Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Photoperiod Experiments in Climate Chambers
2.3. Photosynthesis
2.3.1. Light Curves (LC)
2.3.2. Kautsky Curve Fluorescence Induction Decay (KC)
2.3.3. Fast Fluorescence Transient Analysis (OJIP)
2.4. Statistical Analysis
3. Results
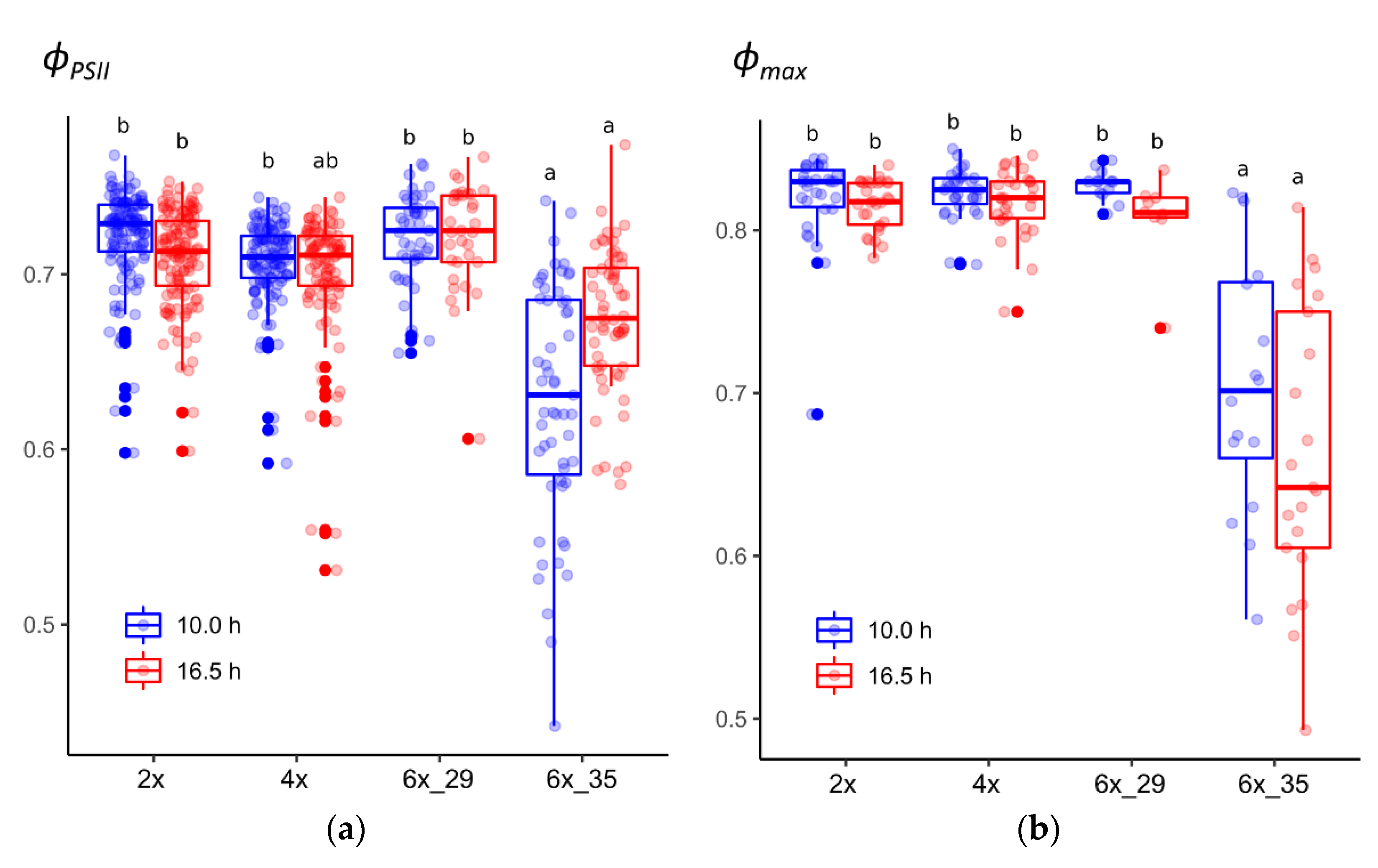
3.1. Cytotype 6x_35 Showed Lower PSII Potential Quantum Yield (ϕPSII) in Both Photoperiods
3.2. Cytotype 6x_35 Showed Lower PSII Maximum Quantum Efficiency (ϕmax) in Both Photoperiods
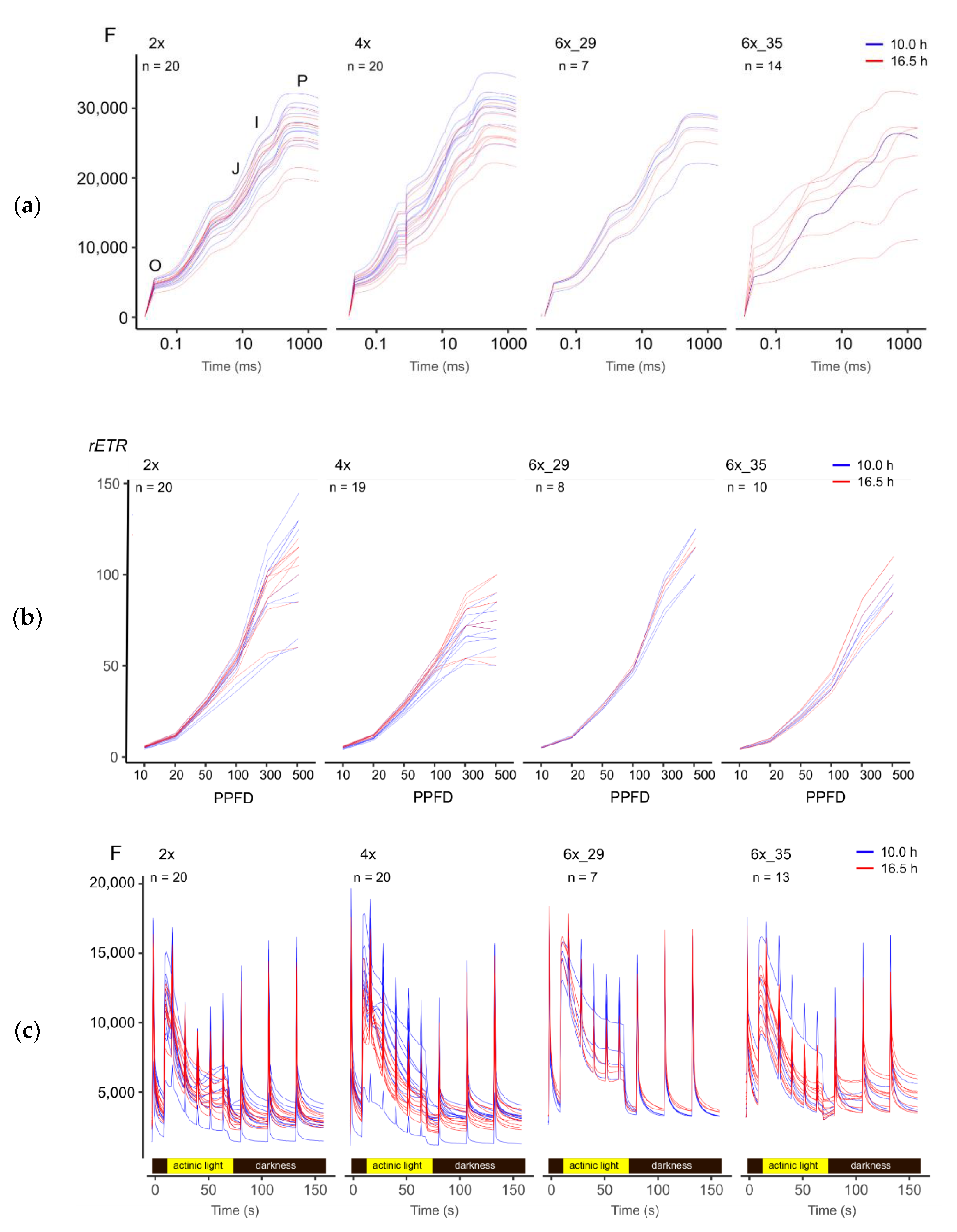
3.3. A Visual Comparison of Fluorescence Experimental Data Hinted at Subtle Differences between Ploidies and Photoperiods
3.4. Photoperiod and Ploidy Effects
3.4.1. OJIP Experiment
3.4.2. Light Curve (LC) Experiment
3.4.3. Kautsky Curce (KC) Experiment
4. Discussion
4.1. Ploidy and Photoperiod Did Not Affect Quantum Yield in General
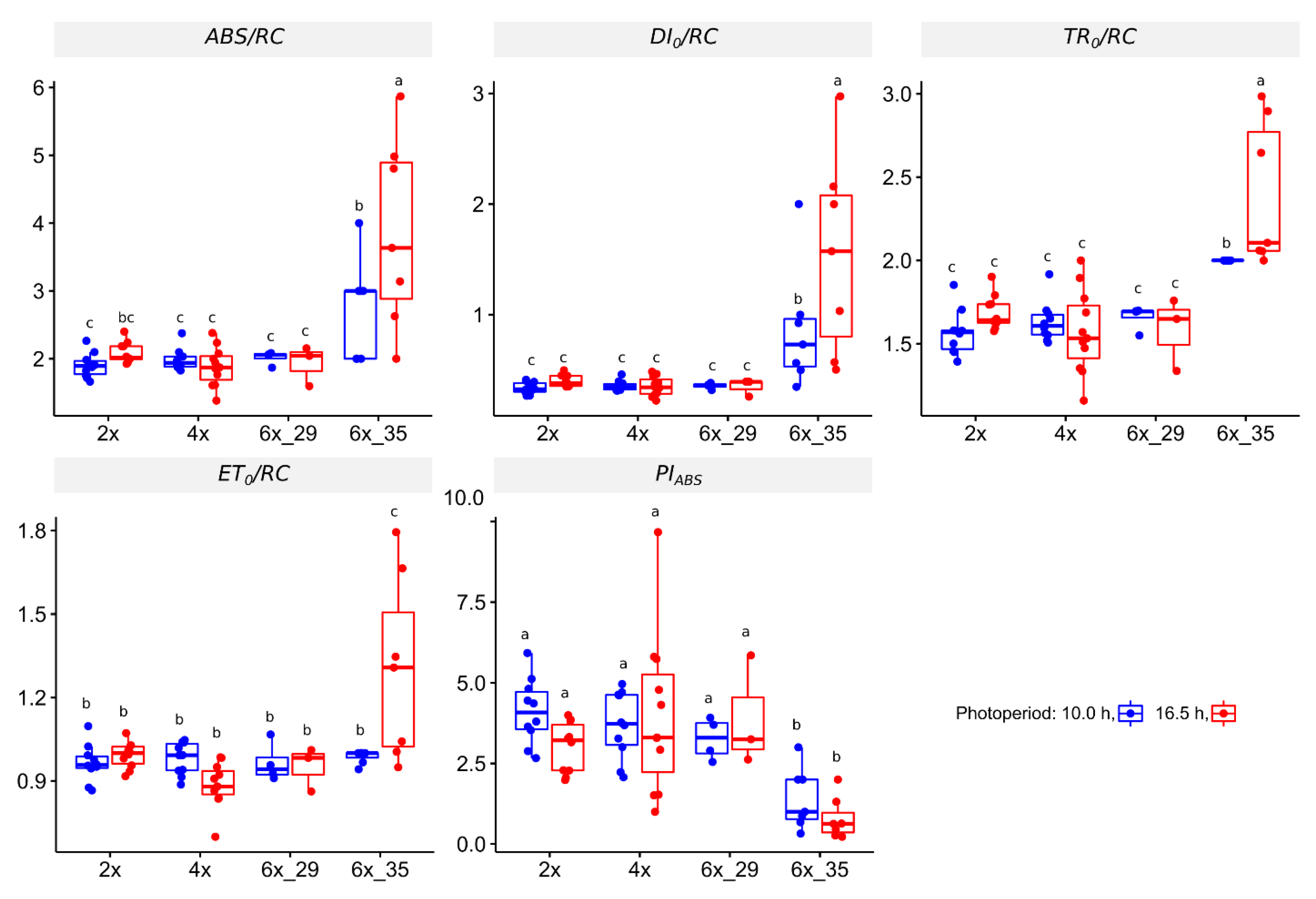
4.2. Fast Fluorescence Transient Analyis (OJIP) Identified Stress in Cytotype 6x_35
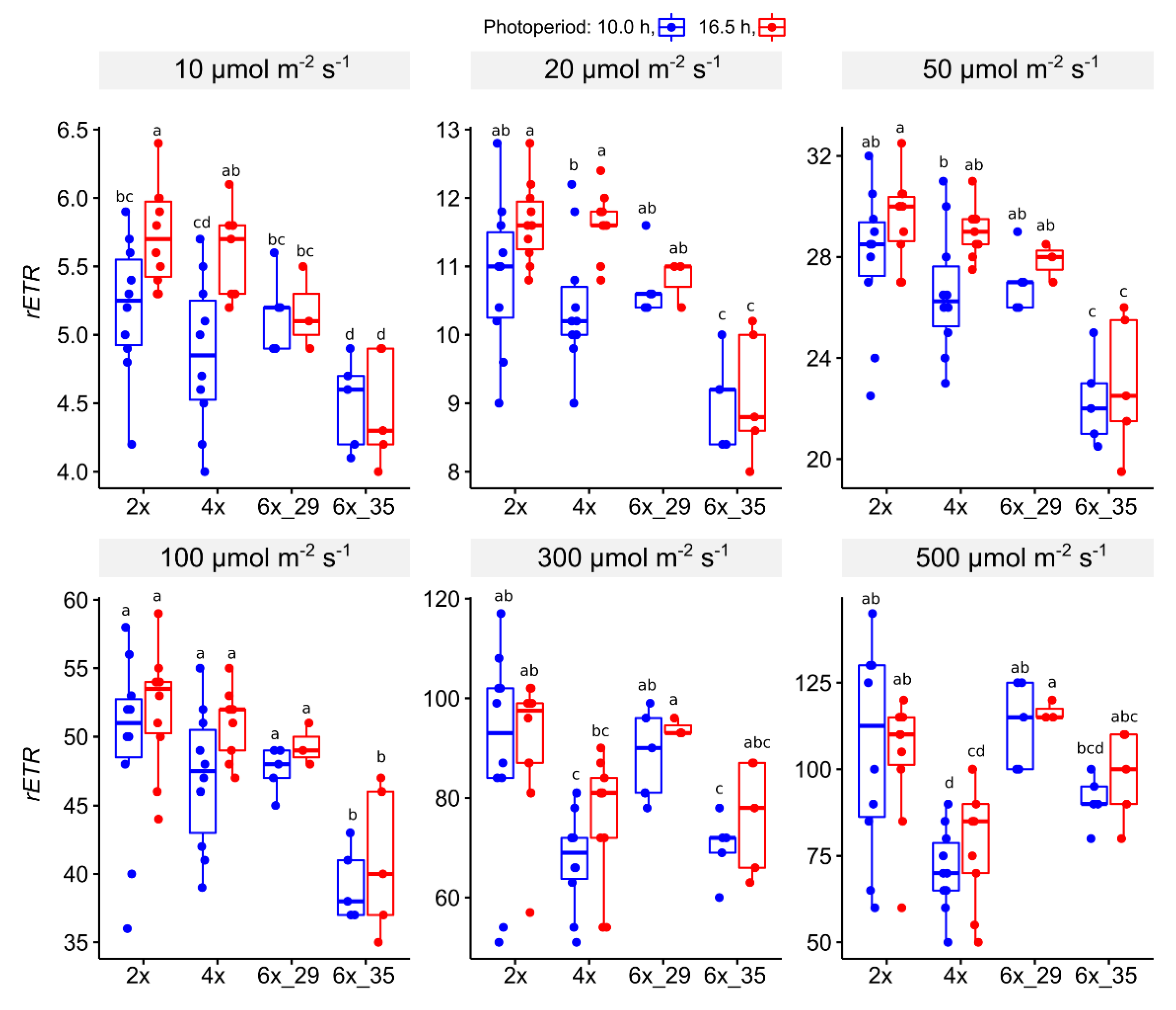
4.3. Light Curve (LC) Experiments Showed That Increasing Actinic Light Caused 4x Cytotypes to Show Lower rETRs and 6x_35 to Resemble 2x and 6x_29 Cytotypes
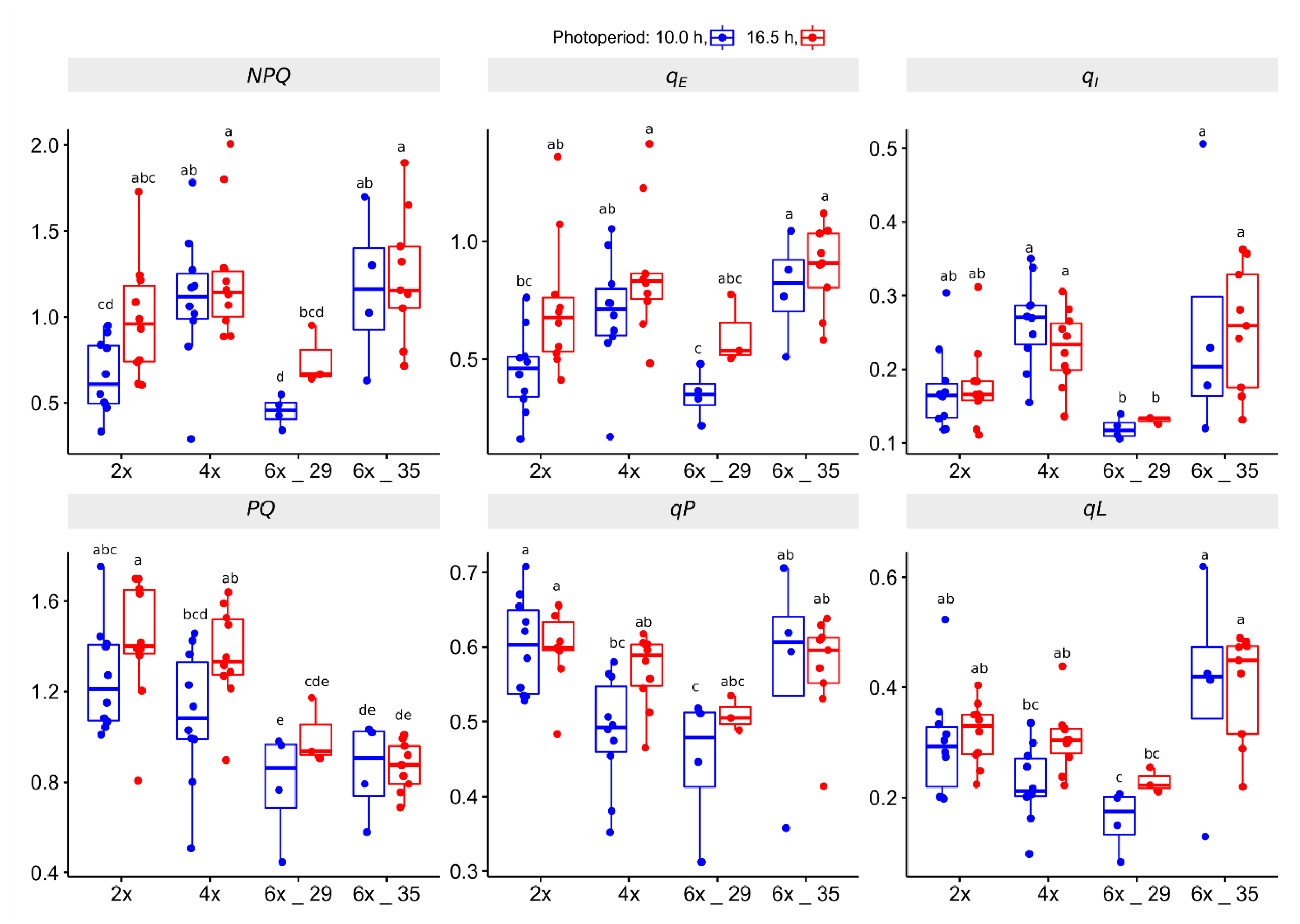
4.4. Kautsky Curve (KC) Analyses Identify Polploids as More Efficient Photosynthesis Quenchers
