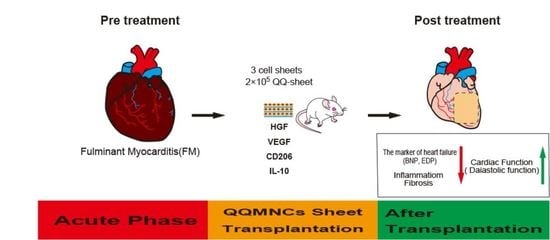
Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
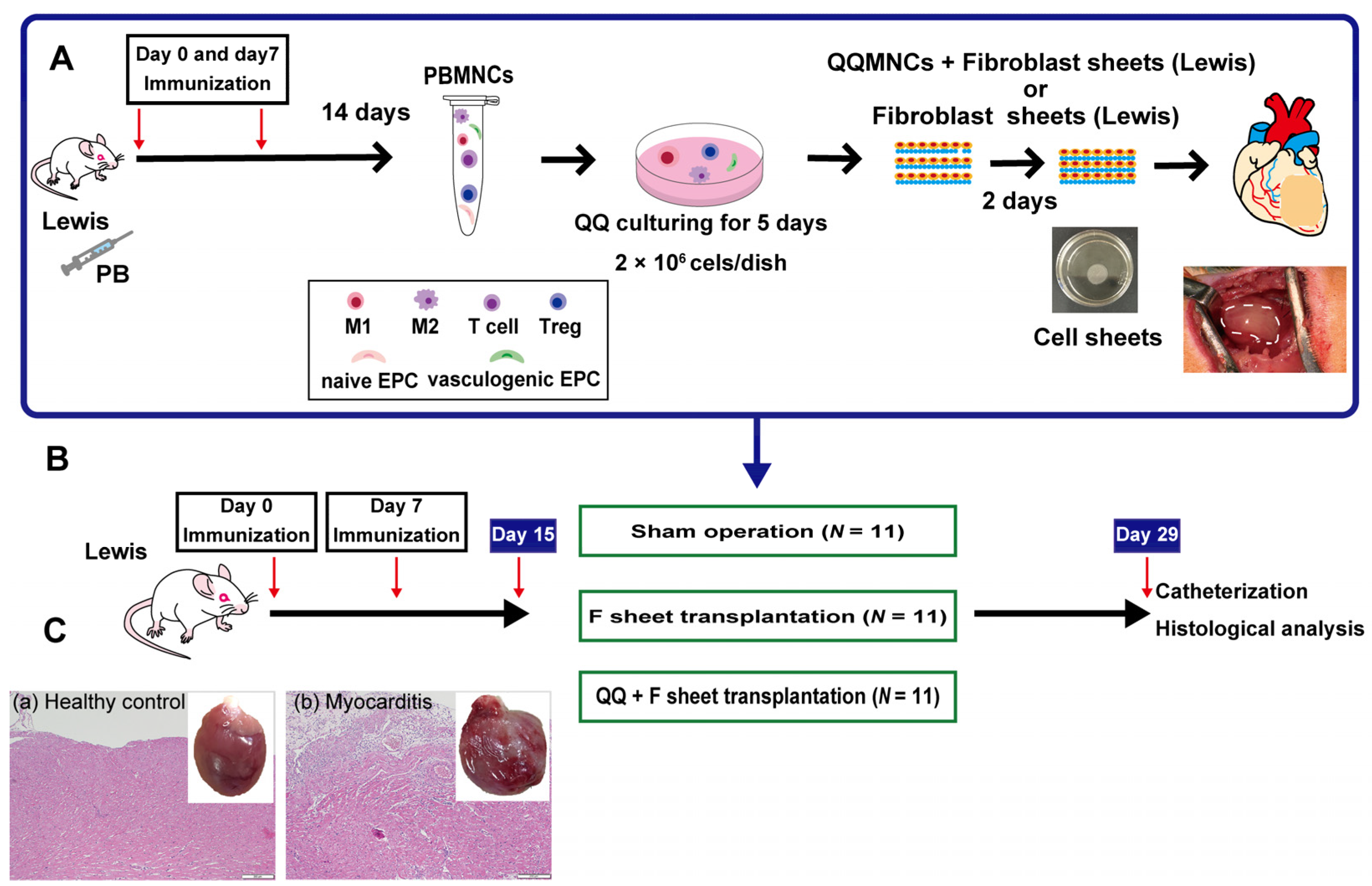
2.1. Study Protocols
2.2. Construction of Rat EAM Model
2.3. PBMNC Isolation and QQ Culture
2.3.1. EPC Colony Formation Assay
2.3.2. Flow Cytometric Analysis
2.4. Preparation of F, M+F and QQ+F Sheets
qRT-PCR
2.5. Transplantation of F and QQ+F Sheets
2.5.1. Cardiac Catheterization
2.5.2. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. QQMNCs in EAM Rats Retained the EPCs Colony-Forming Potential Similar to That in Healthy Rats
3.2. The Restored Cellular Phenotype of QQMNCs Obtained from EAM Rats Was Similar to That from Healthy Rats
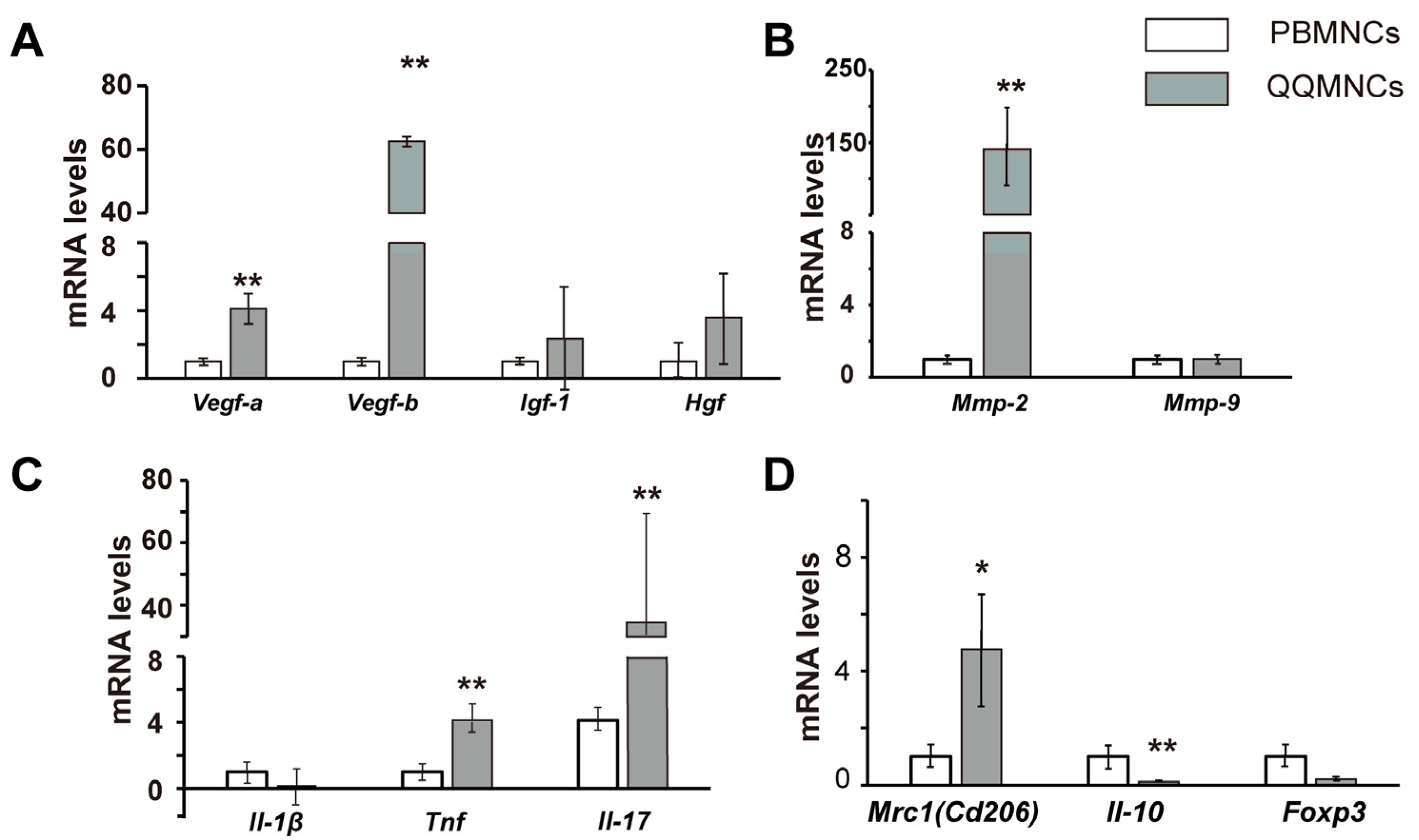
3.3. The Profiles of Gene Expression of Sheet-Free QQMNCs in EAM Rats
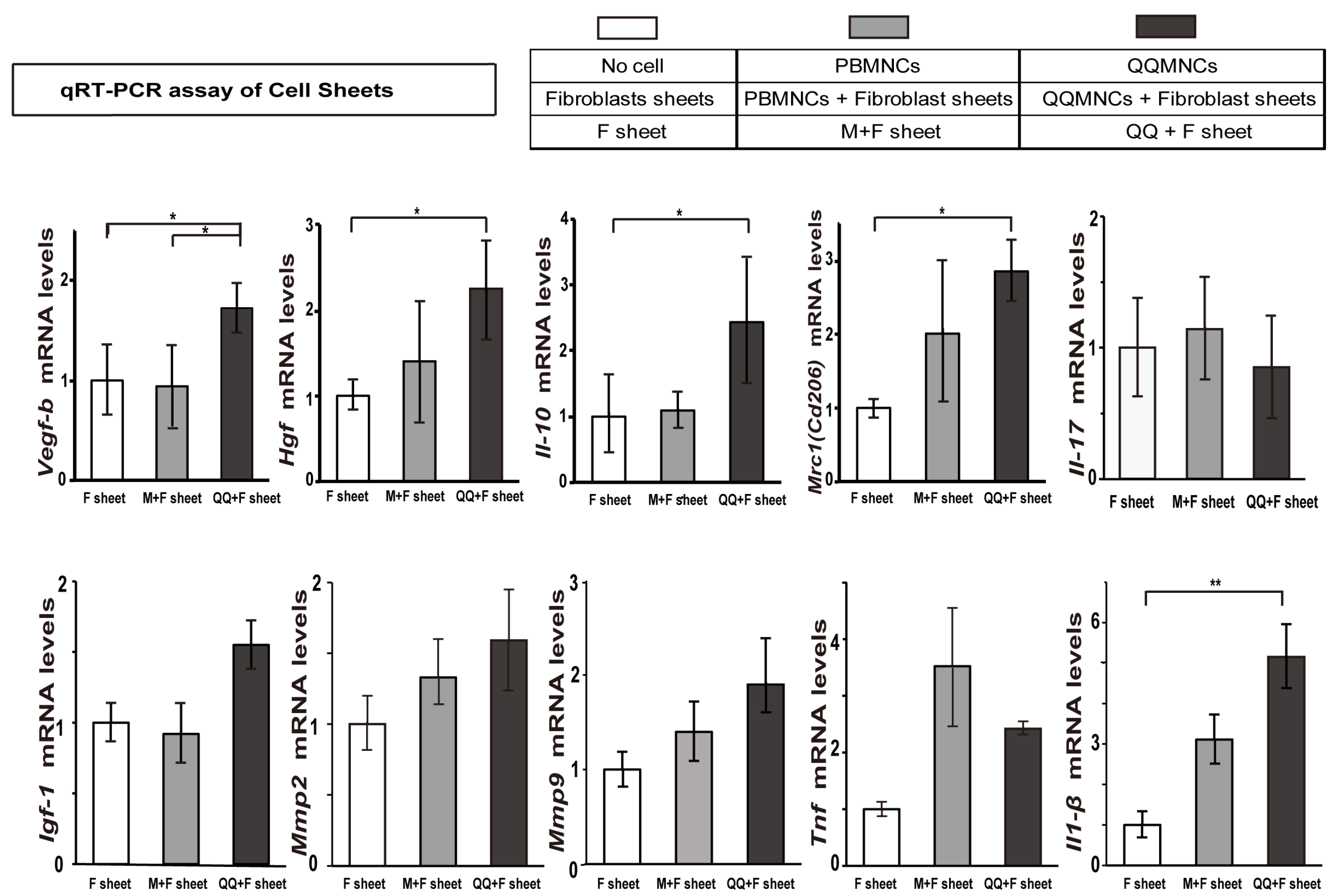
3.4. Favorable Conversion of Gene Expression Profiles in QQ+F Sheet against EAM
3.5. QQ+F Sheets Transplantation Maintained Healthy Diastolic Cardiac Function
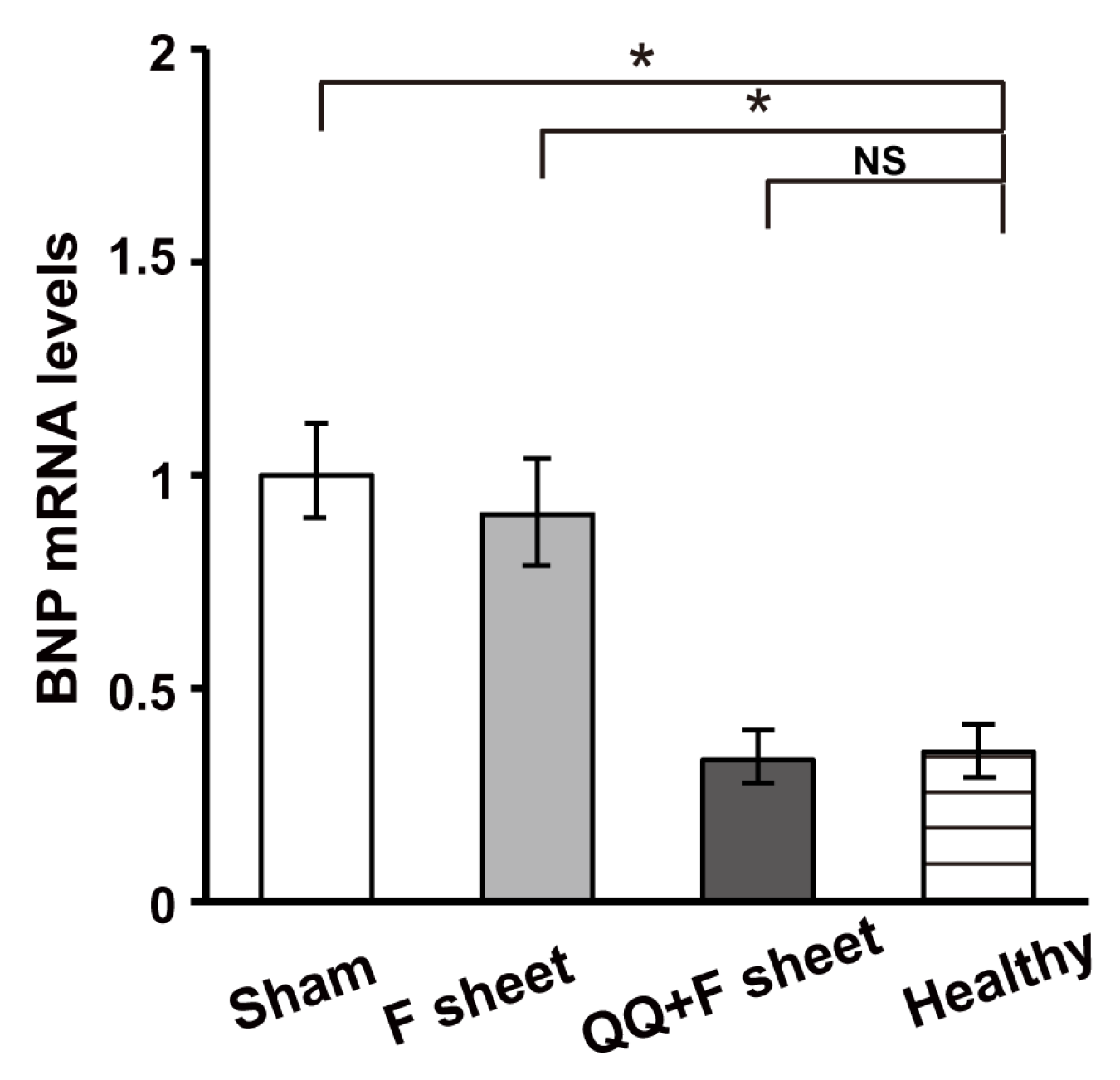
3.6. QQ+F Sheets Grafts in EAM Hearts Inhibited the Bnp Gene Expression Indicating HF
3.7. QQ+F Sheets Grafts Limited LV Remodeling in EAM Hearts
4. Discussion
4.1. Boosted Vascular Regenerative and Anti-Inflammatory Cellular Phenotypes of QQMNCs from EAM Rats
4.2. Manifested Cardioprotective Gene Expression of QQ+F Sheet in EAM Rats
4.3. Favorable Cardioprotective Efficacy of QQ+F Sheets Grafting in EAM Hearts
4.4. Limitation of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veronese, G.; Ammirati, E.; Cipriani, M.; Frigerio, M. Fulminant myocarditis: Characteristics, treatment, and outcomes. Anatol. J. Cardiol. 2018, 199, 279–286. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Sharma, A.N.; Stultz, J.R.; Bellamkonda, N.; Amsterdam, E.A. Fulminant Myocarditis: Epidemiology, Pathogenesis, Diagnosis, and Management. Am. J. Cardiol. 2019, 124, 1954–1960. [Google Scholar] [CrossRef]
