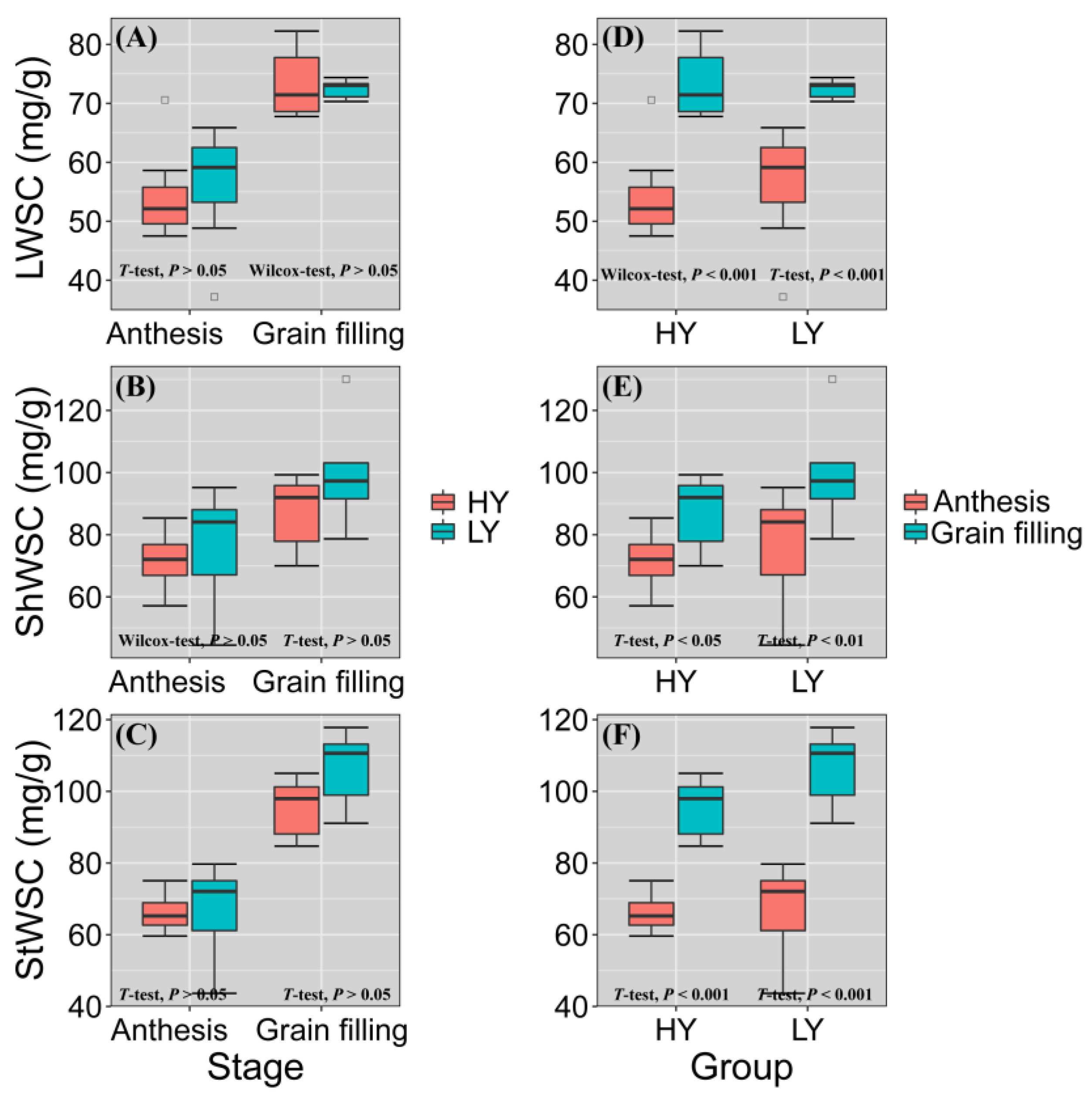
3.4. HY Group Had Lower WSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paul, M.J.; Watson, A.; Griffiths, C.A. Linking fundamental science to crop improvement through understanding source and sink traits and their integration for yield enhancement. J. Exp. Bot. 2020, 71, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.J.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.T.; Edmeades, G.O. Breeding and cereal yield progress. Crop Sci. 2010, 50, 85–98. [Google Scholar] [CrossRef]
- Graybosch, R.A.; Peterson, C.J. Genetic improvement in winter wheat yields in the Great Plains of North America, 1959–2008. Crop Sci. 2010, 50, 1882–1980. [Google Scholar] [CrossRef]
- Woyann, L.G.; Zdziarski, A.D.; Zanella, R.; Rosa, A.C.; de Castro, R.L.; Caierão, E.; Toigo, M.D.C.; Storck, L.; Wu, J.; Benin, G. Genetic gain over 30 years of spring wheat breeding in Brazil. Crop Sci. 2019, 59, 2036–2045. [Google Scholar] [CrossRef]
- Valvo, P.J.L.; Miralles, D.J.; Serrago, R.A. Genetic progress in Argentine bread wheat varieties released between 1918 and 2011: Changes in physiological and numerical yield components. Field Crop. Res. 2018, 221, 314–321. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Royo, C.; Aparicio, N.; Martin-Sanchez, J.A.; Álvaro, F. Genetic improvement of bread wheat yield and associated traits in Spain during the 20th century. J. Agric. Sci.-Camb. 2013, 151, 105–118. [Google Scholar] [CrossRef]
- Wu, W.; Li, C.; Ma, B.; Shah, F.; Liu, Y.; Liao, Y. Genetic progress in wheat yield and associated traits in China since 1945 and future prospects. Euphytica 2014, 196, 155–168. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P.; Manes, Y.; Singh, R.P.; Crossa, J.; Braun, H.J. Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a “Historic” set representing 30 years of breeding. Crop Sci. 2012, 52, 1123–1131. [Google Scholar] [CrossRef]
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Gouis, J.L.; Oury, F.X.; Charmet, G. How changes in climate and agricultural practices influenced wheat production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Sun, S.; Yang, X.; Lin, X.; Sassenrath, G.F.; Li, K. Winter wheat yield gaps and patterns in China. Agron. J. 2018, 110, 319–330. [Google Scholar] [CrossRef]
- Matus, I.; Mellado, M.; Pinares, M.; Madariaga, R.; Pozo, A.D. Genetic progress in winter wheat cultivars released in Chile from 1920 to 2000. Chil. J. Agric. Res. 2012, 72, 303–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiang, C.; Wang, X.; Chen, Y.; Deng, J.; Jiang, C.; Sun, X.; Chen, H.; Li, J.; Piao, W.; et al. New alleles for chlorophyll content and stay-green traits revealed by a genome wide association study in rice (Oryza sativa). Sci. Rep. 2019, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Khosla, R.; Frasier, W.M.; Westfall, D.G.; Inman, D. Economic feasibility of variable-rate nitrogen application utilizing site-specific management zones. Agron. J. 2004, 96, 1572–1580. