Implantable Cardioverter Defibrillator Multisensor Monitoring during Home Confinement Caused by the COVID-19 Pandemic
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HeartLogic Index
2.2. Design of Analysis
2.3. Statistical Analysis
3. Results
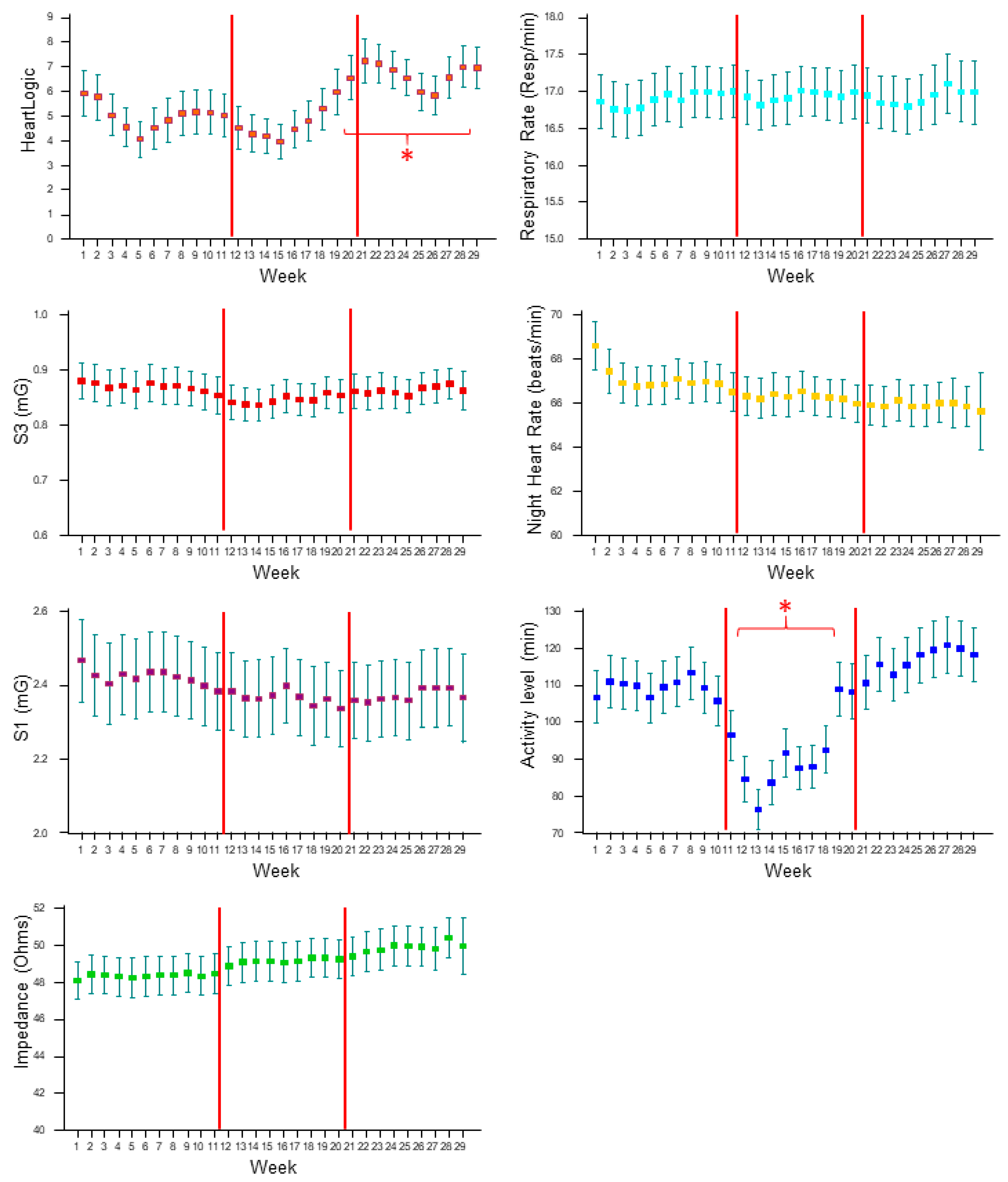
3.1. HeartLogic and Contributing Sensors Trends
3.2. HeartLogic Alerts, Characteristics and Management
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanganu-Bresch, C.; Zerbe, M.J.; Cutrufello, G.; Maci, S.M. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T.; Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2021, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Palmisano, P.; Guerra, F.; Ammendola, E.; Ziacchi, M.; Luigi Pisanò, E.C.; Dell’Era, G.; Aspromonte, V.; Zaccaria, M.; Di Ubaldo, F.; Capucci, A.; et al. Italian Association of Arrhythmology and Cardiac Pacing (AIAC). Physical Activity Measured by Implanted Devices Predicts Atrial Arrhythmias and Patient Outcome: Results of IMPLANTED (Italian Multicentre Observational Registry on Patients with Implantable Devices Remotely Monitored). J. Am. Heart Assoc. 2018, 7, e008146. [Google Scholar]
- Bhatt, A.S.; Moscone, A.; McElrath, E.E.; Varshney, A.S.; Claggett, B.L.; Bhatt, D.L.; Januzzi, J.L.; Butler, J.; Adler, D.S.; Solomon, S.D.; et al. Fewer Hospitalizations for Acute Cardiovascular Conditions during the COVID-19 Pandemic. J. Am. Coll Cardiol. 2020, 76, 280–288. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Angelini, F.; Bocchino, P.P.; Conrotto, F.; Saglietto, A.; Secco, G.G.; Campo, G.; Gallone, G.; Verardi, R.; et al. Reduced Rate of Hospital Admissions for ACS during COVID-19 Outbreak in Northern Italy. N. Engl. J. Med. 2020, 383, 88–89. [Google Scholar] [CrossRef]
- Boriani, G.; Palmisano, P.; Guerra, F.; Bertini, M.; Zanotto, G.; Lavalle, C.; Notarstefano, P.; Accogli, M.; Bisignani, G.; Forleo, G.B.; et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: Results of a survey promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern. Emerg. Med. 2020, 15, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Fulchand, S. COVID-19 and cardiovascular disease. BMJ 2020, 369, m1997. [Google Scholar] [CrossRef]
- Lakkireddy, D.R.; Chung, M.K.; Deering, T.F.; Gopinathannair, R.; Albert, C.M.; Epstein, L.M.; Harding, C.V.; Hurwitz, J.L.; Jeffery, C.C.; Krahn, A.D.; et al. Guidance for Rebooting Electrophysiology Through the COVID-19 Pandemic from the Heart Rhythm Society and the American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology: Endorsed by the American College of Cardiology. JACC Clin. Electrophysiol. 2020, 6, 1053–1066. [Google Scholar]
- Varma, N.; Marrouche, N.F.; Aguinaga, L.; Albert, C.M.; Arbelo, E.; Choi, J.I.; Chung, M.K.; Conte, G.; Dagher, L.; Epstein, L.M.; et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. J. Arrhythm. 2020, 36, 813–826. [Google Scholar] [CrossRef]