- Michler, R.E. Stem cell therapy for heart failure. Cardiol. Rev. 2014, 22, 105–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, G.Y.; Klug, M.G.; Soonpaa, M.H.; Field, L.J. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J. Clin. Investig. 1993, 92, 1548–1554. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Stem cell therapy in heart failure: Where do we stand today? Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165489. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into Endothelial Progenitor Cells: Origin, Classification, Potentials, and Prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Monsanto, M.M.; Wang, B.J.; Ehrenberg, Z.R.; Echeagaray, O.; White, K.S.; Alvarez, R., Jr.; Fisher, K.; Sengphanith, S.; Muliono, A.; Gude, N.A.; et al. Enhancing myocardial repair with CardioClusters. Nat. Commun. 2020, 11, 3955. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Lee, J.Y.; Nguyen, H.; Kaneko, Y.; Borlongan, C.V. Endothelial Progenitor Cells Modulate Inflammation-Associated Stroke Vasculome. Stem Cell Rev. Rep. 2019, 15, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, A.; Soler, M.J.; Martinez-Estrada, O.M.; Vazquez, S.; Marco-Feliu, D.; Vila, J.S.; Vilaro, S.; Lloveras, J. Endothelial progenitor cells are reduced in refractory hypertension. J. Hum. Hypertens. 2008, 22, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tobler, K.; Freudenthaler, A.; Baumgartner-Parzer, S.M.; Wolzt, M.; Ludvik, B.; Nansalmaa, E.; Nowotny, P.J.; Seidinger, D.; Steiner, S.; Luger, A.; et al. Reduction of both number and proliferative activity of human endothelial progenitor cells in obesity. Int. J. Obes. 2010, 34, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Tanaka, R.; Fujimura, S.; Ishikawa, M.; Akimaru, H.; Shizuno, T.; Sato, A.; Okada, Y.; Iida, Y.; Itoh, J.; et al. