Konjac Ceramide (kCer)-Mediated Signal Transduction of the Sema3A Pathway Promotes HaCaT Keratinocyte Differentiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
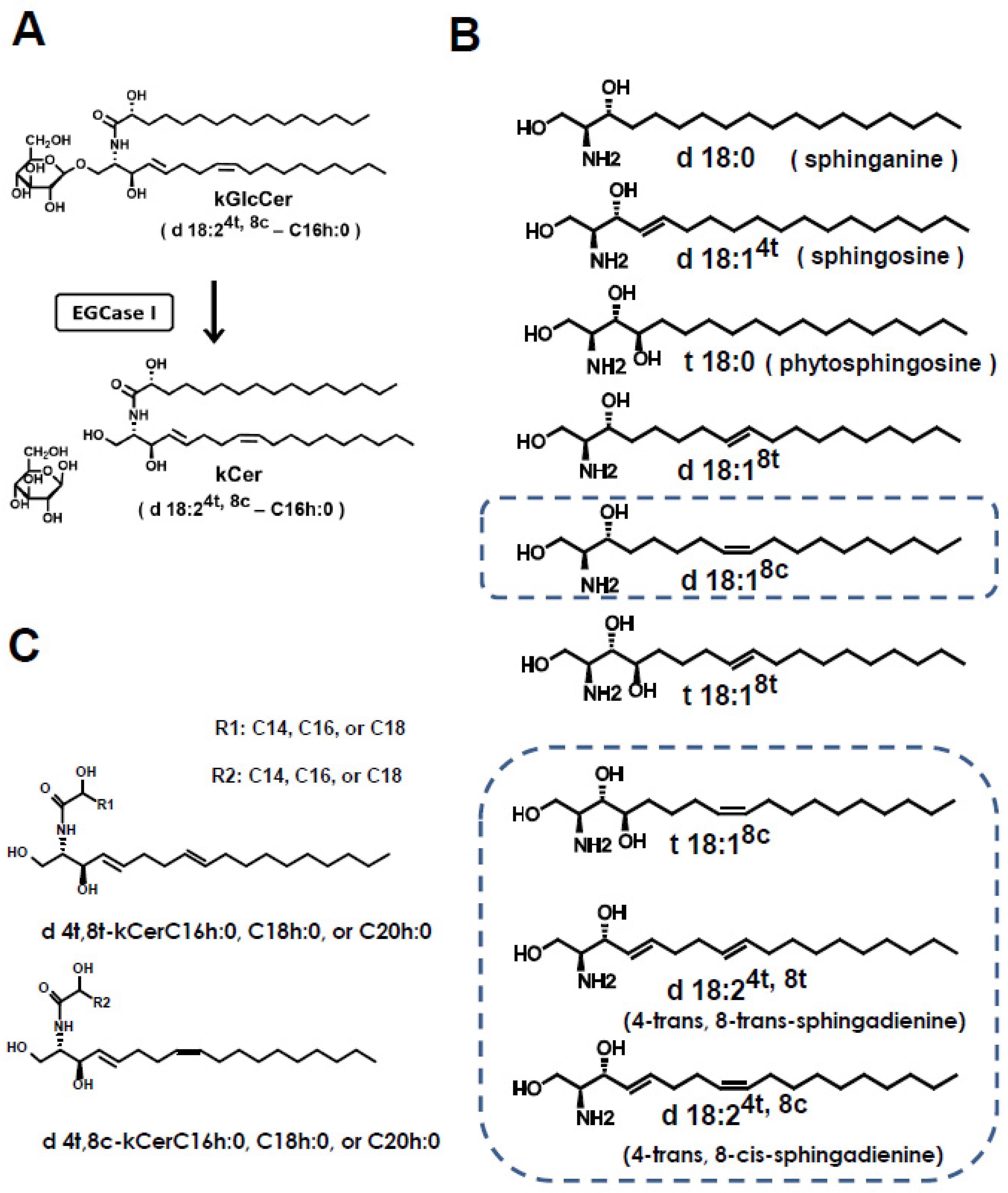
2.2. kCer Preparation
2.3. Short-Term L-Ca Medium HaCaT Migration Model
2.4. Long-Term L-Ca Medium HaCaT Differentiation Model
2.5. RNA Interference and Transfection
2.6. Western Blot Analysis
2.7. Rac1 and RhoA Activation Assay
2.8. [3.H] Dihydrosphingosine (DHS) Labeling Assay
2.9. LC-MS/MS Analysis
2.10. Quantitative RT-PCR (qPCR)
2.11. Statistical Analysis
3. Results
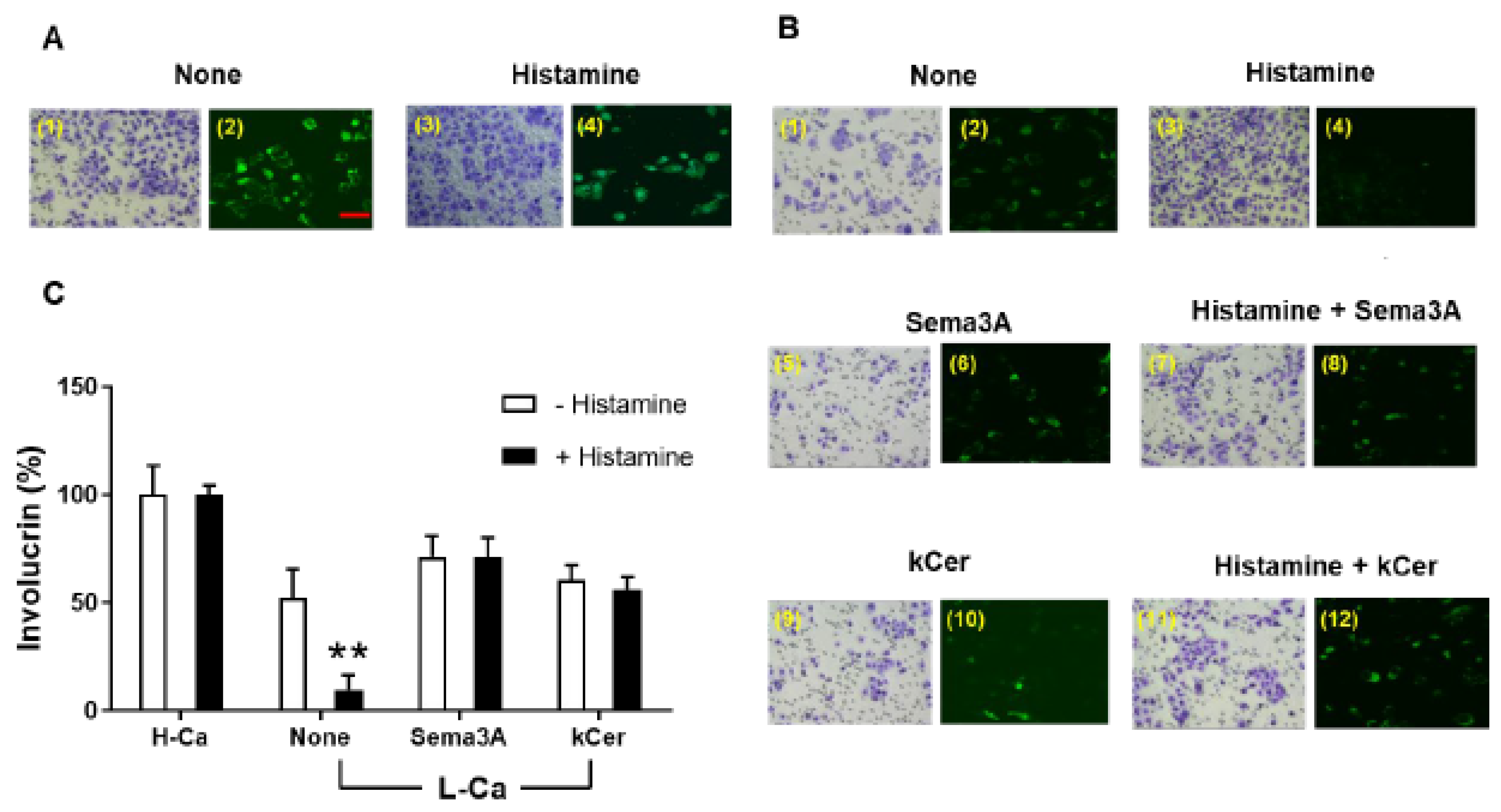
3.1. kCer-Induced HaCaT Cell Migration and Differentiation
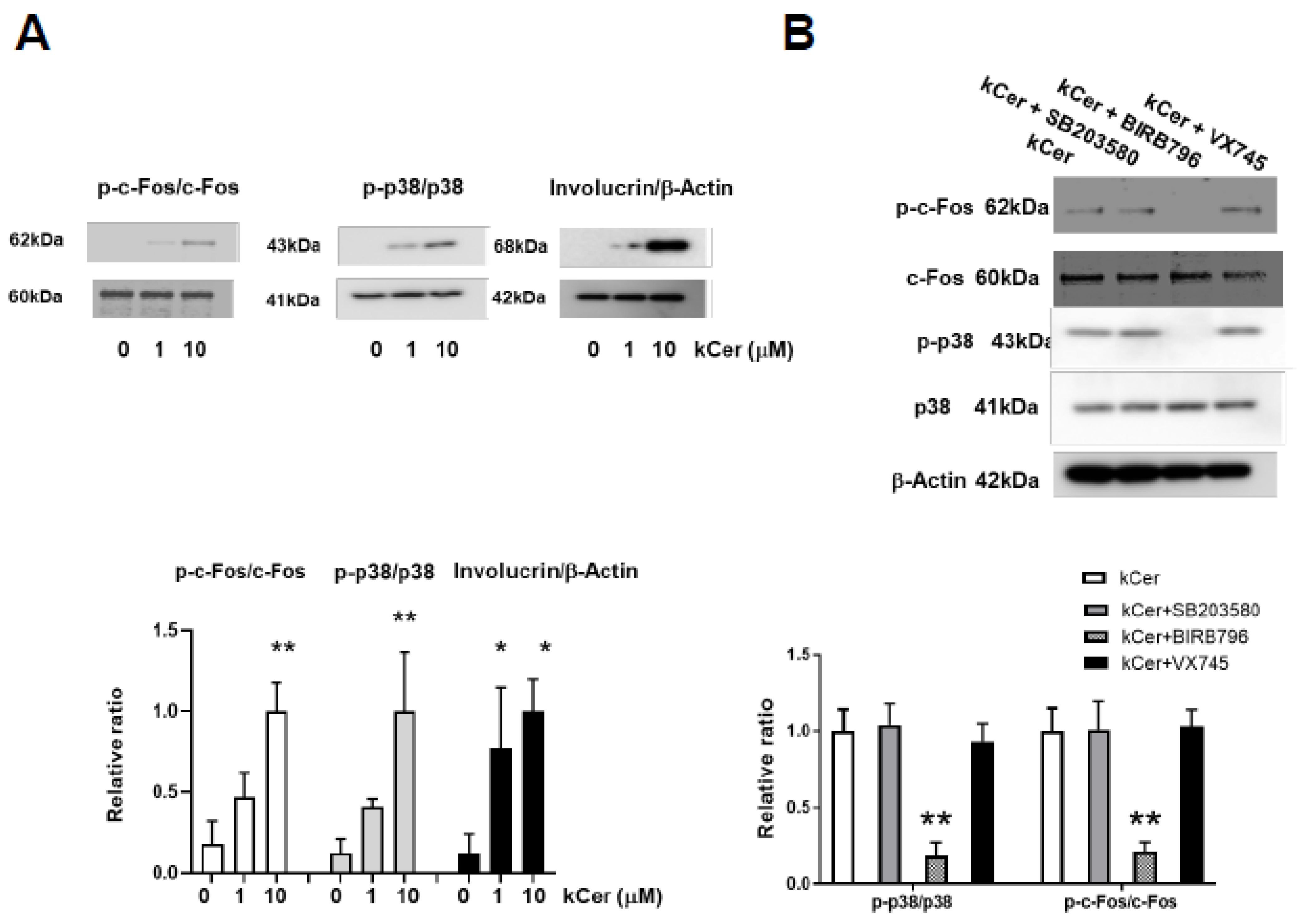
3.2. kCer Activation of Cell Differentiation Pathways
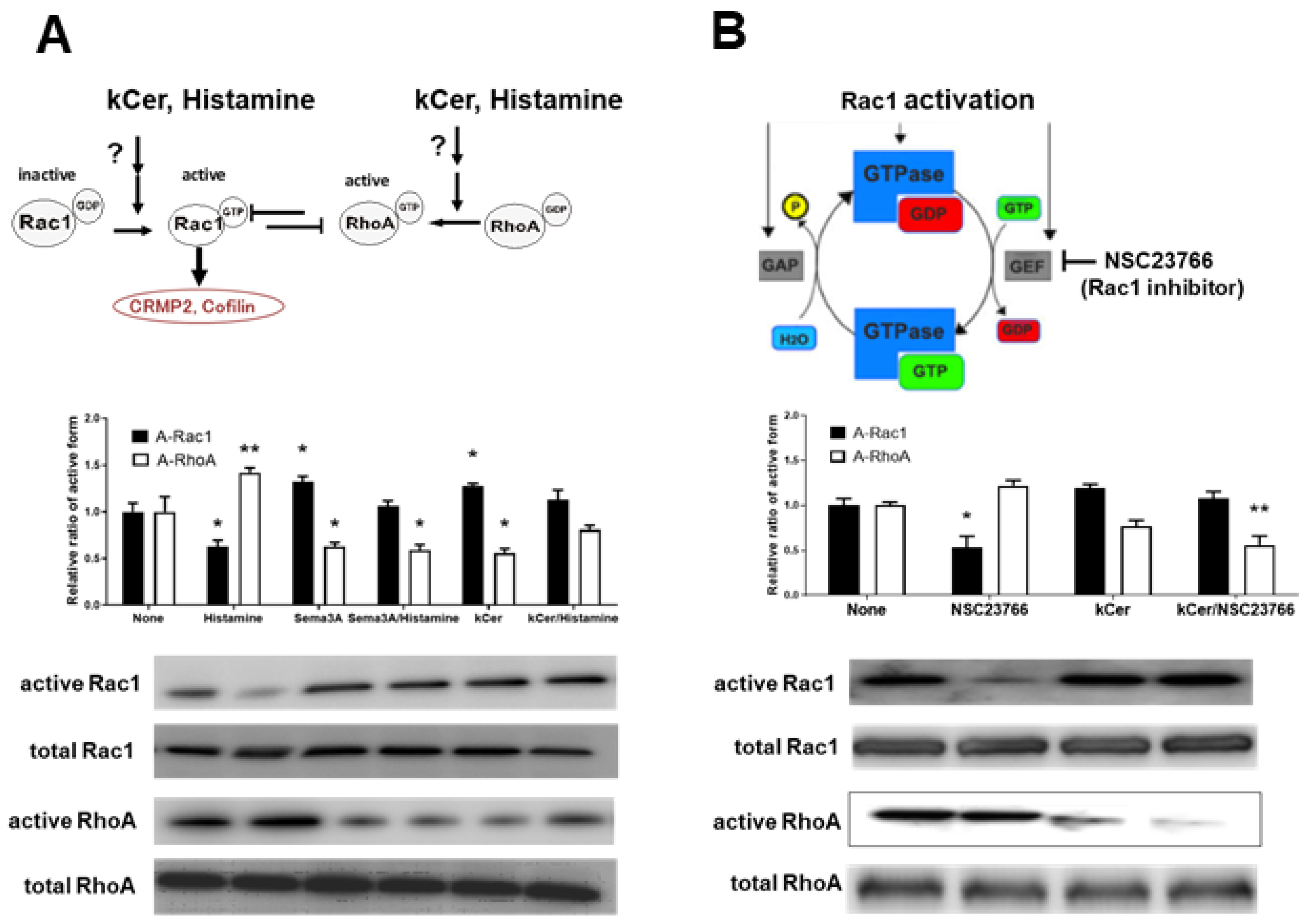
3.3. Effect of kCer on the Balance of Rac1/RhoA Activities
3.4. Effect of kCer on Histamine-Induced RhoA Activation
3.5. Effect of Cell Differentiation on Sphingolipid Metabolism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NGF | nerve growth factor |
| Cer | ceramide |
| GlcCer | glucosylceramide |
| kCer | konjac ceramide |
| CMH | ceramide monohexoside |
| EGCase I | endoglycoceramidase I |
| CRMP2 | collapsin response mediator protein 2 |
| p-CRMP2 | phospho-collapsin response mediator protein 2 |
| Sema3A | semaphorin 3A |
| Nrp1 | neuropilin1 |
| GPCR | G-protein coupled receptor |
| H1R | histamine-activated G-protein coupled receptor 1 |
| H4R | histamine-activated G-protein coupled receptor 4 |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| SM | sphingomyelin |
