Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiments in Laboratory-Controlled Conditions
2.1.1. Design of Trials in Incubators
2.1.2. Collection of Honeybee Hemolymph Samples
2.1.3. Biochemical Hemolymph Parameters
Glucose, Trehalose and Total Lipids Concentrations
2.1.4. Immunological Parameters
Total Proteins and Vitellogenin Concentrations in Hemolymph
Hypopharyngeal Gland Acini Diameters
2.1.5. Carbohydrate-Metabolizing Enzymatic Activity
Activity of Glucose Oxidase in Honeybee Hemolymph
2.2. Sampling of Adult Honeybee’s Mid-Guts during Experiments in Field Conditions
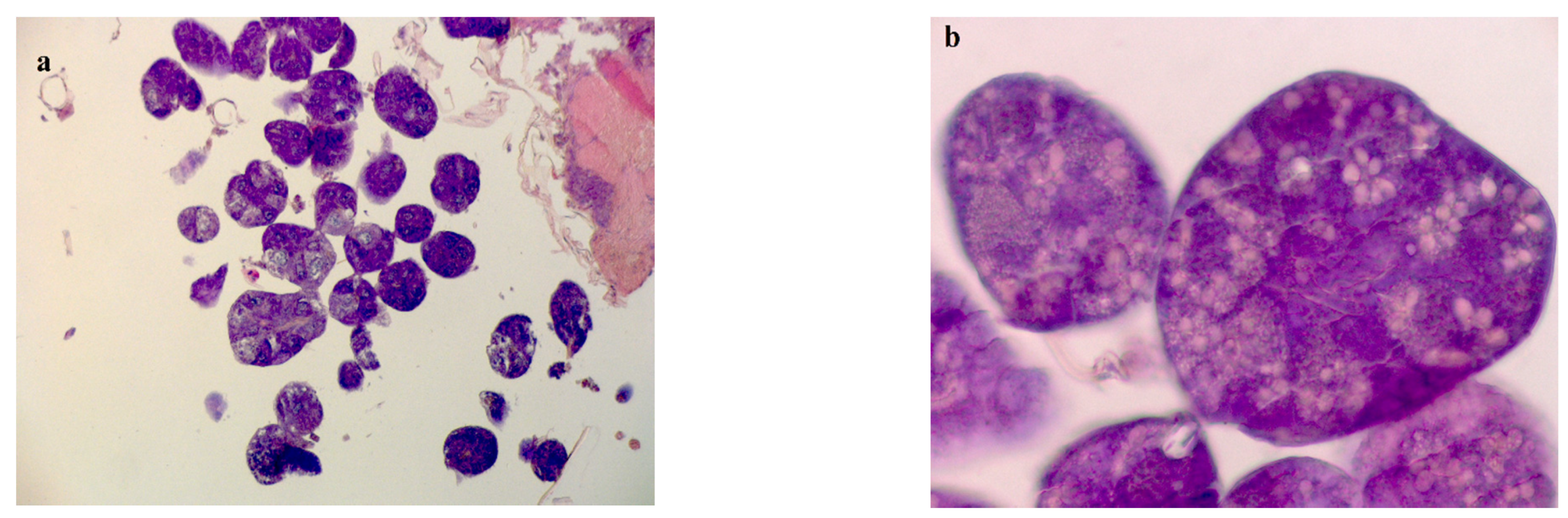
Leucine Aminopeptidase Enzymatic Activity–Histology Approach
2.3. Statistical Analyses
3. Results
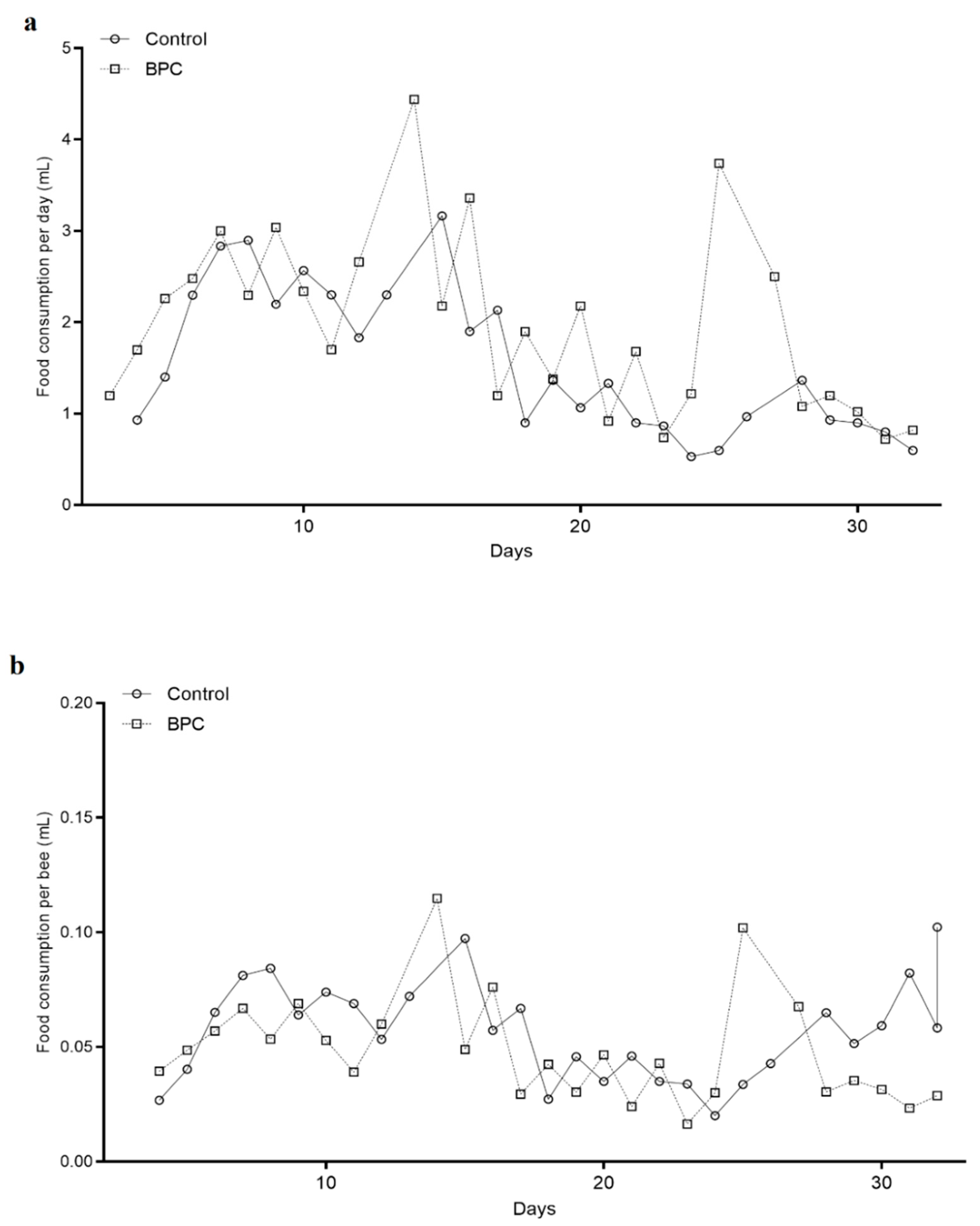
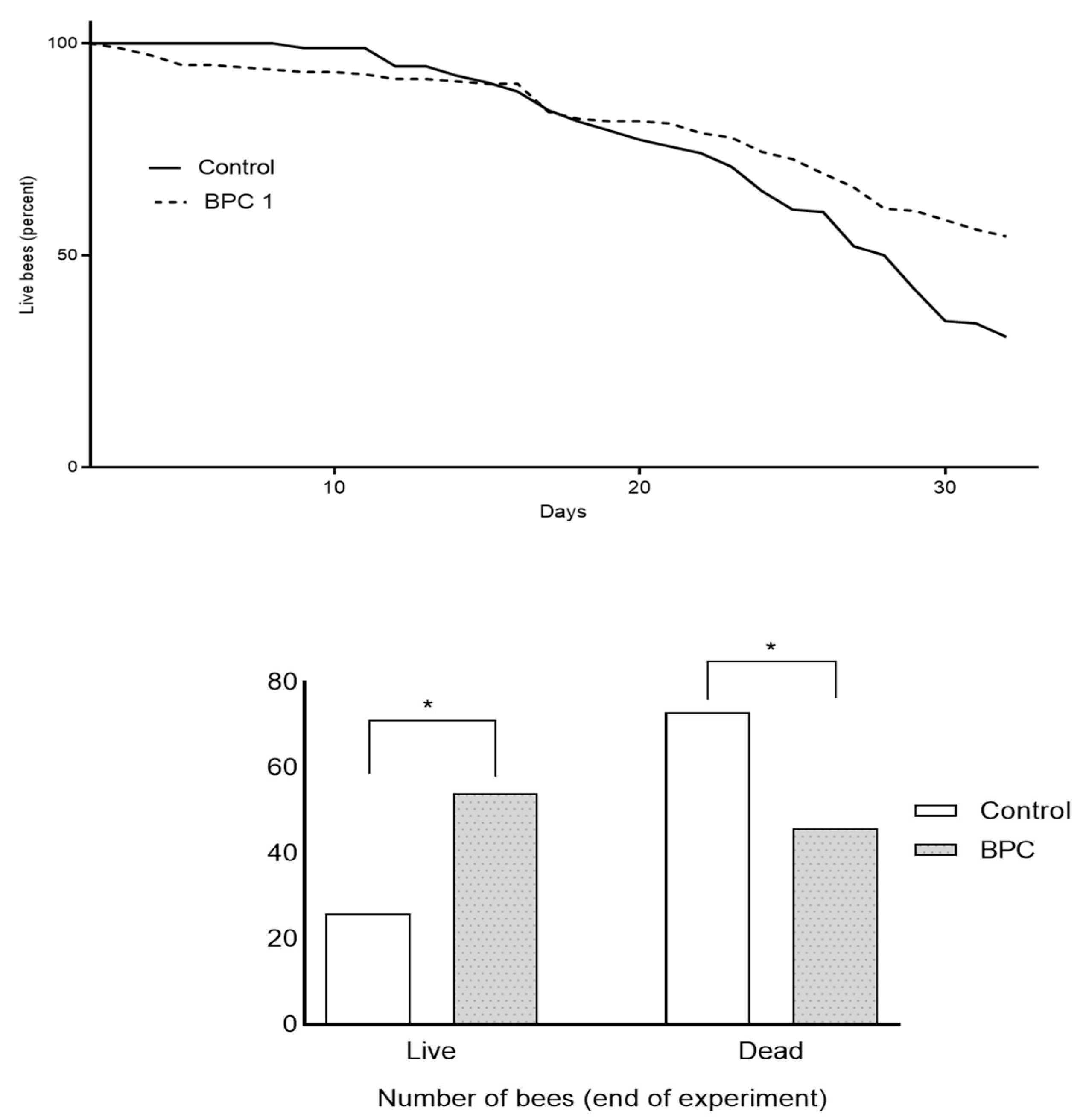
3.1. Consumption of Diets and Survival Rates of Adult Honeybees
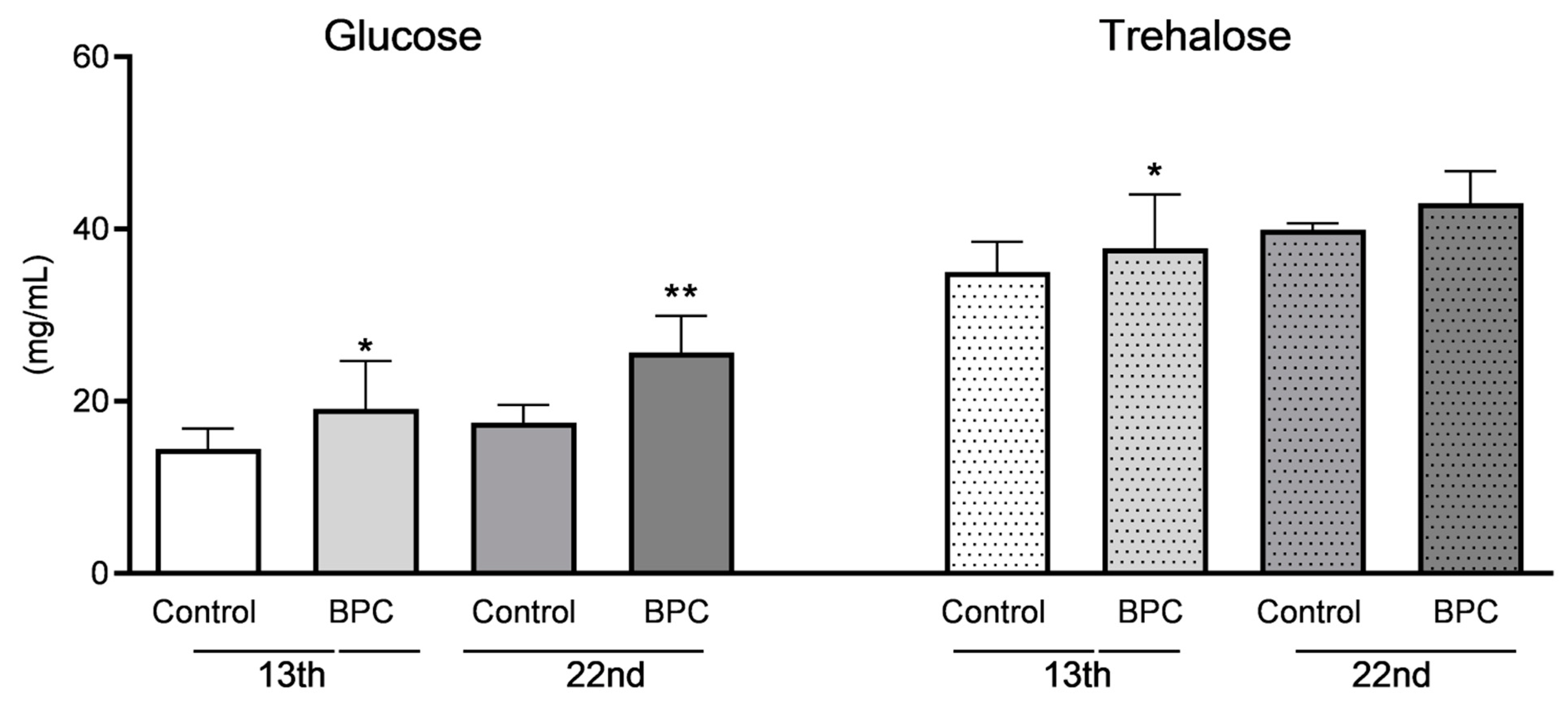
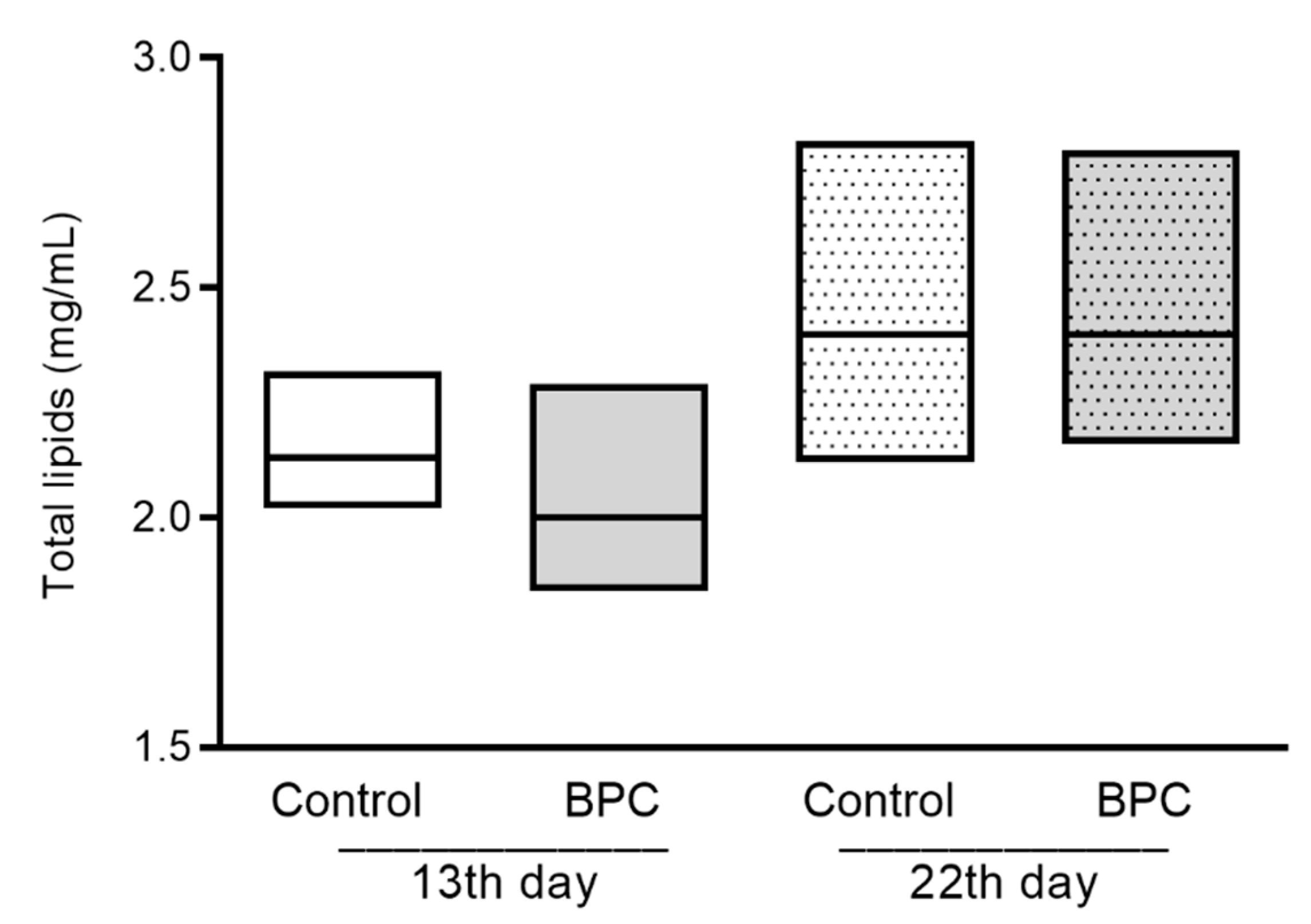
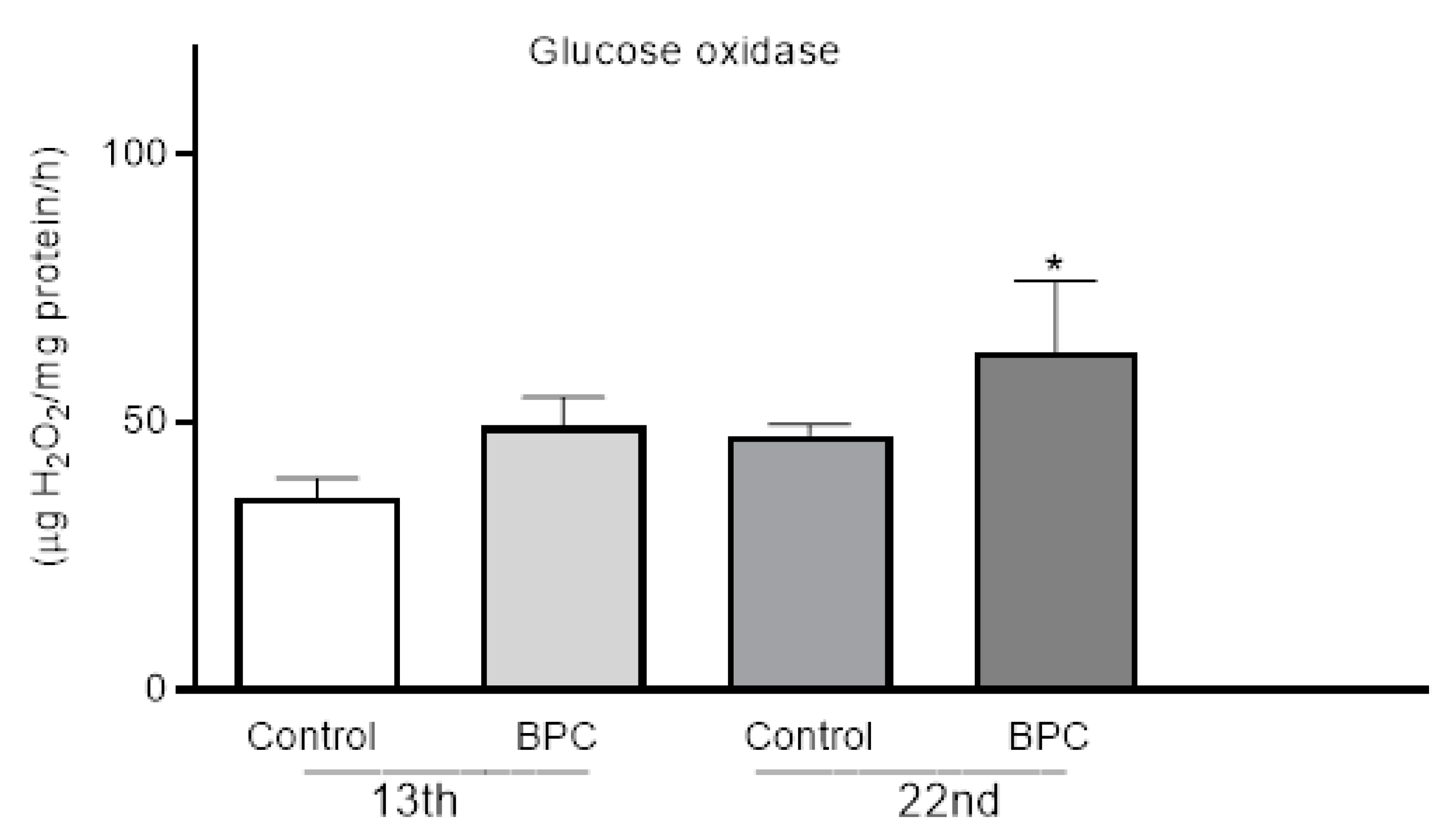
3.2. Biochemical Hemolymph Parameters of Adult Honeybees Fed with BPC
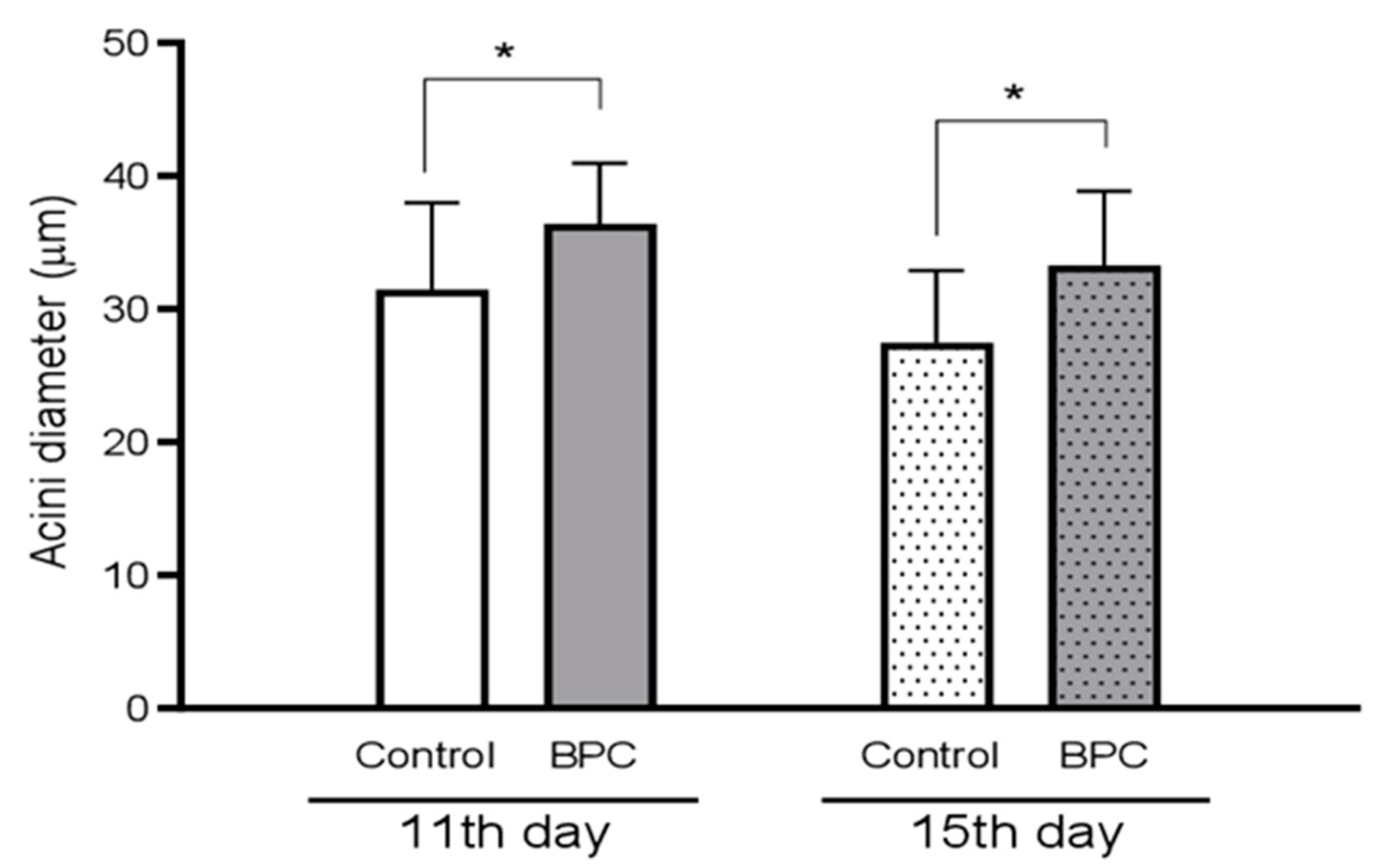
3.3. Effect of Food Supplement BPC 157 on Immunological Parameters
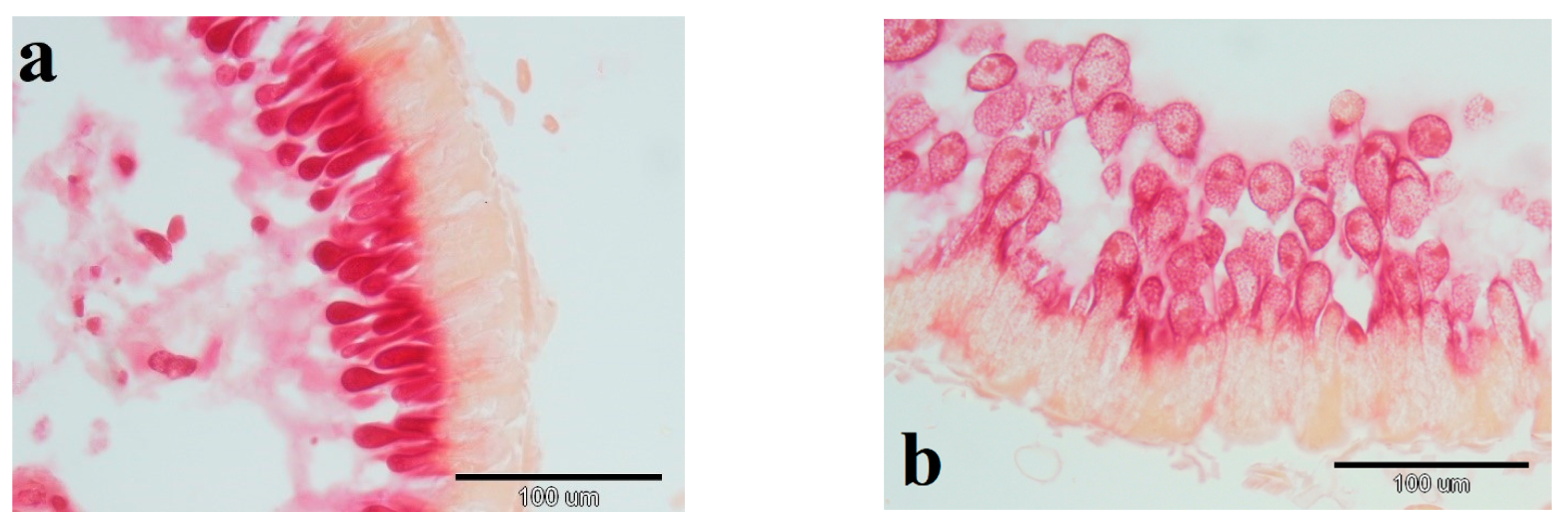
3.4. Leucine Aminopeptidase Activity in Mid-Gut of Bees Fed with BPC 157
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajger, I.T.; Ribarić, J.; Škerl, M.S.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R. Does infection by Nosema ceranae cause “Colony Collapse Disorder” in honey bees (Apis mellifera)? J. Apic. Res. 2010, 49, 80–84. [Google Scholar] [CrossRef]