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Wang, N.; Chen, Y.; Zhang, S. Changes in the yield and associated photosynthetic traits of dry-land winter wheat (Triticum aestivum L.) from the 1940s to the 2010s in Shaanxi Province of China. Field Crop. Res. 2014, 167, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wang, H.; Dong, H.; Qi, X.; Zhao, M.; Fang, Y.; Gao, C.; Hu, L. Progress in genetic improvement of grain yield and related physiological traits of Chinese wheat in Henan Province. Field Crop. Res. 2016, 199, 117–128. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.B.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; He, M.; Fu, J.; Tian, Q.; Yin, Y.; Cao, H. Effects of source sink manipulation on production and distribution of photosynthate after flowering in irrigated and rainfed wheat. Acta Agron. Sin. 1999, 25, 162–168. [Google Scholar] [CrossRef]
- Gent, M.P.N. Photosynthate reserves during grain filling in winter wheat. Agron. J. 1994, 86, 159–167. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; He, M.; Zhang, Y.; Lu, S.; Li, Q.; Shi, S. Allocation of photosynthates and grain growth of two wheat cultivars with different potential grain growth in response to pre- and post-anthesis shading. J. Agron. Crop Sci. 2003, 189, 280–285. [Google Scholar] [CrossRef]
- Jia, S.; Lv, J.; Jiang, S.; Liang, T.; Liu, C.; Jing, Z. Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynthetica 2015, 53, 95–109. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Zhang, Y.; Qin, W.; Wang, Z.; Zhang, Y.; Lu, C.; Lu, Q. In situ measurements of winter wheat diurnal changes in photosynthesis and environmental factors reveal new insight into photosynthesis improvement by super-high-yield cultivation. J. Integr. Agric. 2021, 20, 527–539. [Google Scholar] [CrossRef]
- Austin, R.B.; Morgan, C.L.; Ford, M.A.; Bhagwat, S.G. Flag leaf photosynthesis of Triticum aestivum and related diploid and tetraploid species. Ann. Bot.-Lond. 1982, 49, 177–189. [Google Scholar] [CrossRef]
- Biding, F.; Musgrave, R.B.; Fischer, R.A. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 1977, 270, 431–433. [Google Scholar] [CrossRef]
- Blum, A. Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 1998, 100, 77–83. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H.; Kühbauch, W. Carbon mobilization in shoot parts and roots of wheat during grain filling: Assessment by 13C/12C steady-state labelling, growth analysis and balance sheets of reserves. Plant Cell Environ. 1998, 21, 301–313. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.A.; Madore, M.A.; Waines, J.G. Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci. 2006, 46, 2093–2103. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.A.; Madore, M.A.; Waines, J.G. Genotypic variation for stem reserves and mobilization in wheat: I. Postanthesis changes in internode dry matter. Crop Sci. 2006, 46, 735–746. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; van Herwaarden, A.F.; Jenkins, C.; Weiss, M.; Lewis, D.; Ruuska, S.; Tabe, L.; Fettell, N.A.; Richards, R.A. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Aust. J. Agric. Res. 2008, 59, 891–905. [Google Scholar] [CrossRef]