| Rac1 | Ras-related C3 botulinus toxin substrate 1 |
| RhoA | Ras homology family member A |
| p38MAPK | p38 mitogen-activated protein kinase |
| PKC | protein kinase C |
| PLC | phospholipase C |
| IDR | intrinsically disordered region |
References
- Choi, S.M.; Lee, B.M. Safety and risk assessment of ceramide 3 in cosmetic products. Food Chem. Toxicol. 2015, 84, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Thudichum, J.L.W. The Chemistry of the Brain; Bailliere Tindall Cox: London, UK, 1884; pp. 1829–1901. [Google Scholar]
- Gronnier, J.; Germain, V.; Gouguet, P.; Cacas, J.L.; Mongrand, S. GIPC: Glycosyl Inositol Phospho Ceramides, the major sphingolipids on earth. Plant Signal. Behav. 2016, 11, e1152438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuki, S.; Tamura, N.; Sakai, S.; Tamura, T.; Mukai, K.; Igarashi, Y. Chemoenzymatically preapred konjac ceramide inhibits NGF-induced neurite outgrowth by a semaphorin 3A-like action. Biochem. Biophys. Rep. 2016, 5, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Usuki, S.; Yasutake, Y.; Tamura, N.; Tamura, T.; Tanji, K.; Saitoh, T.; Murai, Y.; Mikami, D.; Yuyama, K.; Monde, K.; et al. Nrp1 is Activated by Konjac Ceramide Binding-Induced Structural Rigidification of the a1a2 Domain. Cells 2020, 9, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral Intake of Glucosylceramide Improves Relatively High level of Transepideramal Water Loss in MIce and Healthy Human Subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, Y.; Kobayashi, U.; Hijikata, A.; Sakaguchi, K.; Goda, H.M.; Tamura, T.; Okino, N.; Ito, M. Preparation and characterization of EGCase I, applicable to the comprehensive analysis of GSLs, using a rhodococcal expression system. J. Lipid Res. 2012, 53, 2242–2251. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, N.; Thoenen, H.; Lindholm, D. TrkA tyrosine residues involved in NGF-induced neurite outgrowth of PC12 cells. Eur. J. Neurosci. 1995, 7, 1125–1133. [Google Scholar] [CrossRef]
- Usuki, S.; Tamura, N.; Tamura, T.; Mukai, K.; Igarashi, Y. Characterization of Konjac Ceramide (kCer) Binding to Sema3A Receptor Nrp1. J. Oleo Sci. 2018, 67, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Usuki, S.; Tamura, N.; Yuyama, K.; Tamura, T.; Mukai, K.; Igarashi, Y. Konjac Ceramide (kCer) Regulates NGF-Induced Neurite Outgrowth via the Sema3A Signaling Pathway. J. Oleo Sci. 2018, 67, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Riese, A.; Eilert, Y.; Meyer, Y.; Arin, M.; Baron, J.M.; Eming, S.; Krieg, T.; Kurschat, P. Epidermal expression of neuropilin 1 protects murine keratinocytes from UVB-induced apoptosis. PLoS ONE 2012, 7, e50944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usuki, S.; Tamura, N.; Tamura, T.; Higashiyama, S.; Tanji, K.; Mitsutake, S.; Inoue, A.; Aoki, J.; Mukai, K.; Igarashi, Y. Konjac ceramide (kCer) regulates keratinocyte migration by Sema3A-like repulsion mechanism. Biochem. Biophys. Rep. 2019, 17, 132–138. [Google Scholar] [CrossRef]
- Shahrabi-Farahani, S.; Wang, L.; Zwaans, B.M.; Santana, J.M.; Shimizu, A.; Takashima, S.; Kreuter, M.; Coultas, L.; D’Amore, P.A.; Arbeit, J.M.; et al. Neuropilin 1 expression correlates with differentiation status of epidermal cells and cutaneous squamous cell carcinomas. Lab. Investig. 