- Higes, M.; Meana, A.; Bartolomé, C.; Botías, C.; Martín-Hernández, R. Nosema ceranae (Microsporidia), a controversial 21st century honey bee pathogen. Environ. Microbiol. Rep. 2013, 5, 17–29. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Hernández, R.M.; Meana, A.; Higes, M. Screening alternative therapies to control Nosemosis type C in honey bee (Apis mellifera iberiensis) colonies. Res. Vet. Sci. 2013, 95, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 Laying down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstuffs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legalcontent/EN/ALL/?uri=celex%3A32009R0470 (accessed on 8 May 2021).
- Anonymous. Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32010R0037 (accessed on 8 May 2021).
- Mutinelli, F. European legislation governing the authorization of veterinary medicinal products with particular reference to the use of drugs for the control of honey bee diseases. Apiacta 2003, 38, 156–168. [Google Scholar]
- Anonymous. Guideline on Veterinary Medicinal Products Controlling Varroa Destructor Parasitosis in Bees. EMA—European Medicines Agency, EMA/CVMP/EWP/459883/2008-Rev.1, Committee for Medicinal Products for Veterinary Use (CVMP). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-veterinary-medicinal-products-controlling-varroa-destructor-parasitosis-bees-revision-1_en.pdf (accessed on 12 May 2021).
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef]
- Rollin, O.; Benelli, G.; Benvenuti, S.; Decourtye, A.; Wratten, S.D.; Canale, A.; Desneux, N. Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review. Agron. Sustain. Dev. 2016, 36, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Iatridou, D.; Pohl, L.; Gajger, I.T.; De Briyne, N.; Bravo, A.; Saunders, J. Mapping the teaching of honeybee veterinary medicine in the European Union and European Free Trade Area. Vet. Rec. Open 2019, 6, e000343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Uribe, M.M.; Fitzgerald, A.; Simone-Finstrom, M. Inducible