- Xue, G.; McIntyre, C.L.; Rattey, A.R.; van Herwaarden, A.F.; Shorter, R. Use of dry matter content as a rapid and low-cost estimate for ranking genotypic differences in water-soluble carbohydrate concentrations in the stem and leaf sheath of Triticum aestivum. Crop Pasture Sci. 2009, 60, 51–59. [Google Scholar] [CrossRef]
- Hoogmoed, M.; Sadras, V.O. The importance of water-soluble carbohydrates in the theoretical framework for nitrogen dilution in shoot biomass of wheat. Field Crop. Res. 2016, 193, 196–200. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Ma, J.; Zhang, P.; Wang, C.; Su, J.; Yang, D. Genetic dissection of stem WSC accumulation and remobilization in wheat (Triticum aestivum L.) under terminal drought stress. BMC Genet. 2020, 21, 50. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Willenbrink, J. Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytol. 2000, 148, 413–422. [Google Scholar] [CrossRef]
- Shearman, V.J.; Sylvester Bradley, R.; Scott, R.K.; Foulkes, M.J. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar] [CrossRef]
- Van Herwaarden, A.F.; Angus, J.F.; Richards, R.A.; Farquhar, G.D. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser II. Carbohydrate and protein dynamics. Aust. J. Agric. Res. 1998, 49, 1083–1093. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osorio, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot.-Lond. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Xue, G.; McIntyre, C.L.; Glassop, D.; Shorter, R. Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol. Biol. 2008, 67, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; van Herwaarden, A.F.; Chapman, S.C. Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration. Field Crop. Res. 2009, 112, 43–54. [Google Scholar] [CrossRef]
- Yang, D.L.; Jing, R.L.; Chang, X.P.; Li, W. Identification of quantitative trait loci and environmental interactions for accumulation and remobilization of water-soluble carbohydrates in wheat (Triticum aestivum L.) stems. Genetics 2007, 176, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Senapati, N.; Brown, H.E.; Semenov, M.A. Raising genetic yield potential in high productive countries: Designing wheat ideotypes under climate change. Agric. For. Meteorol. 2019, 271, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, Y.; Zhang, Y.; Fan, S.; Kong, L. Source-sink modifications affect leaf senescence and grain mass in wheat as revealed by proteomic analysis. BMC Plant Biol. 2020, 20, 1–17. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Kim, J.; Rivera-Amado, C.; Molero, G.; Araus, J.; Savin, R.; Slafer, G.A. Are awns truly relevant for wheat yields? A study of performance of awned/awnless isogenic lines and their response to source–sink manipulations. Field Crop. Res. 2020, 254, 107827. [Google Scholar] [CrossRef]