4.5. Photosynthesis, Habitat Adaptation, and Apomixis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landis, J.B.; Soltis, D.E.; Li, Z.; Marx, H.E.; Barker, M.S.; Tank, D.C.; Soltis, P.S. Impact of whole-genome duplication events on diversification rates in angiosperms. Am. J. Bot. 2018, 105, 348–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S.P. Polyploidy and interspecific hybridization: Partners for adaptation, speciation and evolution in plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hortic. Res. 2020, 7, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourkisti, R.; Froelicher, Y.; Herbette, S.; Morillon, R.; Tomi, F.; Gibernau, M.; Giannettini, J.; Berti, L.; Santini, J. Triploid Citrus genotypes have a better tolerance to natural chilling conditions of photosynthetic capacities and specific leaf volatile organic compounds. Front. Plant Sci. 2020, 11, 330. [Google Scholar] [CrossRef] [Green Version]
- Coate, J.E.; Powell, A.F.; Owens, T.G.; Doyle, J.J. Transgressive physiological and transcriptomic responses to light stress in allopolyploid Glycine dolichocarpa (Leguminosae). Heredity 2013, 110, 160–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, D.A.; Edwards, G.E. Effects of polyploidy on photosynthesis. Photosynth. Res. 1993, 35, 135–147. [Google Scholar] [CrossRef]
- Coate, J.E.; Luciano, A.K.; Seralathan, V.; Minchew, K.J.; Owens, T.G.; Doyle, J.J. Anatomical, biochemical, and photosynthetic responses to recent allopolyploidy in Glycine dolichocarpa (Fabaceae). Am. J. Bot. 2012, 99, 55–67. [Google Scholar] [CrossRef]