versus constitutive social immunity: Examining effects of colony infection on glucose oxidase and defensin-1 production in honeybees. R. Soc. Open Sci. 2017, 4, 170224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.; Shipley, E.; Arnold, K.E. Social immunity in honeybees—Density dependence, diet, and body mass trade-offs. Ecol. Evol. 2018, 8, 4852–4859. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [Green Version]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiwerth, S.; Milavic, M.; Vukojevic, J.; Gojkovic, S.; Krezic, I.; Vuletic, L.B.; Pavlov, K.H.; Petrovic, A.; Sikiric, S.; Vranes, H.; et al. Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Front. Pharmacol. 2021, 12, 627533. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 Rescued NSAID-cytotoxicity Via Stabilizing Intestinal Permeability and Enhancing Cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef]
- Sikiric, P.; Drmic, D.; Sever, M.; Klicek, R.; Blagaic, A.B.; Tvrdeic, A.; Kralj, T.; Kovac, K.K.; Vukojevic, J.; Siroglavic, M.; et al. Fistulas Healing. Stable Gastric Pentadecapeptide BPC 157 Therapy. Curr. Pharm. Des. 2020, 26, 2991–3000. [Google Scholar] [CrossRef]
- Sikiric, P.; Hahm, K.-B.; Blagaic, A.B.; Tvrdeic, A.; Pavlov, K.H.; Petrovic, A.; Kokot, A.; Gojkovic, S.; Krezic, I.; Drmic, D.; et al. Stable Gastric Pentadecapeptide BPC 157, Robert’s Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye’s Stress Coping Response: Progress, Achievements, and the Future. Gut Liver 2020, 14, 153–167. [Google Scholar] [CrossRef]
