Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Variables and Analysis
- Demographic data—patients’ age and sex.
- History of smoking.
- Diagnostic and treatment data—tumor histology, presence or absence of brain metastasis, disease stage and ECOG PS at the beginning of pembrolizumab treatment, PD-L1 TPS and reasons for the end of treatment.
- Effectiveness variables—response to the treatment considered as complete response (CR), partial response (PR), stable disease (SD), disease progression (DP) or unknown, overall survival (OS; months) and progression-free survival (PFS; months).
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Baseline Characteristics of the Study Population
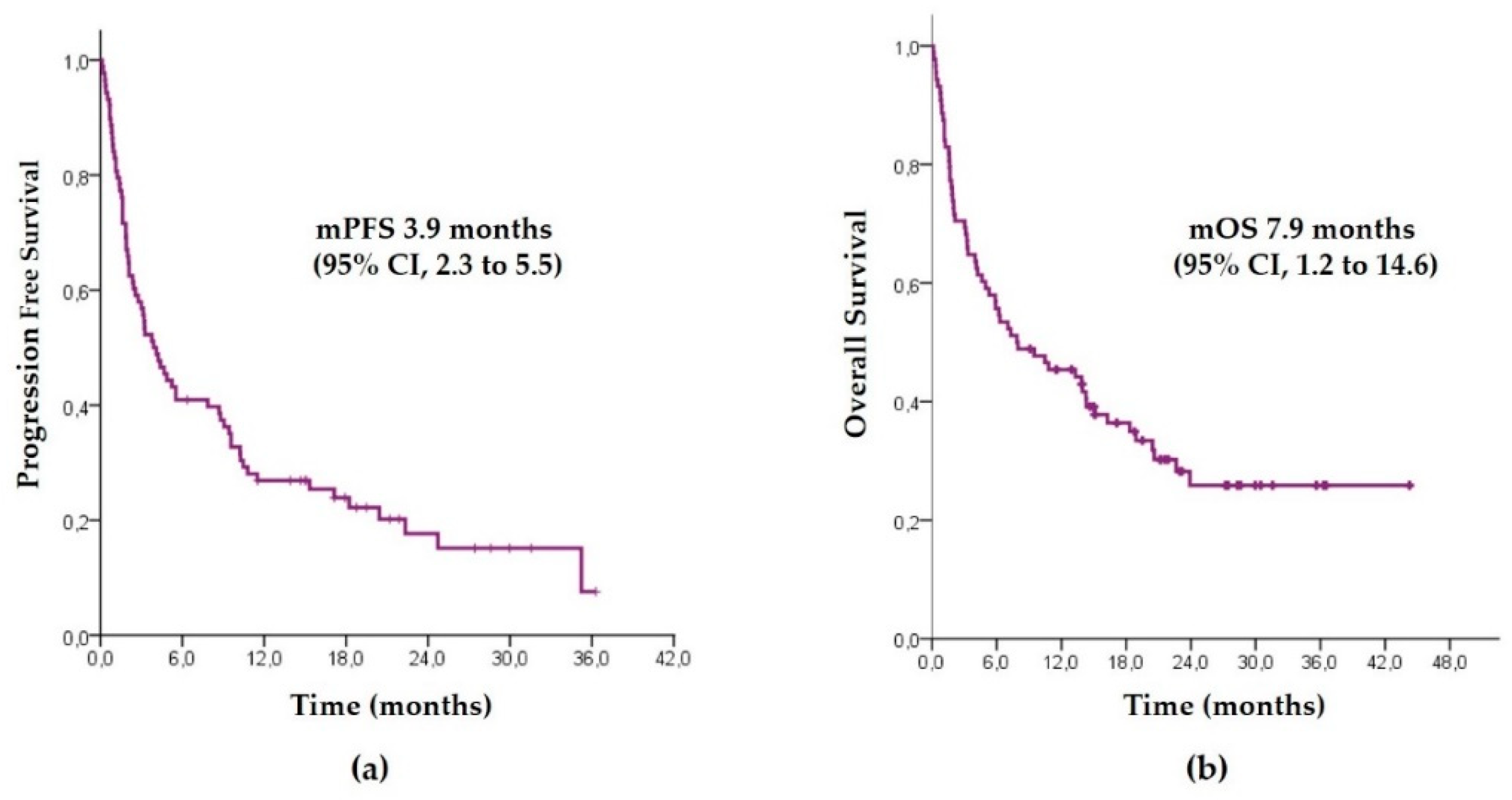
3.2. Pembrolizumab Outcomes in the Overall Population
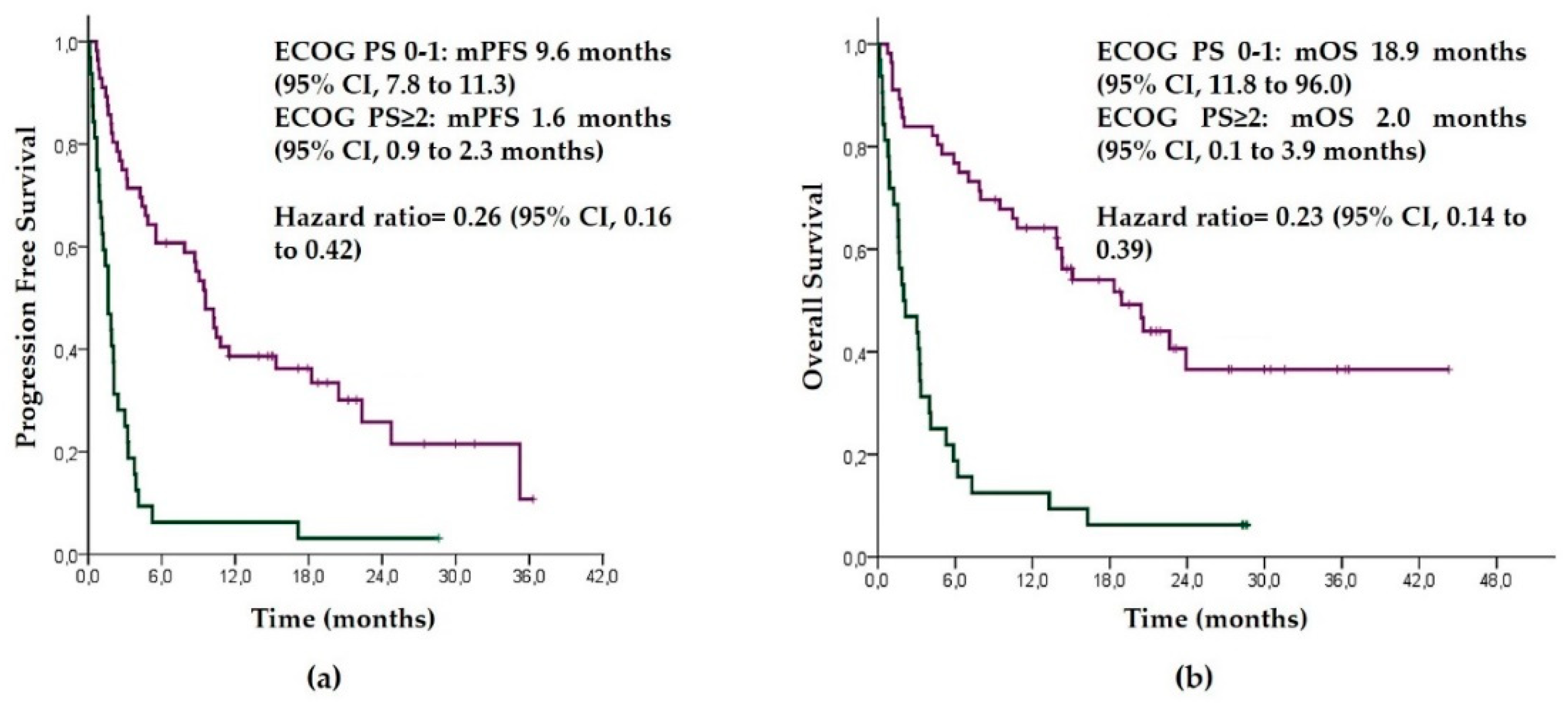
3.3. Results of Pembrolizumab according to ECOG PS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet Lond. Engl. 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet Lond. Engl. 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Boyer, M.; Şendur, M.A.N.; Rodríguez-Abreu, D.; Park, K.; Lee, D.H.; Çiçin, I.; Yumuk, P.F.; Orlandi, F.J.; Leal, T.A.; Molinier, O.; et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (KEYNOTE-042): A Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet Lond. Engl. 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Passaro, A.; Spitaleri, G.; Gyawali, B.; de Marinis, F. Immunotherapy in Non-Small-Cell Lung Cancer Patients With Performance Status 2: Clinical Decision Making With Scant Evidence. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1863–1867. [Google Scholar] [CrossRef] [Green Version]
- Middleton, G.; Brock, K.; Savage, J.; Mant, R.; Summers, Y.; Connibear, J.; Shah, R.; Ottensmeier, C.; Shaw, P.; Lee, S.-M.; et al. Pembrolizumab in Patients with Non-Small-Cell Lung Cancer of Performance Status 2 (PePS2): A Single Arm, Phase 2 Trial. Lancet Respir. Med. 2020, 8, 895–904. [Google Scholar] [CrossRef]