- De Simone, V.; Guardalben, S.; Guarise, P.; Padovani, N.; Giacopelli, D.; Zanotto, G. Home Monitoring trends during COVID-19 infection. J. Arrhythm. 2020, 37, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the, E.S.C. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Slotwiner, D.; Varma, N.; Akar, J.G.; Annas, G.; Beardsall, M.; Fogel, R.I.; Galizio, N.O.; Glotzer, T.V.; Leahy, R.A.; Love, C.J.; et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015, 12, e69–e100. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results from the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Vetrovsky, T.; Frybova, T.; Gant, I.; Semerad, M.; Cimler, R.; Bunc, V.; Siranec, M.; Miklikova, M.; Vesely, J.; Griva, M.; et al. The detrimental effect of COVID-19 nationwide quarantine on accelerometer-assessed physical activity of heart failure patients. ESC Heart Fail. 2020, 7, 2093–2097. [Google Scholar] [CrossRef]
- Calò, L.; Capucci, A.; Santini, L.; Pecora, D.; Favale, S.; Petracci, B.; Molon, G.; Bianchi, V.; Cipolletta, L.; De Ruvo, E.; et al. ICD-measured heart sounds and their correlation with echocardiographic indexes of systolic and diastolic function. J. Interv. Card. Electrophysiol. 2020, 58, 95–101. [Google Scholar] [CrossRef]
- Forleo, G.B.; Santini, L.; Campoli, M.; Malavasi, M.; Scaccia, A.; Menichelli, M.; Riva, U.; Lamberti, F.; Carreras, G.; Orazi, S.; et al. Long-term monitoring of respiratory rate in patients with heart failure: The Multiparametric Heart Failure Evaluation in Implantable Cardioverter-Defibrillator Patients (MULTITUDE-HF) study. J. Interv. Card. Electrophysiol. 2015, 43, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Wang, L.; Chau, E.; Chan, R.H.; Kong, S.L.; Tang, M.O.; Christensen, J.; Stadler, R.W.; Lau, C.P. Intrathoracic impedance monitoring in patients with heart failure: Correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 2005, 112, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Gardner, R.S.; Hariharan, R.; Nair, D.G.; Schulze, C.; An, Q.; Thakur, P.H.; Kwan, B.; Zhang, Y.; Boehmer, J.P. Ambulatory Monitoring of Heart Sounds via an Implanted Device Is Superior to Auscultation for Prediction of Heart Failure Events. J. Card. Fail. 2020, 26, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.; Borer, J.S.; Camm, A.J.; Danchin, N.; Ferrari, R.; Lopez Sendon, J.L.; Steg, P.G.; Tardif, J.C.; Tavazzi, L.; Tendera, M. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007, 50, 823–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.S.; Alvarez-Garcia, J.; Miller, M.A.; Moss, N.; Lala, A. Insights from HeartLogic Multisensor Monitoring During the COVID-19 Pandemic in New York City. JACC Heart Fail. 2020, 8, 1053–1055. [Google Scholar] [CrossRef]