- Gwyer, D.; Wragg, N.; Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019, 377, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Toxicity by NSAIDs. Counteraction by Stable Gastric Pentadecapeptide BPC 157. Curr. Pharm. Des. 2012, 19, 76–83. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Pavlov, K.H.; et al. Occlusion of the Superior Mesenteric Artery in Rats Reversed by Collateral Pathways Activation: Gastric Pentadecapeptide BPC 157 Therapy Counteracts Multiple Organ Dysfunction Syndrome; Intracranial, Portal, and Caval Hypertension; and Aortal Hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded Superior Mesenteric Artery and Vein. Therapy with the Stable Gastric Pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Pavlov, K.H.; Petrovic, A.; Vuletic, L.B.; Milavic, M.; Sikiric, S.; et al. BPC 157 Therapy and the Permanent Occlusion of the Superior Sagittal Sinus in Rat: Vascular Recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef]
- Cesarec, V.; Becejac, T.; Misic, M.; Djakovic, Z.; Olujic, D.; Drmic, D.; Brcic, L.; Rokotov, D.S.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur. J. Pharmacol. 2013, 701, 203–212. [Google Scholar] [CrossRef]
- Chang, C.-H.; Tsai, W.-C.; Hsu, Y.-H.; Pang, J.-H.S. Pentadecapeptide BPC 157 Enhances the Growth Hormone Receptor Expression in Tendon Fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-H.; Tsai, W.-C.; Lin, M.-S.; Hsu, Y.-H.; Pang, J.-H.S. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 774–780. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-J.; Lee, C.-H.; Chueh, H.-Y.; Chang, G.-J.; Huang, H.-Y.; Lin, Y.; Pang, J.-H.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Huang, T.; Gu, J.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Tkalčević, V.I.; Čužić, S.; Brajša, K.; Mildner, B.; Bokulić, A.; Šitum, K.; Perović, D.; Glojnarić, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef]