- Jagadish, K.S.V.; Kavi Kishor, P.B.; Bahuguna, R.N.; von Wirén, N.; Sreenivasulu, N. Staying alive or going to die during terminal senescence—An enigma surrounding yield stability. Front. Plant Sci. 2015, 6, 1070. [Google Scholar] [CrossRef]
- Chang, T.; Zhu, X. Source–sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef] [PubMed]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Field Crop. Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and metabolic regulation of source-sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Slafer, G.A.; Savin, R. Source—sink relationships and grain mass at different positions within the spike in wheat. Field Crop. Res. 1994, 37, 39–49. [Google Scholar] [CrossRef]
- Miralles, D.J.; Slafer, G.A. Sink limitations to yield in wheat: How could it be reduced? J. Agric. Sci.-Camb. 2007, 145, 139–149. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Molero, G.; Trujillo-Negrellos, E.; Reynolds, M.; Foulkes, J. Estimating organ contribution to grain filling and potential for source upregulation in wheat cultivars with a contrasting source-sink balance. Agronomy 2020, 10, 1527. [Google Scholar] [CrossRef]
- Burnett, A.C.; Serbin, S.P.; Rogers, A. Source: Sink imbalance detected with leaf- and canopy-level spectroscopy in a field-grown crop. Plant Cell Environ. 2021, 44, 2466–2479. [Google Scholar] [CrossRef]
- Jin, J.; Liu, X.; Wang, G.; Mi, L.; Shen, Z.; Chen, X.; Herbert, S.J. Agronomic and physiological contributions to the yield improvement of soybean cultivars released from 1950 to 2006 in Northeast China. Field Crop. Res. 2010, 115, 116–123. [Google Scholar] [CrossRef]
- Slafer, G.A.; Satorre, E.H.; Andrade, F.H. Increases in Grain Yield in Bread Wheat from Breeding and Associated Physiological Changes; Marcel Dekker Inc.: New York, NY, USA, 1994; pp. 1–68. [Google Scholar]
- Deng, X.; Gibson, J. Improving eco-efficiency for the sustainable agricultural production: A case study in Shandong, China. Technol. Forecast. Soc. 2019, 144, 394–400. [Google Scholar] [CrossRef]
- Tang, X.W.; Luo, L.P.; Yu, Z.W.; Shi, Y. Differences in population dynamics and dry matter accumulation characteristics of wheat varieties with different yield potential. Shandong Agric. Sci. 2020, 52, 29–33. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, M. Farmers’ perception on combined climatic and market risks and their adaptive behaviors: A case in Shandong Province of China. Environ. Dev. Sustain. 2021, 23, 13042–13061. [Google Scholar] [CrossRef]
- Xiao, Y.G.; Qian, Z.G.; Wu, K.; Liu, J.J.; Xia, X.C.; Ji, W.Q.; He, Z.H. Genetic gains in grain yield and physiological traits of winter wheat in Shandong Province, China, from 1969 to 2006. Crop Sci. 2012, 52, 44–56. [Google Scholar] [CrossRef]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: http://www.r-project.org/ (accessed on 20 December 2021).