- Frost, N.; Kollmeier, J.; Misch, D.; Vollbrecht, C.; Grah, C.; Matthes, B.; Pultermann, D.; Olive, E.; Raspe, M.; Ochsenreither, S.; et al. Pembrolizumab as First-Line Palliative Therapy in PD-L1 Overexpressing (≥50%) NSCLC: Real-World Results with Special Focus on PS ≥ 2, Brain Metastases, and Steroids. Clin. Lung Cancer 2021, in press. [Google Scholar] [CrossRef]
- Tambo, Y.; Sone, T.; Shibata, K.; Nishi, K.; Shirasaki, H.; Yoneda, T.; Araya, T.; Kase, K.; Nishikawa, S.; Kimura, H.; et al. Real-World Efficacy of First-Line Pembrolizumab in Patients With Advanced or Recurrent Non-Small-Cell Lung Cancer and High PD-L1 Tumor Expression. Clin. Lung Cancer 2020, 21, e366–e379. [Google Scholar] [CrossRef]
- Cavaille, F.; Peretti, M.; Garcia, M.E.; Giorgi, R.; Ausias, N.; Vanelle, P.; Barlesi, F.; Montana, M. Real-World Efficacy and Safety of Pembrolizumab in Patients with Non-Small Cell Lung Cancer: A Retrospective Observational Study. Tumori 2021, 107, 32–38. [Google Scholar] [CrossRef]
- Amrane, K.; Geier, M.; Corre, R.; Léna, H.; Léveiller, G.; Gadby, F.; Lamy, R.; Bizec, J.-L.; Goarant, E.; Robinet, G.; et al. First-Line Pembrolizumab for Non-Small Cell Lung Cancer Patients with PD-L1 ≥50% in a Multicenter Real-Life Cohort: The PEMBREIZH Study. Cancer Med. 2020, 9, 2309–2316. [Google Scholar] [CrossRef]
- Velcheti, V.; Chandwani, S.; Chen, X.; Pietanza, M.C.; Burke, T. First-Line Pembrolizumab Monotherapy for Metastatic PD-L1-Positive NSCLC: Real-World Analysis of Time on Treatment. Immunotherapy 2019, 11, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Lilenbaum, R.C.; Cashy, J.; Hensing, T.A.; Young, S.; Cella, D. Prevalence of Poor Performance Status in Lung Cancer Patients: Implications for Research. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2008, 3, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Mazzaschi, G.; Barbieri, F.; Passiglia, F.; Mazzoni, F.; Berardi, R.; Proto, C.; Cecere, F.L.; Pilotto, S.; Scotti, V.; et al. First-Line Pembrolizumab in Advanced Non-Small Cell Lung Cancer Patients with Poor Performance Status. Eur. J. Cancer Oxf. Engl. 2020, 130, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Gill, R.R.; Widick, P.; Bindal, P.; McDonald, D.C.; Shea, M.; Rangachari, D.; Costa, D.B. Association of Performance Status With Survival in Patients With Advanced Non-Small Cell Lung Cancer Treated With Pembrolizumab Monotherapy. JAMA Netw. Open 2021, 4, e2037120. [Google Scholar] [CrossRef]
- Friedlaender, A.; Metro, G.; Signorelli, D.; Gili, A.; Economopoulou, P.; Roila, F.; Banna, G.; De Toma, A.; Camerini, A.; Christopoulou, A.; et al. Impact of Performance Status on Non-Small-Cell Lung Cancer Patients with a PD-L1 Tumour Proportion Score ≥50% Treated with Front-Line Pembrolizumab. Acta Oncol. Stockh. Swed. 2020, 59, 1058–1063. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Jiménez-Aguilar, E.; Hong, F.; Wei, Z.; Nishino, M.; Plodkowski, A.J.; Sawan, P.; Luo, J.; Rizvi, H.; et al. Outcomes to First-Line Pembrolizumab in Patients with PD-L1-High (≥50%) Non-Small Cell Lung Cancer and a Poor Performance Status. J. Immunother. Cancer 2020, 8, e001007. [Google Scholar] [CrossRef]
- Matsubara, T.; Seto, T.; Takamori, S.; Fujishita, T.; Toyozawa, R.; Ito, K.; Yamaguchi, M.; Okamoto, T. Anti-PD-1 Monotherapy for Advanced NSCLC Patients with Older Age or Those with Poor Performance Status. OncoTargets Ther. 2021, 14, 1961–1968. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
| Characteristics | Values, n (%) |
|---|---|
| Sex | |
| Male | 69 (78.4) |
| Female | 19 (21.6) |
| Age median (range), y | 67 (46–85) |
| Smoking history | |
| Yes | 80 (90.9) |
| No | 8 (9.1) |
| Histology | |
| Adenocarcinoma | 60 (68.2) |
| Squamous | 18 (20.5) |