- Vukojević, J.; Siroglavić, M.; Kašnik, K.; Kralj, T.; Stanćić, D.; Kokot, A.; Kolarić, D.; Drmić, D.; Sever, A.Z.; Barišić, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Drmic, D.; Samara, M.; Vidovic, T.; Malekinusic, D.; Antunovic, M.; Vrdoljak, B.; Ruzman, J.; Perisa, M.M.; Pavlov, K.H.; Jeyakumar, J.; et al. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J. Gastroenterol. 2018, 24, 5462–5476. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Drmic, D.; Stupnisek, M.; Kokot, A.; Sever, M.; Zoricic, I.; Zoricic, Z.; Batelja, L.; et al. Stress in Gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr. Pharm. Des. 2017, 23, 4012–4028. [Google Scholar] [CrossRef]
- Hartfelder, K.; Bitondi, M.M.G.; Brent, C.S.; Guidugli-Lazzarini, K.R.; Simões, Z.L.P.; Stabentheiner, A.; Tanaka, E.D.; Wang, Y. Standard methods for physiology and biochemistry research in Apis mellifera. J. Apic. Res. 2013, 52, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Zollner, E.; Kirs, I.K. Uber die quanttive Bestimmung yon Lipoiden (Mikromethode) mittels der vielen natiirliehen Lipoiden (allen bekannten Plasmalipoiden) gemeinsamen Sulfophosphovanillin-Reaktion. Z. Gesamte. Exp. Med. 1962, 135, 545–561. [Google Scholar] [CrossRef]
- Hammond, J.B.W.; Kruger, N.J. The Bradford Method for Protein Quantitation. In New Protein Techniques; Humana Press: Totowa, NJ, USA, 1988; Volume 3, pp. 25–32. [Google Scholar]
- Roulet, F. Methoden der Pathologischen Histologie; Springer: Berlin/Heidelberg, Germany, 1948. [Google Scholar]
- Hrapchak, B.B.; Sheehan, D.C. Enzyme Histochemistry. In Theory and Practice of Histotechnology, 2nd ed.; Mosby: St. Louis, MI, USA, 1980; pp. 304–305. [Google Scholar]
- Gajger, I.T.; Nejedli, S.; Kozarić, Z. The effect of Nozevit on leucine aminopeptidase and esterase activity in the midgut of honey bees (Apis mellifera). Vet. Med. 2013, 58, 422–429. [Google Scholar] [CrossRef]