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J. Am. Heart Assoc. 2014, 3, e000743. [Google Scholar] [CrossRef]
- Mifuji, K.; Ishikawa, M.; Kamei, N.; Tanaka, R.; Arita, K.; Mizuno, H.; Asahara, T.; Adachi, N.; Ochi, M. Angiogenic conditioning of peripheral blood mononuclear cells promotes fracture healing. Bone Jt. Res 2017, 6, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Salybekov, A.A.; Kawaguchi, A.T.; Masuda, H.; Vorateera, K.; Okada, C.; Asahara, T. Regeneration-associated cells improve recovery from myocardial infarction through enhanced vasculogenesis, anti-inflammation, and cardiomyogenesis. PLoS ONE 2018, 13, e0203244. [Google Scholar] [CrossRef]
- Tanaka, R.; Ito-Hirano, R.; Fujimura, S.; Arita, K.; Hagiwara, H.; Mita, T.; Itoh, M.; Kawaji, H.; Ogawa, T.; Watada, H.; et al. Ex vivo conditioning of peripheral blood mononuclear cells of diabetic patients promotes vasculogenic wound healing. Stem Cells Transl. Med. 2021, 10, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Kashiyama, N.; Miyagawa, S.; Fukushima, S.; Kawamura, T.; Kawamura, A.; Yoshida, S.; Nakamura, Y.; Harada, A.; Masuda, H.; Toda, K.; et al. Vasculogenically conditioned peripheral blood mononuclear cells inhibit mouse immune response to induced pluripotent stem cell-derived allogeneic cardiac grafts. PLoS ONE 2019, 14, e0217076. [Google Scholar] [CrossRef]
- Guo, R.; Morimatsu, M.; Feng, T.; Lan, F.; Chang, D.; Wan, F.; Ling, Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res. 2020, 11, 19. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Okano, T. Cell Sheet Tissue Engineering for Heart Failure. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016; pp. 19–24. [Google Scholar] [CrossRef]
