Contractile and Structural Properties of Detrusor from Children with Neurogenic Lower Urinary Tract Dysfunction
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Tissue Samples, Ethics and Preparations
2.2. Solutions
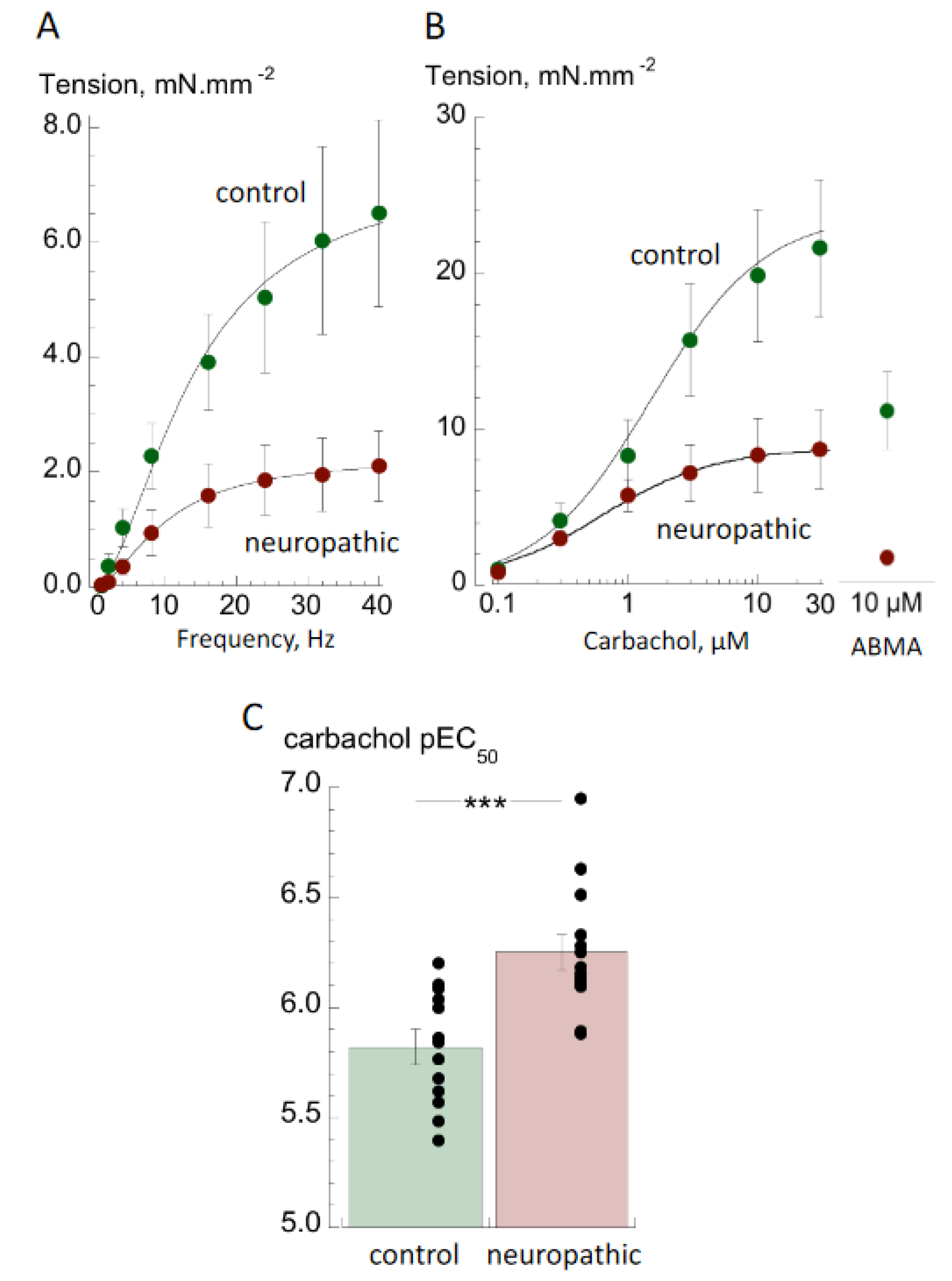
2.3. Active Tension Recording
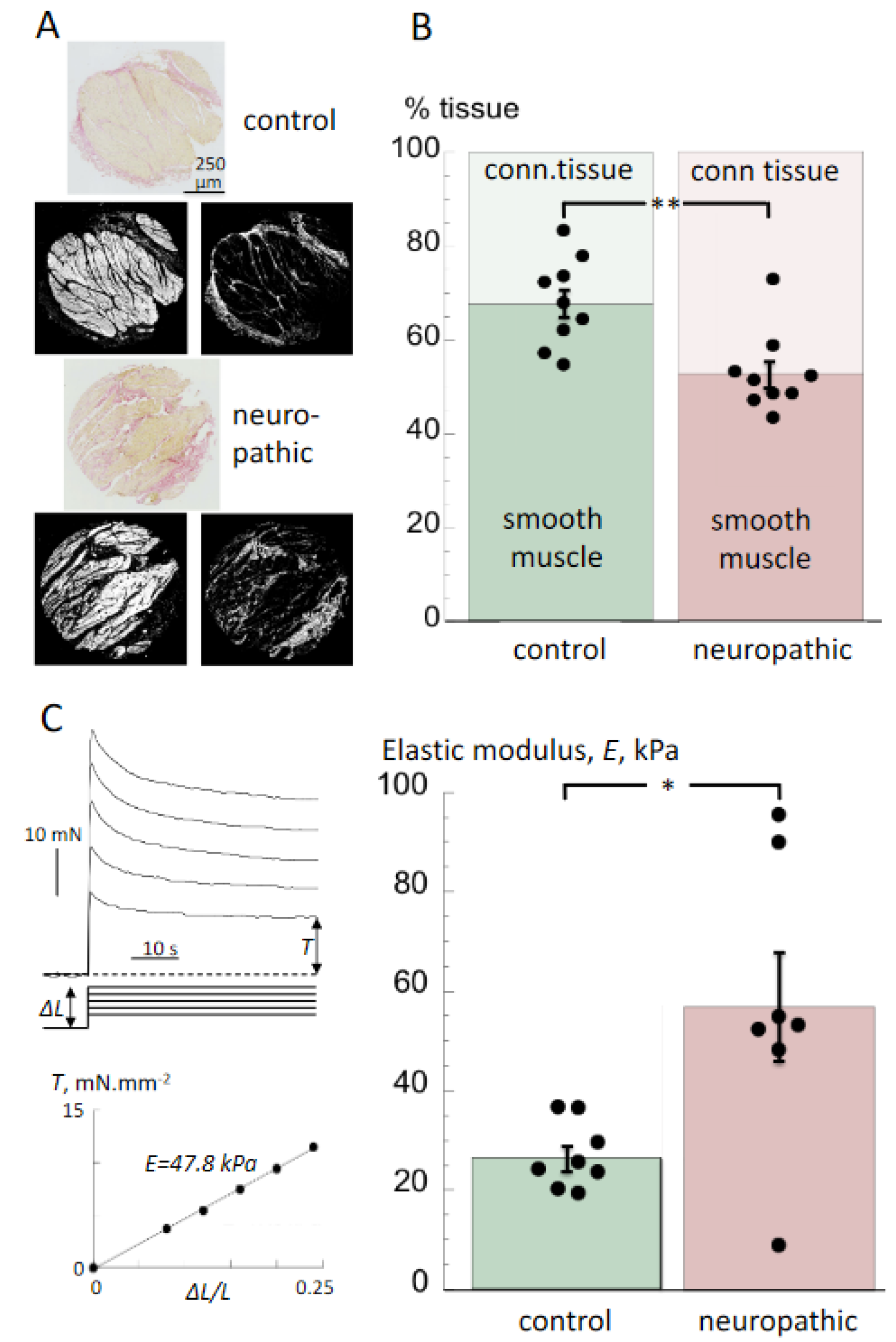
2.4. Histology
2.5. Biomechanical Measurements
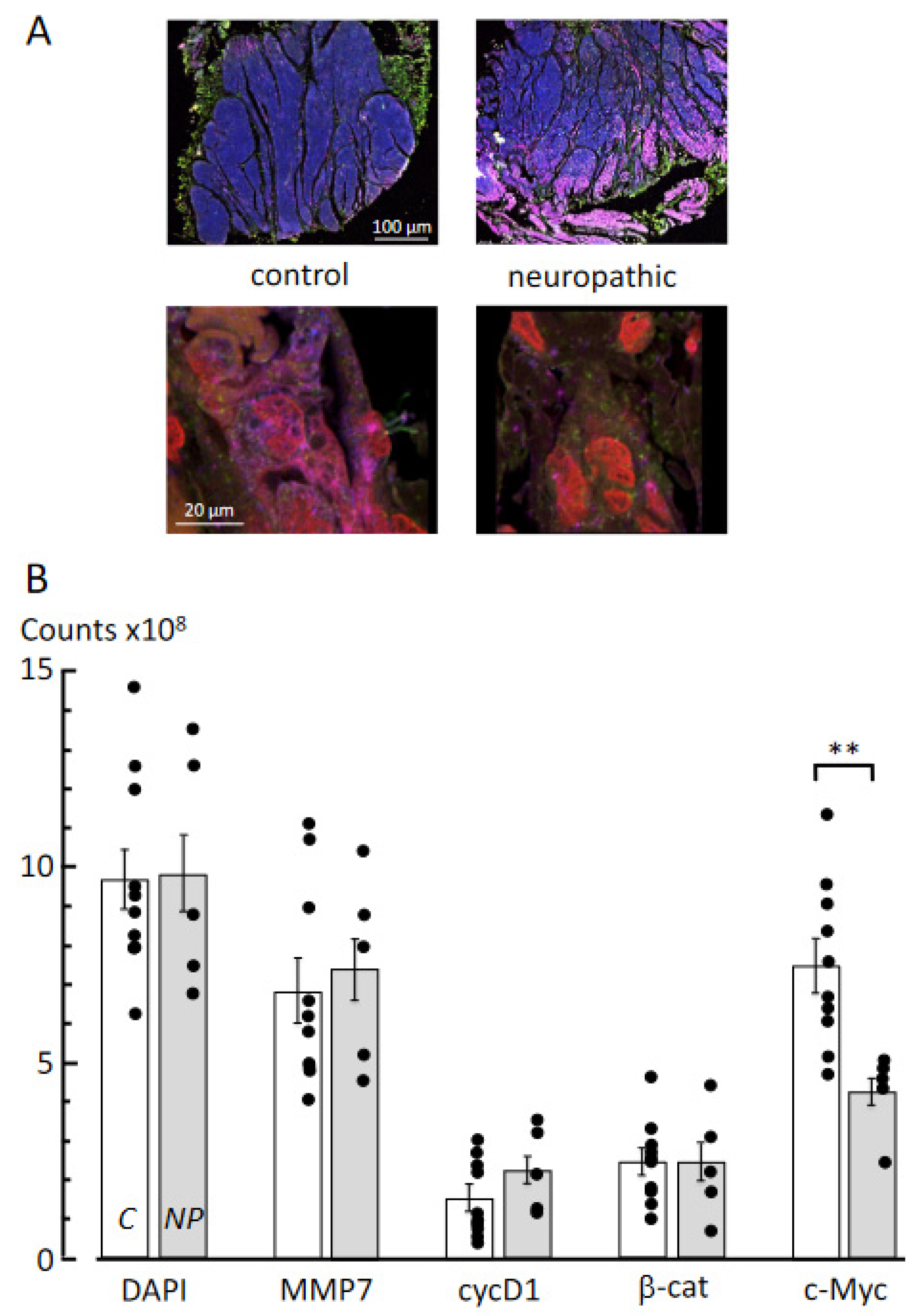
2.6. Multiplex Immunofluorescence Labelling and Quantitative Image Intensity Analyses
2.7. Data Presentation and Analysis
3. Results
3.1. Contractile Properties
3.2. Histological Properties and Detrusor Stiffness
3.3. Multiplex Immunofluorescence Labelling
4. Discussion
4.1. Contractile Properties of Detrusor from Bladders with Neurogenic LUT (NLUT) Dysfunction
4.2. Histological and Biomechanical Properties of Tissue from Bladders with Neurogenic LUT Dysfunction
4.3. Molecular Pathways Underlying Fibrosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greene, N.D.; Copp, A.J. Neural tube defects. Ann. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef]
- Cho, P.S.; Bauer, S.B.; Pennison, M.; Rosoklija, I.; Bellows, A.L.; Logvinenko, T.; Khoshbin, S.; Borer, J.G. Sacral agenesis and neurogenic bladder: Long-term outcomes of bladder and kidney function. J. Pediatr. Urol. 2016, 12, 158.e1–158.e7. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Adzick, N.S. Fetal myelomeningocele: Natural history, pathophysiology, and in-utero intervention. Semin. Fetal. Neonatal. Med. 2010, 15, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nijman, R.J. Neurogenic and non-neurogenic bladder dysfunction. Curr. Opin. Urol. 2001, 11, 577–583. [Google Scholar] [CrossRef]
- Sakakibara, R.; Hattori, T.; Uchiyama, T.; Kamura, K.; Yamanishi, T. Uroneurological assessment of spina bifida cystica and occulta. Neurourol. Urodyn. 2003, 22, 328–334. [Google Scholar] [CrossRef]