- Pietropaoli, M.; Skerl, M.S.; Cazier, J.; Riviere, M.-P.; Tiozzo, B.; Eggenhoeffner, R.; Gregorc, A.; Haefeker, W.; Higes, M.; Ribarits, A.; et al. BPRACTICES Project: Towards a Sustainable European Beekeeping. Bee World 2020, 97, 66–69. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Evaluating Effects of a Critical Micronutrient (24-Methylenecholesterol) on Honey Bee Physiology. Ann. Entomol. Soc. Am. 2020, 113, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Gajger, I.T.; Vlainić, J.; Šoštarić, P.; Prešern, J.; Bubnič, J.; Škerl, M.I.S. Effects on Some Therapeutical, Biochemical, and Immunological Parameters of Honey Bee (Apis mellifera) Exposed to Probiotic Treatments, in Field and Laboratory Conditions. Insects 2020, 11, 638. [Google Scholar] [CrossRef]
- Stabler, D.; Al-Esawy, M.; Chennells, J.A.; Perri, G.; Robinson, A.; Wright, G.A. Regulation of dietary intake of protein and lipid by nurse-age adult worker honeybees. J. Exp. Biol. 2021, 224, 230615. [Google Scholar] [CrossRef]
- Cariveau, D.P.; Powell, J.; Koch, H.; Winfree, R.; Moran, N.A. Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus). ISME J. 2014, 8, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Medina, L.A.; Koch, H.; Sing, K.-W.; Soh, E.J.Y.; Ascher, J.S.; Jaffé, R.; Moran, N.A. Dynamic microbiome evolution in social bees. Sci. Adv. 2017, 3, e1600513. [Google Scholar] [CrossRef] [Green Version]
- Lazzeri, A.M.; Mangia, N.; Mura, M.E.; Floris, I.; Satta, A.; Ruiu, L. Potential of novel food-borne Lactobacillus isolates against the honeybee pathogen Paenibacillus larvae. Biocontrol. Sci. Technol. 