| NSCLC poorly differentiated | 6 (6.8) |
| Others | 4 (4.5) |
| Disease stage | |
| IIIB | 2 (2.3) |
| IV | 86 (97.7) |
| Brain metastases | |
| Yes | 14 (15.9) |
| No | 74 (84.1) |
| ECOG PS | |
| 0 | 18 (20.5) |
| 1 | 38 (43,2) |
| 2 | 25 (28.4) |
| 3 | 7 (8.0) |
| PD-L1 TPS% | |
| <90% | 62 (70.5) |
| ≥90% | 26 (29.5) |
| Characteristics | ECOG 0–1 n (%) | ECOG ≥ 2 n (%) | p-Value |
|---|---|---|---|
| Sex | 0.304 | ||
| Male | 42 (75) | 27 (84.4) | |
| Female | 14 (25) | 5 (15.6) | |
| Age median (range), y | 66.8 (46–84) | 66.1 (49–85) | 0.68 |
| Smoking history | 50 (89.3) | 30 (93.8) | 0.483 |
| Histology | 0.331 | ||
| Adenocarcinoma | 41(73.2) | 19 (54.4) | |
| Squamous | 11 (19.6) | 7 (21.9) | |
| NSCLC poorly differentiated | 2 (3.6) | 4 (12.5) | |
| Others | 2 (3.6) | 2 (6.3) | |
| Disease stage | 0.582 | ||
| IIIB | 1 (1.8) | 1 (3.1) | |
| IV | 55 (98.2) | 31 (96.9) | |
| Brain metastases | 0.607 | ||
| Yes | 8 (14.3) | 6 (18.8) | |
| No | 48 (85.7) | 26 (81.3) | |
| PD-L1 TPS% | 0.453 | ||
| <90% | 41(73.2) | 21 (65.6) | |
| ≥90% | 15 (26.8) | 11 (34.4) |
| Response | N (%) |
|---|---|
| CR | - |
| PR | 28 (31.8) |
| SD | 17 (19.3) |
| PD | 21 (23.9) |
| NE | 22 (25.0) |
| Response | ECOG PS 0–1 | ECOG PS ≥ 2 |
|---|---|---|
| PR, n (%) | 23 (26.1) | 5 (5.7) |
| SD, n (%) | 16 (18.2) | 1 (1.1) |
| PD, n (%) | 11 (12.5) | 10 (11.4) |
| Progression Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Median (95% CI) | HR (95% CI) | p-Value | Median (95% CI) | HR (95% CI) | p-Value |
| Sex | 0.75 (0.42–1.34) | 0.33 | 0.64 (0.33–1.23) | 0.175 | ||
| Female | 9.06 (1.72–4.98) | 18.9 (4.4–33.9) | ||||
| male | 3.2 (1.41–4.98) | 7.03 (4.56–9.47) | ||||
| Age | 0.96 (0.59–1.56) | 0.86 | 0.98 (0.59–1.65) | 0.965 | ||
| <70 | 3.76 (1.33–6.21) | 7.9 (0–17.3) | ||||
| ≥70 | 3.9 (0.71–7.09) | 7.03 (1.94–12.13) | ||||
| Smoking history | 0.775 (0.33–1.79) | 0.538 | 0.69 (0.27–1.71) | 0.42 | ||