2014, 94, 752–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gschwandtner, M.; Mildner, M.; Mlitz, V.; Gruber, F.; Eckhart, L.; Werfel, T.; Gutzmer, R.; Elias, P.M.; Tschachler, E. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy 2013, 68, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Deyrieux, A.F.; Wilson, V.G. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology 2007, 54, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Fujimoto, S.; Uratsuji, H.; Saeki, H.; Kagami, S.; Tsunemi, Y.; Komine, M.; Tamaki, K. CCR4 and CCR10 are expressed on epidermal keratinocytes and are involved in cutaneous immune reaction. Cytokine 2008, 44, 172–178. [Google Scholar] [CrossRef]
- Nakahara, K.; Ohkuni, A.; Kitamura, T.; Abe, K.; Naganuma, T.; Ohno, Y.; Zoeller, R.A.; Kihara, A. The Sjogren-Larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Mol. Cell 2012, 46, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Mikami, D.; Sakai, S.; Nishimukai, M.; Yuyama, K.; Mukai, K.; Igarashi, Y. Structure-dependent absorption of atypical sphingoid long-chain bases from digestive tract into lymph. Lipids Health Dis. 2021, 20, 24. [Google Scholar] [CrossRef]
- Bolado-Carrancio, A.; Rukhlenko, O.S.; Nikonova, E.; Tsyganov, M.A.; Wheeler, A.; Garcia-Munoz, A.; Kolch, W.; von Kriegsheim, A.; Kholodenko, B.N. Periodic propagating waves coordinate RhoGTPase network dynamics at the leading and trailing edges during cell migration. Elife 2020, 9, e58165. [Google Scholar] [CrossRef]