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and Nosema ceranae Infection. Microb. Ecol. 2020, 80, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Engels, W.; Kaatz, H.; Zillikens, A.; Simões, Z.L.P.; Truve, A.; Engels, W.; Zillikens, M.C.; Truve, K. Honey bee reproduction: Vitellogenin and caste-specific regulation of fertility. In Advances in Invertebrate Reproduction; Hoshi, M., Yamashita, O., Eds.; Elsiever: Amsterdam, The Netherlands, 1990; pp. 495–502. [Google Scholar]
- Corby-Harris, V.; Jones, B.M.; Walton, A.; Schwan, M.R.; Anderson, K.E. Transcriptional markers of sub-optimal nutrition in developing Apis mellifera nurse workers. BMC Genom. 2014, 15, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive Markers of Honey Bee Colony Collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, S.; Liu, Q. Vitellogenin Functions as a Multivalent Pattern Recognition Receptor with an Opsonic Activity. PLoS ONE 2008, 3, e1940. [Google Scholar] [CrossRef] [Green Version]
- Cardoso-Júnior, C.A.M.; Oldroyd, B.P.; Ronai, I. Vitellogenin expression in the ovaries of adult honeybee workers provides insights into the evolution of reproductive and social traits. Insect Mol. Biol. 2021, 30, 277–286. [Google Scholar] [CrossRef]
- Evans, J.D.; Lopez, D.L. Bacterial Probiotics Induce an Immune Response in the Honey Bee (Hymenoptera: Apidae). J. Econ. Entomol. 2004, 97, 752–756. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maes, P.W.; Rodrigues, P.A.P.; Oliver, R.; Mott, B.M.; Anderson, K.E. Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol. Ecol. 2016, 25, 5439–5450. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.-L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef]
- Matašin, Ž.; Nejedli, S.; Tlak Gajger, I. Leucine aminopeptidase activity in the midgut of nosema diseased honeybees (Apis mellifera). Vet. Arhiv. 2012, 82, 599–607. [Google Scholar]