- Conraads, V.M.; Tavazzi, L.; Santini, M.; Oliva, F.; Gerritse, B.; Yu, C.M.; Cowie, M.R. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: The SENSE-HF trial. Eur. Heart J. 2011, 32, 2266–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maines, M.; Landolina, M.; Lunati, M.; Lonardi, G.; Pappone, A.; Proclemer, A.; Zanotto, G.; Santini, M.; Varbaro, A.; Vimercati, M.; et al. Intrathoracic and ventricular impedances are associated with changes in ventricular volume in patients receiving defibrillators for, C.R.T. Pacing. Clin. Electrophysiol. 2010, 33, 64–73. [Google Scholar] [CrossRef]
- Ogura, A.; Izawa, K.P.; Tawa, H.; Kureha, F.; Wada, M.; Harada, N.; Ikeda, Y.; Kimura, K.; Kondo, N.; Kanai, M.; et al. Impact of the COVID-19 pandemic on phase 2 cardiac rehabilitation patients in Japan. Heart Vessels 2021, 36, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, C.J.; Thomas, G.; Middeldorp, M.E.; Harper, C.; Elliott, A.D.; Ray, N.; Lau, D.H.; Campbell, K.; Sanders, P. Ventricular arrhythmia burden during the coronavirus disease 2019 (COVID-19) pandemic. Eur. Heart J. 2021, 42, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Santini, L.; D’Onofrio, A.; Dello Russo, A.; Calò, L.; Pecora, D.; Favale, S.; Petracci, B.; Molon, G.; Bianchi, V.; De Ruvo, E.; et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin. Cardiol. 2020, 43, 691–697. [Google Scholar] [CrossRef]
- Capucci, A.; Santini, L.; Favale, S.; Pecora, D.; Petracci, B.; Calò, L.; Molon, G.; Cipolletta, L.; Bianchi, V.; Schirripa, V.; et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: A retrospective case series report. ESC Heart Fail. 2019, 6, 308–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calò, L.; Bianchi, V.; Ferraioli, D.; Santini, L.; Dello Russo, A.; Carriere, C.; Santobuono, V.E.; Andreoli, C.; La Greca, C.; Arena, G.; et al. Multiparametric Implantable Cardioverter-Defibrillator Algorithm for Heart Failure Risk Stratification and Management: An Analysis in Clinical Practice. Circ. Heart Fail. 2021, 14, e008134. [Google Scholar] [CrossRef]
- Treskes, R.W.; Beles, M.; Caputo, M.L.; Cordon, A.; Biundo, E.; Maes, E.; Egorova, A.D.; Schalij, M.J.; Van Bockstal, K.; Grazioli-Gauthier, L.; et al. Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Fail. 2021, 8, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- De Juan Bagudá, J.; Gavira Gómez, J.J.; Pachón Iglesias, M.; Cózar León, R.; Escolar Pérez, V.; González Fernández, Ó.; Rivas Gándara, N.; Goirigolzarri Artaza, J.; Díaz Molina, B.; Macías Gallego, A.; et al. Remote heart failure management using the HeartLogic algorithm. RE-HEART Registry. Rev. Esp. Cardiol. 2021. [Google Scholar] [CrossRef]
- Egolum, U.O.; Parikh, K.; Lekavich, C.; Wosik, J.; Frazier-Mills, C.; Fudim, M. Applications of the Multisensor HeartLogic Heart Failure Monitoring Algorithm during the COVID-19 Global Pandemic. JACC Case Rep. 2020, 2, 2265–2269. [Google Scholar] [CrossRef]

| Parameter | Total N = 349 |
|---|---|