- Lamb, E.; Shirtliffe, S.; May, W. Structural equation modeling in the plant sciences: An example using yield components in oat. Can. J. Plant Sci. 2011, 91, 603–619. [Google Scholar] [CrossRef]
- McLeod, E.M.; Banerjee, S.; Bork, E.W.; Hall, L.M.; Hare, D.D. Structural equation modeling reveals complex relationships in mixed forage swards. Crop Prot. 2015, 78, 106–113. [Google Scholar] [CrossRef]
- Wright, S. Correlation or causation. J. Agric. Res. 1921, 20, 557–585. [Google Scholar]
- Dhungana, P.; Eskridge, K.M.; Baenziger, P.S.; Campbell, B.T.; Gill, K.S.; Dweikat, I. Analysis of genotype-by-environment interaction in wheat using a structural equation model and chromosome substitution lines. Crop Sci. 2007, 47, 477–484. [Google Scholar] [CrossRef]
- Alwin, D.F.; Hauser, R.M. The decomposition of effects in path analysis. Am. Sociol. Rev. 1975, 40, 37–47. [Google Scholar] [CrossRef]
- Finkelstein, D. A beginner’s guide to structural equation modeling. Technometrics 2005, 47, 522. [Google Scholar] [CrossRef]
- Araus, J.L.; Brown, H.R.; Febrero, A.; Bort, J.; Serret, M.D. Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant Cell Environ. 1993, 16, 383–392. [Google Scholar] [CrossRef]
- Murchie, E.H.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2008, 181, 532–552. [Google Scholar] [CrossRef] [PubMed]
- Tambussi, E.A.; Bort, J.; Guiamet, J.J.; Nogués, S.; Araus, J.L.; Araus. The photosynthetic role of ears in C3 cereals: Metabolism, water use efficiency and contribution to grain yield. Crit. Rev. Plant Sci. 2007, 26, 1–16. [Google Scholar] [CrossRef]
- Fischer, R.A.; Hillerislambers, D. Effect of environment and cultivar on source limitation to grain weight in wheat. Aust. J. Agric. Res. 1978, 29, 443–458. [Google Scholar] [CrossRef]
- Zhang, H.; Turner, N.C.; Poole, M.L. Source-sink balance and manipulating sink-source relations of wheat indicate that the yield potential of wheat is sink-limited in high-rainfall zones. Crop Pasture Sci. 2010, 61, 852–861. [Google Scholar] [CrossRef]
- Wang, S.H.; Jing, Q.; Dai, T.B.; Jiang, D.; Cao, W.X. Evolution characteristics of flag leaf photosynthesis and grain yield of wheat cultivars bred in different years. Chin. J. Appl. Ecol. 2008, 19, 1255–1260. [Google Scholar]
- Jiang, G.M.; Sun, J.Z.; Liu, H.Q.; Qu, C.M.; Wang, K.J.; Guo, R.J.; Bai, K.Z.; Gao, L.M.; Kuang, T.Y. Changes in the rate of photosynthesis accompanying the yield increase in wheat cultivars released in the past 50 years. J. Plant Res. 2003, 116, 347–354. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef]
- Gaju, O.; DeSilva, J.; Carvalho, P.; Hawkesford, M.J.; Griffiths, S.; Greenland, A.; Foulkes, M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crop. Res. 2016, 193, 1–15. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, J.; Furbank, R.; Griffiths, S.; King, J.; Murchie, E.; Parry, M.; Slafer, G. Achieving yield gains in wheat. Plant Cell Environ. 2012, 35, 1799–1823. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhang, Y.; Fischer, T.; Zhao, Z.; Zhou, X.; Wang, Z.; Wang, E. The contribution of spike photosynthesis to wheat yield needs to be considered in process-based crop models. Field Crop. Res. 2020, 257, 107931. [Google Scholar] [CrossRef]