- Münzbergová, Z.; Haisel, D. Effects of polyploidization on the contents of photosynthetic pigments are largely population-specific. Photosynth. Res. 2019, 140, 289–299. [Google Scholar] [CrossRef]
- Schoenfelder, K.P.; Fox, D.T. The expanding implications of polyploidy. J. Cell Biol. 2015, 209, 485–491. [Google Scholar] [CrossRef]
- Jeong, S.; Clark, S.E. Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 2005, 169, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Bauerle, W.L.; Oren, R.; Way, D.A.; Qian, S.S.; Stoy, P.C.; Thornton, P.E.; Bowden, J.D.; Hoffman, F.M.; Reynolds, R.F. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. PNAS 2012, 109, 8612–8617. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Kume, A.; Hanba, Y.T. Seasonal variations in photosynthetic functions of the urban landscape tree species Gingko biloba: Photoperiod is a key trait. Trees 2021, 35, 273–285. [Google Scholar] [CrossRef]
- Wu, Z.; Skelvåg, A.O.; Baadshaug, O.H. Quantification of photoperiodic effects on growth of Phleum pratense. Ann. Bot. 2004, 94, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulpice, R.; Flis, A.; Ivakov, A.A.; Apelt, F.; Krohn, N.; Encke, B.; Abel, C.; Feil, R.; Lunn, J.E.; Stitt, M. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol. Plant 2014, 7, 137–155. [Google Scholar] [CrossRef]