- Stein, R.; Bogaert, G.; Dogan, H.S.; Hoen, L.; Kocvara, R.; Nijman, R.J.M.; Quadackers, J.S.L.T.; Rawashdeh, Y.F.; Silay, M.S.; Tekgul, S.; et al. EAU/ESPU guidelines on the management of neurogenic bladder in children and adolescent part I diagnostics and conservative treatment. Neurourol. Urodyn. 2020, 39, 45–57. [Google Scholar] [CrossRef]
- Tanaka, H.; Kakizaki, H.; Kobayashi, S.; Shibata, T.; Ameda, K.; Koyanagi, T. The relevance of urethral resistance in children with myelodysplasia: Its impact on upper urinary tract deterioration and the outcome of conservative management. J. Urol. 1999, 161, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Malakounides, G.; Lee, F.; Murphy, F.; Boddy, S.-A. Single centre experience: Long term outcomes in spina bifida patients. J. Pediatr. Urol. 2013, 9, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.W.; Carr, M.C.; Adzick, N.S.; Burrows, P.K.; Thomas, J.C.; Thom, E.A.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Farmer, D.; et al. Bladder Function After Fetal Surgery for Myelomeningocele. Pediatrics 2015, 136, e906–e913. [Google Scholar] [CrossRef] [PubMed]
- Janzen, J.; Vuong, P.N.; Bersch, U.; Michel, D.; Zaech, G.A. Bladder tissue biopsies in spinal cord injured patients: Histopathologic aspects of 61 cases. Neurourol. Urodyn. 1998, 17, 525–530. [Google Scholar] [CrossRef]
- Deveaud, C.M.; Macarak, E.J.; Kucich, U.; Ewalt, D.H.; Abrams, W.R.; Howard, P.S. Molecular analysis of collagens in bladder fibrosis. J. Urol. 1998, 160, 1518–1527. [Google Scholar] [CrossRef]
- Speakman, M.; Brading, A.; Gilpin, C.; Dixon, J.; Gilpin, S.; Gosling, J. Bladder Outflow Obstruction—A Cause of Denervation Supersensitivity. J. Urol. 1987, 138, 1461–1466. [Google Scholar] [CrossRef]