- Molero, G.; Reynolds, M.P. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crop. Res. 2020, 255, 107866. [Google Scholar] [CrossRef]
- Gámez, A.L.; Vicente, R.; Sanchez-Bragado, R.; Jauregui, I.; Morcuende, R.; Goicoechea, N.; Aranjuelo, I. Differential flag leaf and ear photosynthetic performance under elevated (CO2) conditions during grain filling period in durum wheat. Front. Plant Sci. 2020, 11, 587958. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Vicente, R.N.; Molero, G.; Serret, M.D.; Maydup, M.A.L.N.; Araus, J.L. New avenues for increasing yield and stability in C3 cereals: Exploring ear photosynthesis. Curr. Opin. Plant Biol. 2020, 56, 223–234. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.P.; Araus, J.L. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ δ13C. J. Exp. Bot. 2014, 65, 5401–5413. [Google Scholar] [CrossRef]
- McIntyre, C.L.; Seung, D.; Casu, R.E.; Rebetzke, G.J.; Shorter, R.; Xue, G.P. Genotypic variation in the accumulation of water soluble carbohydrates in wheat. Funct. Plant Biol. 2012, 39, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, J.; Deng, X. The spike weight contribution of the photosynthetic area above the upper internode in a winter wheat under different nitrogen and mulching regimes. Crop J. 2019, 7, 89–100. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Su, B. Accumulation and remobilization of stem reserves in wheat (review). J. China Agric. Univ. 1994, 20, 369–374. [Google Scholar]
- Takahashi, T.; Chevalier, P.; Rupp, R. Storage and remobilization of soluble carbohydrates after heading in different plant parts of a winter wheat cultivar. Plant Prod. Sci. 2001, 4, 160–165. [Google Scholar] [CrossRef][Green Version]
- Takahashi, T.; Tsuchihashi, N.; Nakaseko, K. Grain filling mechanisms in spring wheat. I. Grain filling phases according to the development of plant organs. Jpn. J. Crop Sci. 1993, 62, 560–564. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.H.; Sui, X.X.; Xia, X.C.; Zhang, X.K.; Zhang, G.S. Genetic improvement of grain yield and associated traits in the northern China winter wheat region from 1960 to 2000. Crop Sci. 2007, 47, 245–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.Z.; Cai, S.B.; He, Z.H.; Zhang, X.K.; Xia, X.C.; Zhang, G.S. Genetic improvement of grain yield and associated traits in the southern China winter wheat region: 1949 to 2000. Euphytica 2007, 157, 465–473. [Google Scholar] [CrossRef]
- Aisawi, K.A.B.; Reynolds, M.P.; Singh, R.P.; Foulkes, M.J. The physiological basis of the genetic progress in yield potential of CIMMYT spring wheat cultivars from 1966 to 2009. Crop Sci. 2015, 55, 1749–1764. [Google Scholar] [CrossRef]
- Wang, J.; Yu, B.; Wang, R.; Wang, L.; Geng, R.; Chen, Y.; Li, M. Evolution and genetic analysis of yield characters of wheat varieties in Shandong province. Shandong Agric. Sci. 2007, 2, 5–7. [Google Scholar] [CrossRef]