- Webb, A.A.R.; Seki, M.; Satake, A.; Caldana, C. Continuous dynamic adjustment of the plant circadian oscillator. Nat. Commun. 2019, 10, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2020, 186, 79–92. [Google Scholar] [CrossRef]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 2012, 1817, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Garab, G.; Adams, W., III; Govindjee. Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Sato, S.; Kamiyama, M.; Iwata, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicon esculentum by disrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Ma, X.; Su, Z.; Ma, H. Molecular genetic analyses of abiotic stress responses during plant reproductive development. J. Exp. Bot. 2020, 71, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Asker, S.E.; Jerling, L. Apomixis in Plants; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Mateo de Arias, M.; Gao, L.; Sherwood, D.A.; Dwivedi, K.K.; Price, B.J.; Jamison, M.; Kowallis, B.M.; Carman, J.G. Whether gametophytes are reduced or unreduced in angiosperms might be determined metabolically. Genes 2020, 11, 1449. [Google Scholar] [CrossRef]
- Klatt, S.; Hadacek, F.; Hodač, L.; Brinkmann, G.; Eilerts, M.; Hojsgaard, D.; Hörandl, E. Photoperiod extension enhances sexual megaspore formation and triggers metabolic reprogramming in facultative apomictic Ranunculus auricomus. Front. Plant Sci. 2016, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Ulum, F.B.; Costa Castro, C.; Hörandl, E. Ploidy-dependent effects of light stress on the mode of reproduction in the Ranunculus auricomus complex (Ranunculaceae). Front. Plant Sci. 