- Imanishi, Y.; Miyagawa, S.; Maeda, N.; Fukushima, S.; Kitagawa-Sakakida, S.; Daimon, T.; Hirata, A.; Shimizu, T.; Okano, T.; Shimomura, I.; et al. Induced adipocyte cell-sheet ameliorates cardiac dysfunction in a mouse myocardial infarction model: A novel drug delivery system for heart failure. Circulation 2011, 124, S10–S17. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Funakoshi, S.; Yoshida, Y.; Yamanaka, S.; Shimizu, T.; Okano, T.; et al. Enhanced Therapeutic Effects of Human iPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci. Rep. 2017, 7, 8824. [Google Scholar] [CrossRef]
- Kawamura, M.; Paulsen, M.J.; Goldstone, A.B.; Shudo, Y.; Wang, H.; Steele, A.N.; Stapleton, L.M.; Edwards, B.B.; Eskandari, A.; Truong, V.N.; et al. Tissue-engineered smooth muscle cell and endothelial progenitor cell bi-level cell sheets prevent progression of cardiac dysfunction, microvascular dysfunction, and interstitial fibrosis in a rodent model of type 1 diabetes-induced cardiomyopathy. Cardiovasc. Diabetol. 2017, 16, 142. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shimizu, T.; Yamato, M.; Tono, K.; Masuda, H.; Asahara, T.; Kasanuki, H.; Okano, T. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J. Artif. Organs Off. J. Jpn. Soc. Artif. Organs 2008, 11, 141–147. [Google Scholar] [CrossRef]
- Kodama, M.; Matsumoto, Y.; Fujiwara, M.; Masani, F.; Izumi, T.; Shibata, A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin. Immunol. Immunopathol. 1990, 57, 250–262. [Google Scholar] [CrossRef]
- Kodama, M.; Hanawa, H.; Saeki, M.; Hosono, H.; Inomata, T.; Suzuki, K.; Shibata, A. Rat dilated cardiomyopathy after autoimmune giant cell myocarditis. Circ. Res. 1994, 75, 278–284. [Google Scholar] [CrossRef]