- Fry, C.H.; Bayliss, M.; Young, J.S.; Hussain, M. Influence of age and bladder dysfunction on the contractile properties of isolated human detrusor smooth muscle. BJU Int. 2010, 108, E91–E96. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, R.; Wang, X.; He, D. Imbalance between matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinase-1 (TIMP-1) contributes to bladder compliance changes in rabbits with partial bladder outlet obstruction (PBOO). BJU Int. 2013, 112, E391–E397. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Körberg, I.B.; Hofmeister, W.; Markljung, E.; Cao, J.; Nilsson, D.; Ludwig, M.; Draaken, M.; Holmdahl, G.; Barker, G.; Reutter, H.; et al. WNT3 involvement in human bladder exstrophy and cloaca development in zebrafish. Hum. Mol. Genet. 2015, 24, 5069–5078. [Google Scholar] [CrossRef]
- Johal, N.; Arthurs, C.; Cuckow, P.; Cao, K.; Wood, D.; Ahmed, A.; Fry, C. Functional, histological and molecular characteristics of human exstrophy detrusor. J. Pediatr. Urol. 2019, 15, 154.e1–154.e9. [Google Scholar] [CrossRef]
- Johal, N.; Cao, K.; Arthurs, C.; Millar, M.; Thrasivoulou, C.; Ahmed, A.; Jabr, R.I.; Wood, D.; Cuckow, P.; Fry, C.H. Contractile function of detrusor smooth muscle from children with posterior urethral valves—The role of fibrosis. J. Pediatr. Urol. 2021, 17, 100.e1–100.e10. [Google Scholar] [CrossRef]
- Johal, N.; Wood, D.N.; Wagg, A.S.; Cuckow, P.; Fry, C.H. Functional properties and connective tissue content of pediatric human detrusor muscle. Am. J. Physiol. Physiol. 2014, 307, F1072–F1079. [Google Scholar] [CrossRef][Green Version]