2020, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Selva, J.P.; Zappacosta, D.; Carballo, J.; Rodrigo, J.M.; Bellido, A.; Gallo, C.A.; Gallardo, J.; Echenique, V. Genes modulating the increase in sexuality in the facultative diplosporous grass Eragrostis curvula under water stress conditions. Genes 2020, 11, 969. [Google Scholar] [CrossRef]
- Karunarathne, P.; Reutemann, A.V.; Schedler, M.; Glücksberg, A.; Martínez, E.J.; Honfi, A.I.; Hojsgaard, D.H. Sexual modulation in a polyploid grass: A reproductive contest between environmentally inducible sexual and genetically dominant apomictic pathways. Sci. Rep. 2020, 10, 8319. [Google Scholar] [CrossRef]
- Hörandl, E.; Hadacek, F. The oxidative damage initiation hypothesis for meiosis. Plant Reprod. 2013, 26, 351–367. [Google Scholar] [CrossRef] [Green Version]
- de Storme, N.; Geelen, D. Sexual polyploidization in plants cytological mechanisms and molecular regulation. New Phytol. 2013, 198, 670–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barke, B.H.; Karbstein, K.; Daubert, M.; Hörandl, E. The relation of meiotic behaviour to hybridity, polyploidy and apomixis in the Ranunculus auricomus complex (Ranunculaceae). BMC Plant Biol. 2020, 20, 523. [Google Scholar] [CrossRef] [PubMed]
- Hojsgaard, D.; Greilhuber, J.; Pellino, M.; Paun, O.; Sharbel, T.F.; Hörandl, E. Emergence of apospory and bypass of meiosis via apomixis after sexual hybridisation and polyploidisation. New Phytol. 2014, 204, 1000–1012. [Google Scholar] [CrossRef] [Green Version]