- Jackson, B.; Peyrollier, K.; Pedersen, E.; Basse, A.; Karlsson, R.; Wang, Z.; Lefever, T.; Ochsenbein, A.M.; Schmidt, G.; Aktories, K.; et al. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol. Biol. Cell 2011, 22, 593–605. [Google Scholar] [CrossRef]
- Pillai, S.; Bikle, D.D.; Mancianti, M.L.; Cline, P.; Hincenbergs, M. Calcium regulation of growth and differentiation of normal human keratinocytes: Modulation of differentiation competence by stages of growth and extracellular calcium. J. Cell Physiol. 1990, 143, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]
- Mehic, D.; Bakiri, L.; Ghannadan, M.; Wagner, E.F.; Tschachler, E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J. Investig. Dermatol. 2005, 124, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Qiu, L.; Song, H.; Dang, N. MAPK Pathway Involved in Epidermal Terminal Differentiation of Normal Human Epidermal Keratinocytes. Open Med. 2018, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kurschat, P.; Bielenberg, D.; Rossignol-Tallandier, M.; Stahl, A.; Klagsbrun, M. Neuron restrictive silencer factor NRSF/REST is a transcriptional repressor of neuropilin-1 and diminishes the ability of semaphorin 3A to inhibit keratinocyte migration. J. Biol. Chem. 2006, 281, 2721–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.; Cao, Y.; Xing, F.; Tao, M.; Fu, H.; Luo, H.; Yang, X. A Preliminary Study of the Effect of Semaphorin 3A and Acitretin on the Proliferation, Migration, and Apoptosis of HaCaT Cells. Indian J. Dermatol. 2019, 64, 250. [Google Scholar] [CrossRef] [PubMed]
- Kamata, Y.; Tominaga, M.; Umehara, Y.; Honda, K.; Kamo, A.; Moniaga, C.S.; Komiya, E.; Toyama, S.; Suga, Y.; Ogawa, H.; et al. Calcium-Inducible MAPK/AP-1 Signaling Drives Semaphorin 3A Expression in Normal Human Epidermal Keratinocytes. J. Investig. Dermatol. 2020, 140, 1346–1354.e1345. [Google Scholar] [CrossRef]