- Masuda, H.; Iwasaki, H.; Kawamoto, A.; Akimaru, H.; Ishikawa, M.; Ii, M.; Shizuno, T.; Sato, A.; Ito, R.; Horii, M.; et al. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Transl. Med. 2012, 1, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Yamato, M.; Isoi, Y.; Akutsu, T.; Setomaru, T.; Abe, K.; Kikuchi, A.; Umezu, M.; Okano, T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res. 2002, 90, e40. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, A.T.; Salybekov, A.A.; Yamano, M.; Kitagishi, H.; Sekine, K.; Tamaki, T. PEGylated carboxyhemoglobin bovine (SANGUINATE) ameliorates myocardial infarction in a rat model. Artif. Organs 2018, 42, 1174–1184. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in Cardiomyocytes and Heart Diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Yang, L.; Liu, Y.; He, J.; Yang, L.; Zhang, Q.; Liu, F.; Li, J.; Liu, J.; Sumi, S.; et al. Resveratrol attenuates doxorubicin-induced cardiotoxicity in rats by up-regulation of vascular endothelial growth factor B. J. Nutr. Biochem. 2020, 79, 108132. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Nakamura, T.; Matsumoto, K.; Sawa, Y.; Matsuda, H.; Nakamura, T. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc. Res. 2001, 51, 41–50. [Google Scholar] [CrossRef]
- Prat-Vidal, C.; Crisostomo, V.; Moscoso, I.; Baez-Diaz, C.; Blanco-Blazquez, V.; Gomez-Mauricio, G.; Albericio, G.; Aguilar, S.; Fernandez-Santos, M.E.; Fernandez-Aviles, F.; et al. Intracoronary Delivery of Porcine Cardiac Progenitor Cells Overexpressing IGF-1 and HGF in a Pig Model of Sub-Acute Myocardial Infarction. Cells 