- Pellino, M.; Hojsgaard, D.; Hörandl, E.; Sharbel, T.F. Chasing the Apomictic Factors in the Ranunculus auricomus Complex: Exploring Gene Expression Patterns in Microdissected Sexual and Apomictic Ovules. Genes 2020, 11, 728. [Google Scholar] [CrossRef]
- Barke, B.H.; Daubert, M.; Hörandl, E. Establishment of apomixis in diploid F2 hybrids and Inheritance of apospory from F1 to F2 hybrids of the Ranunculus auricomus complex. Front. Plant Sci. 2018, 9, 1111. [Google Scholar] [CrossRef]
- Nogler, G.A. Genetics of apospory in apomictic Ranunculus auricomus. V: Conclusion. Bot. Helv. 1984, 94, 411–422. [Google Scholar]
- Hodač, L.; Scheben, A.P.; Hojsgaard, D.; Paun, O.; Hörandl, E. ITS polymorphisms shed light on hybrid evolution in apomictic plants: A case study on the Ranunculus auricomus complex. PLoS ONE 2014, 9, e103003. [Google Scholar] [CrossRef] [PubMed]
- Hörandl, E.; Dobeš, C.; Lambrou, M. Chromosomen- und Pollenuntersuchungen an östereichischen Arten des apomiktischen Ranunculus auricomus-Komplexes. Bot. Helv. 1997, 107, 195–209. [Google Scholar]
- Hörandl, E.; Greilhuber, J.; Klimova, K.; Paun, O.; Temsch, E.; Emadzade, K.; Hodalova, I. Reticulate evolution and taxonomic concepts in the Ranunculus auricomus complex (Ranunculaceae): Insights from analysis of morphological, karyological and molecular data. Taxon 2009, 58, 1194–1215. [Google Scholar] [CrossRef] [PubMed]