- Dussaubat, C.; Brunet, J.-L.; Higes, M.; Colbourne, J.K.; López, J.; Choi, J.-H.; Martin-Hernandez, R.; Botías, C.; Cousin, M.; Mcdonnell, C.; et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar]
- Fowler, J.H.; Narváez-Vásquez, J.; Aromdee, D.N.; Pautot, V.; Holzer, F.M.; Walling, L.L. Leucine Aminopeptidase Regulates Defense and Wound Signaling in Tomato Downstream of Jasmonic Acid. Plant Cell 2009, 21, 1239–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajger, I.T.; Dar, S. Plant Allelochemicals as Sources of Insecticides. Insects 2021, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.S.; Gu, Y.-Q.; Pautot, V.; Bray, E.A.; Walling, L.L. Leucine Aminopeptidase RNAs, Proteins, and Activities Increase in Response to Water Deficit, Salinity, and the Wound Signals Systemin, Methyl Jasmonate, and Abscisic Acid1. Plant Physiol. 1999, 120, 979–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlak Gajger, I.; Smodiš Škerl, M.I.; Šoštarić, P.; Šuran, J.; Sikirić, P.; Vlainić, J. Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157. Biology 2021, 10, 891. https://doi.org/10.3390/biology10090891
Tlak Gajger I, Smodiš Škerl MI, Šoštarić P, Šuran J, Sikirić P, Vlainić J. Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157. Biology. 2021; 10(9):891. https://doi.org/10.3390/biology10090891
Chicago/Turabian StyleTlak Gajger, Ivana, Maja Ivana Smodiš Škerl, Petra Šoštarić, Jelena Šuran, Predrag Sikirić, and Josipa Vlainić. 2021. "Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157" Biology 10, no. 9: 891. https://doi.org/10.3390/biology10090891
APA StyleTlak Gajger, I., Smodiš Škerl, M. I., Šoštarić, P., Šuran, J., Sikirić, P., & Vlainić, J. (2021). Physiological and Immunological Status of Adult Honeybees (Apis mellifera) Fed Sugar Syrup Supplemented with Pentadecapeptide BPC 157. Biology, 10(9), 891. https://doi.org/10.3390/biology10090891