- Harrison, S.C.W.; Hunnam, G.R.; Farman, P.; Ferguson, D.R.; Doyle, P.T. Bladder Instability and Denervation in Patients with Bladder Outflow Obstruction. BJU Int. 1987, 60, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Sellers, D.J.; McKay, N.G.; Chapple, C.R.; Chess-Williams, R. Muscarinic receptor function, density and G-protein coupling in the overactive diabetic rat bladder. Auton. Autacoid. Pharmacol. 2006, 26, 303–309. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sugita, S.; Nagayama, K. Tensile properties of smooth muscle cells, elastin, and collagen fibers. In Vascular Engineering 2016; Tanishita, K., Yamamoto, K., Eds.; Springer: Tokyo, Japan, 2016. [Google Scholar]
- Akhtar, R.; Sherratt, M.J.; Cruickshank, J.K.; Derby, B. Characterizing the elastic properties of tissues. Mater. Today 2011, 14, 96–105. [Google Scholar] [CrossRef]
- Leal da Cruz, M.; Liguori, R.; Garrone, G.; Leslie, B.; Ottoni, S.L.; Carvalheiro, S.; Moron, A.F.; Ortiz, V.; Macedo, A. Categorization of bladder dynamics and treatment after fetal myelomeningocele mepair: First 50 cases prospectively assessed. J. Urol. 2014, 193, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Shah, M.A.; Babu, D.M. Symptomatic lower urinary tract dysfunction in sacral agenesis: Potentially high risk? Indian J. Urol. 2018, 34, 56–61. [Google Scholar] [CrossRef]
- Compérat, E.; Reitz, A.; Delcourt, A.; Capron, F.; Denys, P.; Chartier-Kastler, E. Histologic Features in the Urinary Bladder Wall Affected from Neurogenic Overactivity—A Comparison of Inflammation, Oedema and Fibrosis With and Without Injection of Botulinum Toxin Type A. Eur. Urol. 2006, 50, 1058–1064. [Google Scholar] [CrossRef]
- Keller, E.E.; Patras, I.; Hutu, I.; Roider, K.; Sievert, K.; Aigner, L.; Janetschek, G.; Lusuardi, L.; Zimmermann, R.; Bauer, S. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourol. Urodyn. 2020, 39, 586–593. [Google Scholar] [CrossRef]
- Young, J.S.; Matharu, R.; Carew, M.A.; Fry, C.H. Inhibition of stretching-evoked ATP release from bladder mucosa by anticholinergic agents. BJU Int. 2012, 110, E397–E401. [Google Scholar] [CrossRef] [PubMed]