| Yes | 3.7 (2.08–5.44) | 7.03 (2.03–12.04) | ||||
| No | 4.4 (0–14.7) | 10.8 (0–28.3) | ||||
| ECOG PS | 0.25 (0.16–0.42) | <0.001 | 0.23 (0.13–0.38) | <0.001 | ||
| 0–1 | 9.57 (7.82–11.32) | 18.9 (11.77–26.03) | ||||
| ECOG ≥ 2 | 1.63 (0.99–2.28) | 2.0 (0.11–3.89) | ||||
| Histology | 0.79 (0.48–1.31) | 0.36 | 0.73 (0.43–1.23) | 0.238 | ||
| Adenocarcinoma | 4.4 (2.48–6.32) | 9.47 (0.19–18.74) | ||||
| Non-adenocarcinoma | 2.1 (0.46–3.74) | 5.9 (0.67–11.3) | ||||
| PD-L1 TPS | 0.77 (0.46– 1.28) | 0.32 | 0.75 (0.44–1.28) | 0.283 | ||
| <90% | 4.1 (2.29–5.9) | 9.47 (1.26–17.67) | ||||
| ≥90% | 3.27 (0–7.8) | 5.3 (0–11.17) | ||||
| Brain Metastasis | 0.70 (0.38–1.29) | 0.25 | 0.62 (0.33–1.17) | 0.139 | ||
| No | 4.23 (1.98–6.48) | 10.47 (3.52–17.42) | ||||
| Yes | 3.2 (1.12–5.28) | 4.1 (2.33–5.87) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez Galán, R.; Prado-Mel, E.; Pérez-Moreno, M.A.; Caballano-Infantes, E.; Flores Moreno, S. Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population. Biology 2021, 10, 890. https://doi.org/10.3390/biology10090890
Jiménez Galán R, Prado-Mel E, Pérez-Moreno MA, Caballano-Infantes E, Flores Moreno S. Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population. Biology. 2021; 10(9):890. https://doi.org/10.3390/biology10090890
Chicago/Turabian StyleJiménez Galán, Rocío, Elena Prado-Mel, María Antonia Pérez-Moreno, Estefanía Caballano-Infantes, and Sandra Flores Moreno. 2021. "Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population" Biology 10, no. 9: 890. https://doi.org/10.3390/biology10090890
APA StyleJiménez Galán, R., Prado-Mel, E., Pérez-Moreno, M. A., Caballano-Infantes, E., & Flores Moreno, S. (2021). Influence of Performance Status on the Effectiveness of Pembrolizumab Monotherapy in First-Line for Advanced Non-Small-Cell Lung Cancer: Results in a Real-World Population. Biology, 10(9), 890. https://doi.org/10.3390/biology10090890