- Calderini, D.F.; Dreccer, M.F.; Slafer, G.A. Genetic improvement in wheat yield and associated traits. A re-examination of previous results and the latest trends. Plant Breed. 1995, 114, 108–112. [Google Scholar] [CrossRef]
- Sadras, V.O.; Lawson, C. Genetic gain in yield and associated changes in phenotype, trait plasticity and competitive ability of South Australian wheat varieties released between 1958 and 2007. Crop Pasture Sci. 2011, 62, 533–549. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Trujillo-Negrellos, E.; Molero, G.; Reynolds, M.P.; Sylvester-Bradley, R.; Foulkes, M.J. Optimizing dry-matter partitioning for increased spike growth, grain number and harvest index in spring wheat. Field Crop. Res. 2019, 240, 154–167. [Google Scholar] [CrossRef]
- Calderini, D.F.; Castillo, F.M.; Arenas-M, A.; Molero, G.; Reynolds, M.P.; Craze, M.; Bowden, S.; Milner, M.J.; Wallington, E.J.; Dowle, A.; et al. Overcoming the trade-off between grain weight and number in wheat by the ectopic expression of expansin in developing seeds leads to increased yield potential. New Phytol. 2020, 230, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integr. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
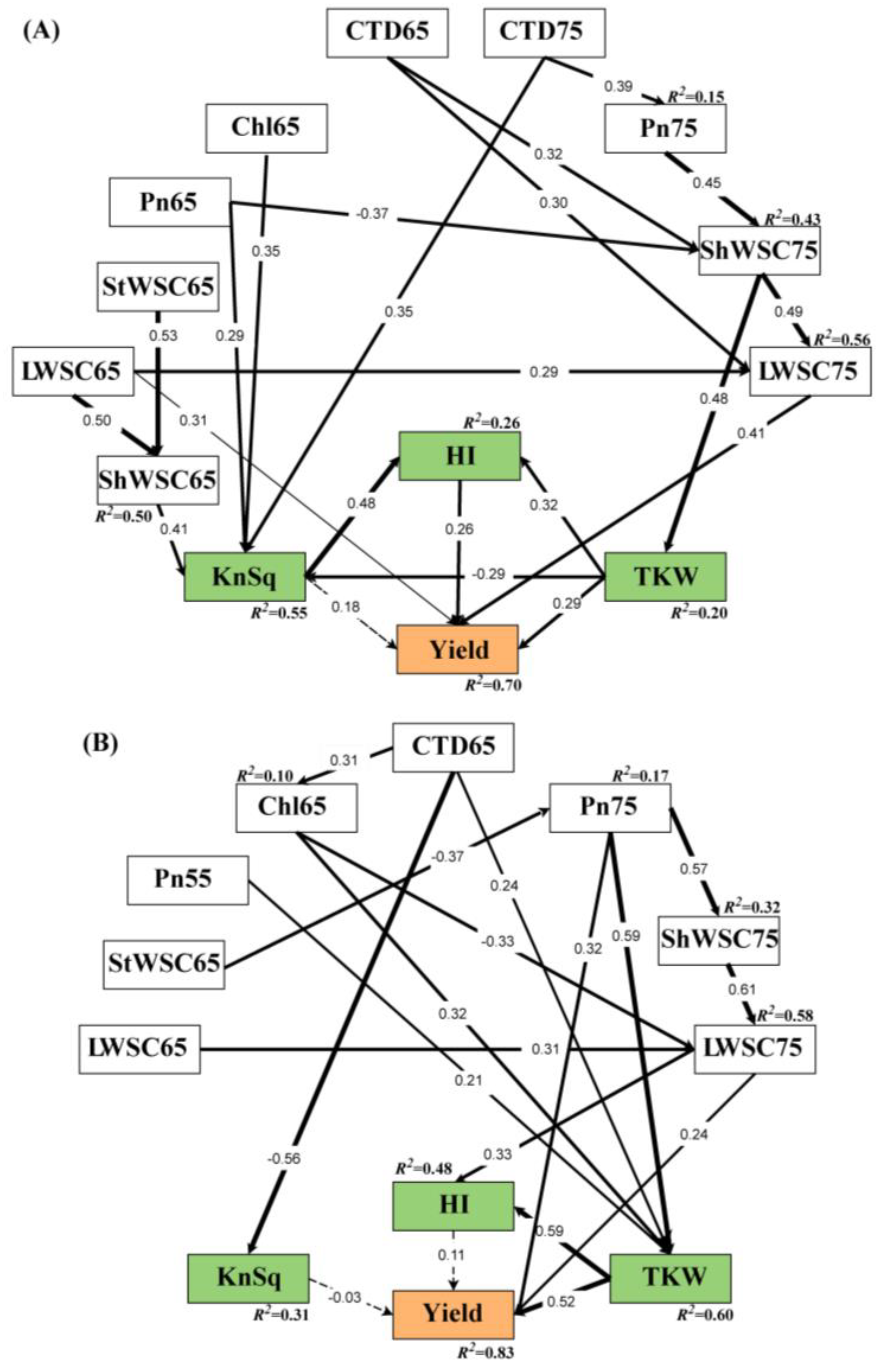
| Pn75 | ShWSC65 | ShWSC75 | LWSC75 | TKW | KnSq | HI | Yield | ||
|---|---|---|---|---|---|---|---|---|---|
| Direct effects | CTD65 | 0.000 | 0.000 | 0.301 ** | 0.315 ** | 0.000 | 0.000 | 0.000 | 0.000 |
| CTD75 | 0.387 ** | 0.000 | 0.000 | 0.000 | 0.000 | 0.348 ** | 0.000 | 0.000 | |
| Chl65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.348 ** | 0.000 | 0.000 | |
| Pn65 | 0.000 | 0.000 | −0.374 *** | 0.000 | 0.000 | 0.292 ** | 0.000 | 0.000 | |
| Pn75 | 0.000 | 0.000 | 0.448 *** | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| LWSC65 | 0.000 | 0.503 *** | 0.000 | 0.294 ** | 0.000 | 0.000 | 0.000 | 0.315 *** | |
| ShWSC65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.408 *** | 0.000 | 0.000 | |
| StWSC65 | 0.000 | 0.533 *** | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| LWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.407 *** | |
| ShWSC75 | 0.000 | 0.000 | 0.000 | 0.494 *** | 0.448 *** | 0.000 | 0.000 | 0.000 | |
| TKW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | −0.288 ** | 0.319 ** | 0.293 ** | |
| KnSq | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.480 *** | 0.175 | |
| HI | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.256 ** | |
| Indirect effects | CTD65 | 0.000 | 0.000 | 0.000 | 0.154 | 0.135 | −0.037 | 0.024 | 0.235 ** |
| CTD75 | 0.000 | 0.000 | 0.173 | 0.089 | 0.078 | −0.021 | 0.180 | 0.166 | |
| Chl65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.166 | 0.107 | |
| Pn65 | 0.000 | 0.000 | 0.000 | −0.192 | −0.167 | 0.046 | 0.109 | −0.038 | |
| Pn75 | 0.000 | 0.000 | 0.000 | 0.230 ** | 0.200 * | −0.055 | 0.036 | 0.153 | |
| LWSC65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.196 * | 0.098 | 0.186 | |