- Hanna-Mitchell, A.T.; Wolf-Johnston, A.S.; Barrick, S.R.; Kanai, A.J.; Chancellor, M.B.; de Groat, W.C.; Birder, L.A. Effect of botulinum toxin A on urothelial-release of ATP and expression of SNARE targets within the urothelium. Neurourol. Urodyn. 2015, 34, 79–84. [Google Scholar] [PubMed]
- Pascali, M.P.; Mosiello, G.; Boldrini, R.; Salsano, M.L.; Castelli, E.; De Gennaro, M. Effects of Botulinum Toxin Type A in the Bladder Wall of Children with Neurogenic Bladder Dysfunction: A Comparison of Histological Features Before and After Injections. J. Urol. 2011, 185, 2552–2557. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, S.; Yagmur, I.; Parlar, Y.; Özel, Ş.K.; Akyildiz, C.; Avanoglu, A.; Ulman, I. Botulinum injection is useless on fibrotic neuropathic bladders. J. Pediatr. Urol. 2015, 11, 27.e1–27.e4. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Tingskov, S.J.; Djurhuus, J.C.; Nørregaard, R.; Olsen, L.H. Can bladder fibrosis in congenital urinary tract obstruction be reversed? J. Pediatr. Urol. 2017, 13, 574–580. [Google Scholar] [CrossRef]
- Fry, C.H.; Chakrabarty, B.; Hashitani, H.; Andersson, K.; McCloskey, K.; Jabr, R.I.; Drake, M. New targets for overactive bladder—ICI-RS 2109. Neurourol. Urodyn. 2020, 39, S113–S121. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Zabbarova, I.V.; Birder, L.A.; Wipf, P.; Getchell, S.E.; Tyagi, P.; Fry, C.H.; Drake, M.J.; Kanai, A.J. Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice. Neurourol. Urodyn. 2018, 37, 2441–2451. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Penn, L. Reflecting on 25 years with MYC. Nat. Rev. Cancer 2008, 8, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Miao, N.; Wang, B.; Xu, J.; Gan, X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Yang, L.; et al. c-Myc promotes