- Ritto, D.; Tanasawet, S.; Singkhorn, S.; Klaypradit, W.; Hutamekalin, P.; Tipmanee, V.; Sukketsiri, W. Astaxanthin induces migration in human skin keratinocytes via Rac1 activation and RhoA inhibition. Nutr. Res. Pract. 2017, 11, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Kanda, N.; Watanabe, S. Histamine enhances the production of nerve growth factor in human keratinocytes. J. Investig. Dermatol. 2003, 121, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.H.; Liao, S.L.; Wang, S.H.; Wang, C.C.; Yang, C.H. Simvastatin and ROCK Inhibitor Y-27632 Inhibit Myofibroblast Differentiation of Graves’ Ophthalmopathy-Derived Orbital Fibroblasts via RhoA-Mediated ERK and p38 Signaling Pathways. Front. Endocrinol. 2020, 11, 607968. [Google Scholar] [CrossRef]
- Bishop, A.L.; Hall, A. Rho GTPases and their effector proteins. Biochem. J. 2000, 348 Pt 2, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.K.; Lou, M.; Zheng, Y.; Lang, R.A. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. Proc. Natl. Acad. Sci. USA 2011, 108, 18289–18294. [Google Scholar] [CrossRef] [Green Version]
- Papp, H.; Czifra, G.; Lazar, J.; Gonczi, M.; Csernoch, L.; Kovacs, L.; Biro, T. Protein kinase C isozymes regulate proliferation and high cell density-mediated differentiation in HaCaT keratinocytes. Exp. Dermatol. 