| ShWSC65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.202 ** | 0.131 | |
| StWSC65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.208 ** | 0.104 | 0.067 | |
| LWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| ShWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | −0.123 | 0.080 | 0.341 *** | |
| TKW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | −0.137 | −0.004 | |
| KnSq | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.133 | |
| HI | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Chl65 | Pn75 | LWSC75 | ShWSC75 | KnSq | TKW | HI | Yield | ||
|---|---|---|---|---|---|---|---|---|---|
| Direct effects | CTD65 | 0.314 * | 0.000 | 0.000 | 0.000 | −0.559 *** | 0.237 * | 0.000 | 0.000 |
| Chl65 | 0.000 | 0.000 | −0.327 *** | 0.000 | 0.000 | 0.315 ** | 0.000 | 0.000 | |
| Pn55 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.205 * | 0.000 | 0.000 | |
| Pn75 | 0.000 | 0.000 | 0.000 | 0.569 *** | 0.000 | 0.593 *** | 0.000 | 0.319 *** | |
| StWSC65 | 0.000 | −0.365 ** | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| LWSC65 | 0.000 | 0.000 | 0.314 *** | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| LWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.326** | 0.238 ** | |
| ShWSC75 | 0.000 | 0.000 | 0.615 *** | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| KnSq | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | −0.029 | |
| TKW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.587 *** | 0.514 *** | |
| HI | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.110 | |
| Indirect effects | CTD65 | 0.000 | 0.000 | −0.102 | 0.000 | 0.000 | 0.100 | 0.165 | 0.184 |
| Chl65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.079 | 0.094 | |
| Pn55 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.121 | 0.120 | |
| Pn75 | 0.000 | 0.000 | 0.349 *** | 0.000 | 0.000 | 0.000 | 0.462 *** | 0.440 *** | |
| StWSC65 | 0.000 | 0.000 | −0.127 | −0.208 ** | 0.000 | −0.217 ** | −0.169 | −0.278 ** | |
| LWSC65 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.103 | 0.087 | |
| LWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.036 | |
| ShWSC75 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.201** | 0.170 | |
| KnSq | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| TKW | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.065 | |
| HI | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zheng, B.; He, Y. Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes. Biology 2022, 11, 42. https://doi.org/10.3390/biology11010042
Zhang C, Zheng B, He Y. Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes. Biology. 2022; 11(1):42. https://doi.org/10.3390/biology11010042
Chicago/Turabian StyleZhang, Cong, Bangyou Zheng, and Yong He. 2022. "Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes" Biology 11, no. 1: 42. https://doi.org/10.3390/biology11010042
APA StyleZhang, C., Zheng, B., & He, Y. (2022). Improving Grain Yield via Promotion of Kernel Weight in High Yielding Winter Wheat Genotypes. Biology, 11(1), 42. https://doi.org/10.3390/biology11010042