| Male gender, n (%) | 283 (81) |
| Age, years | 69 ± 11 |
| Ischemic etiology, n (%) | 156 (45) |
| NYHA class | |
| − Class I, n (%) − Class II, n (%) − Class III, n (%) − Class IV, n (%) | 21 (6) 188 (54) 131 (37) 9 (3) |
| LV ejection fraction, % | 31 ± 8 |
| AF history, n (%) | 133 (38) |
| Valvular disease, n (%) | 63 (18) |
| Coronary artery disease, n (%) | 166 (48) |
| Diabetes, n (%) | 99 (28) |
| COPD, n (%) | 59 (17) |
| Chronic kidney disease, n (%) | 101 (29) |
| Hypertension, n (%) | 210 (60) |
| β-Blocker use, n (%) | 329 (94) |
| ACE-inhibitor, ARB or ARNI use, n (%) | 321 (92) |
| MRA use, n (%) | 209 (60) |
| Diuretic use, n (%) | 324 (93) |
| Antiarrhythmic use, n (%) | 84 (24) |
| Anticoagulant therapy use, n (%) | 142 (41) |
| Ivabradine use, n (%) | 28 (8) |
| CRT device, n (%) | 269 (77) |
| Primary prevention, n (%) | 329 (94) |
| Alerts, n | Rate [95% CI], Alerts/100 pt-Weeks | Alert Duration, Days | Maximum Index Value | Alerts with Actions | Remote Management | |
|---|---|---|---|---|---|---|
| Pre-lockdown (weeks 1–11) | 35 | 0.91 (0.64–1.27) | 47 (29–60) | 28 ± 11 | 11 (31%) | 31 (89%) |
| Lockdown (weeks 12–20) | 49 | 1.56 (1.15–2.06) | 39 (28–57) | 30 ± 15 | 11 (22%) | 44 (90%) |
| Post-lockdown (weeks 21–29) | 43 | 1.37 (0.99–1.84) | 36 (25–57) | 24 ± 12 | 12 (28%) | 38 (88%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziacchi, M.; Calò, L.; D’Onofrio, A.; Manzo, M.; Dello Russo, A.; Santini, L.; Giubilato, G.; Carriere, C.; Santobuono, V.E.; Savarese, G.; et al. Implantable Cardioverter Defibrillator Multisensor Monitoring during Home Confinement Caused by the COVID-19 Pandemic. Biology 2022, 11, 120. https://doi.org/10.3390/biology11010120
Ziacchi M, Calò L, D’Onofrio A, Manzo M, Dello Russo A, Santini L, Giubilato G, Carriere C, Santobuono VE, Savarese G, et al. Implantable Cardioverter Defibrillator Multisensor Monitoring during Home Confinement Caused by the COVID-19 Pandemic. Biology. 2022; 11(1):120. https://doi.org/10.3390/biology11010120
Chicago/Turabian StyleZiacchi, Matteo, Leonardo Calò, Antonio D’Onofrio, Michele Manzo, Antonio Dello Russo, Luca Santini, Giovanna Giubilato, Cosimo Carriere, Vincenzo Ezio Santobuono, Gianluca Savarese, and et al. 2022. "Implantable Cardioverter Defibrillator Multisensor Monitoring during Home Confinement Caused by the COVID-19 Pandemic" Biology 11, no. 1: 120. https://doi.org/10.3390/biology11010120
APA StyleZiacchi, M., Calò, L., D’Onofrio, A., Manzo, M., Dello Russo, A., Santini, L., Giubilato, G., Carriere, C., Santobuono, V. E., Savarese, G., La Greca, C., Arena, G., Talarico, A., Pisanò, E., Giammaria, M., Pangallo, A., Campari, M., Valsecchi, S., & Diemberger, I. (2022). Implantable Cardioverter Defibrillator Multisensor Monitoring during Home Confinement Caused by the COVID-19 Pandemic. Biology, 11(1), 120. https://doi.org/10.3390/biology11010120