2003, 12, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Dashti, S.R.; Efimova, T.; Eckert, R.L. MEK7-dependent activation of p38 MAP kinase in keratinocytes. J. Biol. Chem. 2001, 276, 8059–8063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonak, C.; Mildner, M.; Klosner, G.; Paulitschke, V.; Kunstfeld, R.; Pehamberger, H.; Tschachler, E.; Trautinger, F. The hsp27kD heat shock protein and p38-MAPK signaling are required for regular epidermal differentiation. J. Dermatol. Sci. 2011, 61, 32–37. [Google Scholar] [CrossRef]
- Eckert, R.L.; Efimova, T.; Dashti, S.R.; Balasubramanian, S.; Deucher, A.; Crish, J.F.; Sturniolo, M.; Bone, F. Keratinocyte survival, differentiation, and death: Many roads lead to mitogen-activated protein kinase. J. Investig. Dermatol. Symp. Proc. 2002, 7, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Cursons, J.; Gao, J.; Hurley, D.G.; Print, C.G.; Dunbar, P.R.; Jacobs, M.D.; Crampin, E.J. Regulation of ERK-MAPK signaling in human epidermis. BMC Syst. Biol. 2015, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Efimova, T.; Broome, A.M.; Eckert, R.L. A regulatory role for p38 delta MAPK in keratinocyte differentiation. Evidence for p38 delta-ERK1/2 complex formation. J. Biol. Chem. 2003, 278, 34277–34285. [Google Scholar] [CrossRef] [Green Version]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [Green Version]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Pang, H.B.; Braun, G.B.; Friman, T.; Aza-Blanc, P.; Ruidiaz, M.E.; Sugahara, K.N.; Teesalu, T.; Ruoslahti, E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5, 4904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
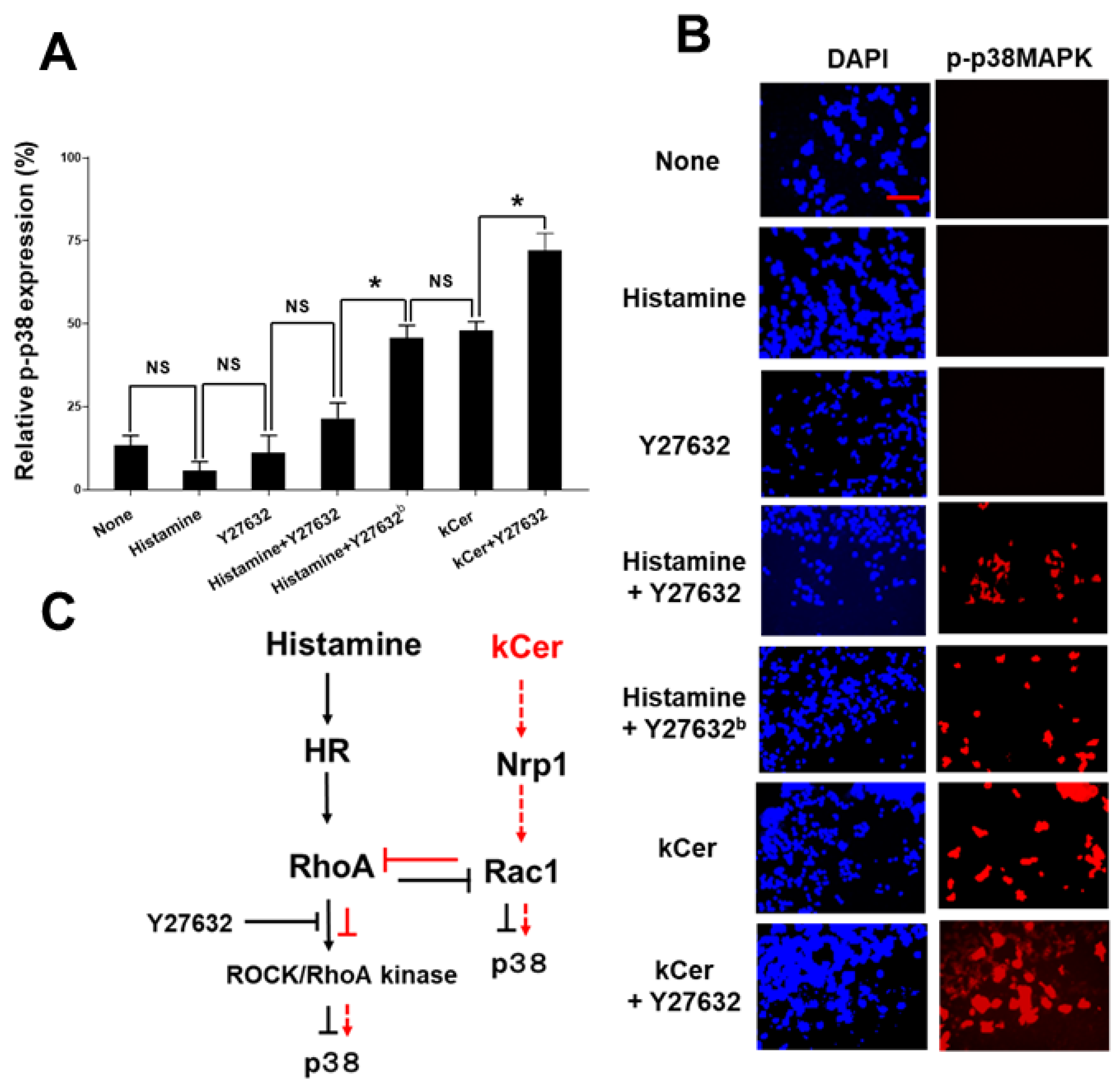
 , suppression via histamine.
, suppression via histamine.
 , suppression via histamine.