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Chandra, S.; Ehrlich, K.C.; Lacey, M.; Baribault, C.; Ehrlich, M. Epigenetics and expression of key genes associated with cardiac fibrosis: NLRP3, MMP2, MMP9, CCN2/CTGF and AGT. Epigenomics 2021, 13, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Farnsworth, C.W.; Bailey, A.L.; Jaffe, A.S.; Scott, M.G. Diagnostic concordance between NT-proBNP and BNP for suspected heart failure. Clin. Biochem. 2018, 59, 50–55. [Google Scholar] [CrossRef]
- Tsukada, S.; Kwon, S.M.; Matsuda, T.; Jung, S.Y.; Lee, J.H.; Lee, S.H.; Masuda, H.; Asahara, T. Identification of mouse colony-forming endothelial progenitor cells for postnatal neovascularization: A novel insight highlighted by new mouse colony-forming assay. Stem Cell Res. Ther. 2013, 4, 20. [Google Scholar] [CrossRef]
- Gupta, R.; Liu, L.; Zhang, X.; Fan, X.; Krishnamurthy, P.; Verma, S.; Tongers, J.; Misener, S.; Ashcherkin, N.; Sun, H.; et al. IL-10 provides cardioprotection in diabetic myocardial infarction via upregulation of Heme clearance pathways. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Su, Z.; Sun, C.; Yin, J.; Yuan, H.; Sandoghchian, S.; Jiao, Z.; Wang, S.; Xu, H. IL-17 contributes to cardiac fibrosis following experimental autoimmune myocarditis by a PKCbeta/Erk1/2/NF-kappaB-dependent signaling pathway. Int. Immunol. 2012, 24, 605–612. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, M.; Li, H.H.; Long, Q.; Liang, W.; Wen, S.; Wang, M.; Guo, H.P.; Cheng, X.; Liao, Y.H. Autophagy contributes to IL-17-induced plasma cell differentiation in experimental autoimmune myocarditis. Int. Immunopharmacol. 