renal fibrosis by inducing integrin αv-mediated transforming growth factor-β signaling. Kidney Int. 2017, 92, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Cubero, F.J.; Nevzorova, Y.A. c-MYC—Making Liver Sick: Role of c-MYC in Hepatic Cell Function, Homeostasis and Disease. Genes 2017, 8, 123. [Google Scholar] [CrossRef]
- Hofmann, J.W.; Zhao, X.; De Cecco, M.; Peterson, A.L.; Pagliaroli, L.; Manivannan, J.; Hubbard, G.B.; Ikeno, Y.; Zhang, Y.; Feng, B.; et al. Reduced Expression of MYC Increases Longevity and Enhances Healthspan. Cell 2015, 160, 477–488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johal, N.; Cao, K.X.; Xie, B.; Millar, M.; Davda, R.; Ahmed, A.; Kanai, A.J.; Wood, D.N.; Jabr, R.I.; Fry, C.H. Contractile and Structural Properties of Detrusor from Children with Neurogenic Lower Urinary Tract Dysfunction. Biology 2021, 10, 863. https://doi.org/10.3390/biology10090863
Johal N, Cao KX, Xie B, Millar M, Davda R, Ahmed A, Kanai AJ, Wood DN, Jabr RI, Fry CH. Contractile and Structural Properties of Detrusor from Children with Neurogenic Lower Urinary Tract Dysfunction. Biology. 2021; 10(9):863. https://doi.org/10.3390/biology10090863
Chicago/Turabian StyleJohal, Navroop, Kevin X. Cao, Boyu Xie, Michael Millar, Reena Davda, Aamir Ahmed, Anthony J. Kanai, Dan N. Wood, Rita I. Jabr, and Christopher H. Fry. 2021. "Contractile and Structural Properties of Detrusor from Children with Neurogenic Lower Urinary Tract Dysfunction" Biology 10, no. 9: 863. https://doi.org/10.3390/biology10090863
APA StyleJohal, N., Cao, K. X., Xie, B., Millar, M., Davda, R., Ahmed, A., Kanai, A. J., Wood, D. N., Jabr, R. I., & Fry, C. H. (2021). Contractile and Structural Properties of Detrusor from Children with Neurogenic Lower Urinary Tract Dysfunction. Biology, 10(9), 863. https://doi.org/10.3390/biology10090863