, suppression via histamine.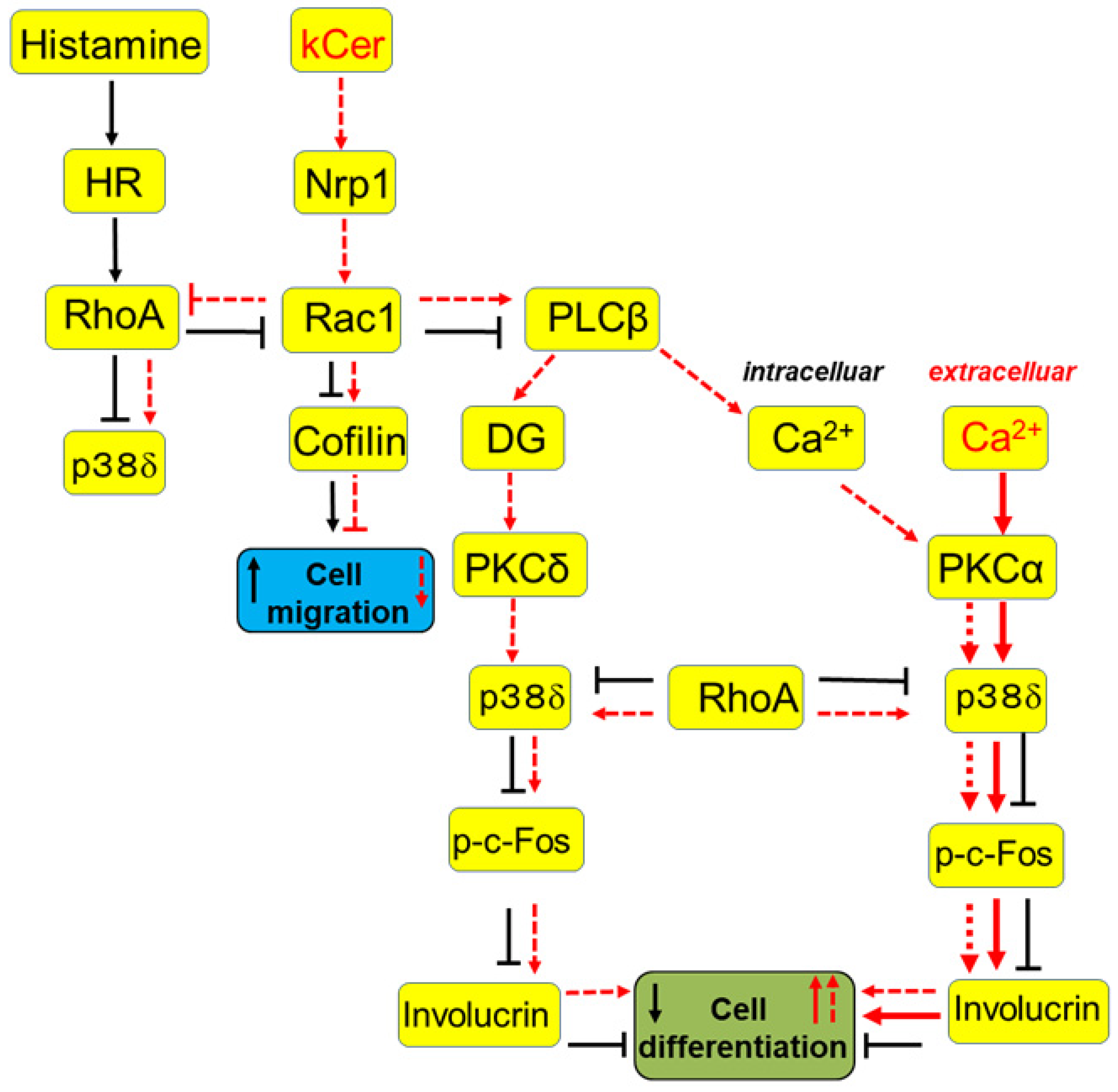
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usuki, S.; Tamura, N.; Tamura, T.; Yuyama, K.; Mikami, D.; Mukai, K.; Igarashi, Y. Konjac Ceramide (kCer)-Mediated Signal Transduction of the Sema3A Pathway Promotes HaCaT Keratinocyte Differentiation. Biology 2022, 11, 121. https://doi.org/10.3390/biology11010121
Usuki S, Tamura N, Tamura T, Yuyama K, Mikami D, Mukai K, Igarashi Y. Konjac Ceramide (kCer)-Mediated Signal Transduction of the Sema3A Pathway Promotes HaCaT Keratinocyte Differentiation. Biology. 2022; 11(1):121. https://doi.org/10.3390/biology11010121
Chicago/Turabian StyleUsuki, Seigo, Noriko Tamura, Tomohiro Tamura, Kohei Yuyama, Daisuke Mikami, Katsuyuki Mukai, and Yasuyuki Igarashi. 2022. "Konjac Ceramide (kCer)-Mediated Signal Transduction of the Sema3A Pathway Promotes HaCaT Keratinocyte Differentiation" Biology 11, no. 1: 121. https://doi.org/10.3390/biology11010121
APA StyleUsuki, S., Tamura, N., Tamura, T., Yuyama, K., Mikami, D., Mukai, K., & Igarashi, Y. (2022). Konjac Ceramide (kCer)-Mediated Signal Transduction of the Sema3A Pathway Promotes HaCaT Keratinocyte Differentiation. Biology, 11(1), 121. https://doi.org/10.3390/biology11010121