2014, 18, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Hanawa, H.; Yoshida, T.; Hayashi, M.; Liu, H.; Ding, L.; Otaki, K.; Hao, K.; Yoshida, K.; Kato, K.; et al. Alteration of IL-17 related protein expressions in experimental autoimmune myocarditis and inhibition of IL-17 by IL-10-Ig fusion gene transfer. Circ. J. 2008, 72, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hu, L.Q.; Li, H.Q.; Wu, J.; Bian, N.N.; Yan, G. Beneficial effects of andrographolide in a rat model of autoimmune myocarditis and its effects on PI3K/Akt pathway. Korean J. Physiol. Pharm. 2019, 23, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Takehana, H.; Matsuda, C.; Yokoyama, H.; Kohno, K.; Suzuki, K.; Inomata, T. Experimental autoimmune myocarditis and its pathomechanism. Herz 2000, 25, 274–278. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekine, K.; Kawaguchi, A.T.; Miyazawa, M.; Hanawa, H.; Matsuda, S.; Tamaki, T.; Asahara, T.; Masuda, H. Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model. Biology 2022, 11, 106. https://doi.org/10.3390/biology11010106
Sekine K, Kawaguchi AT, Miyazawa M, Hanawa H, Matsuda S, Tamaki T, Asahara T, Masuda H. Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model. Biology. 2022; 11(1):106. https://doi.org/10.3390/biology11010106
Chicago/Turabian StyleSekine, Kaori, Akira T. Kawaguchi, Masaki Miyazawa, Haruo Hanawa, Shinichi Matsuda, Tetsuro Tamaki, Takayuki Asahara, and Haruchika Masuda. 2022. "Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model" Biology 11, no. 1: 106. https://doi.org/10.3390/biology11010106
APA StyleSekine, K., Kawaguchi, A. T., Miyazawa, M., Hanawa, H., Matsuda, S., Tamaki, T., Asahara, T., & Masuda, H. (2022). Transplantation of Fibroblast Sheets with Blood Mononuclear Cell Culture Exerts Cardioprotective Effects by Enhancing Anti-Inflammation and Vasculogenic Potential in Rat Experimental Autoimmune Myocarditis Model. Biology, 11(1), 106. https://doi.org/10.3390/biology11010106