- Karbstein, K.; Tomasello, S.; Hodač, L.; Lorberg, E.; Daubert, M.; Hörandl, E. Moving beyond assumptions: Polyploidy and environmental effects explain a geographical parthenogenesis scenario in European plants. Mol. Ecol. 2021, 30, 2659–2675. [Google Scholar] [CrossRef]
- Pellino, M.; Hojsgaard, D.; Schmutzer, T.; Scholz, U.; Hörandl, E.; Vogel, H.; Sharbel, T.F. Asexual genome evolution in the apomictic Ranunculus auricomus complex: Examining the effects of hybridization and mutation accumulation. Mol. Ecol. 2013, 22, 5908–5921. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation, and Adaptation; Yunus, M., Ed.; Taylor & Francis: London, UK, 2000; pp. 445–483. ISBN 0748408215. [Google Scholar]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. In vivo assessment of stress impact on plant’s vitality: Applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In Mycorrhiza: State of the Art, Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematics, 3rd ed.; Varma, A., Ed.; Springer: Berlin, Germany, 2008; pp. 679–703. ISBN 978-3-540-78824-9. [Google Scholar]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Fiaz, S.; Wang, X.; Younas, A.; Alharthi, B.; Riaz, A.; Ali, H. Apomixis and strategies to induce apomixis to preserve hybrid vigor for multiple generations. GM Crops Food 2021, 12, 57–70. [Google Scholar] [CrossRef]
- Schmidt, A. Controlling apomixis: Shared features and distinct characteristics of gene regulation. Genes 2020, 11, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodač, L.; Barke, B.H.; Hörandl, E. Mendelian segregation of leaf phenotypes in experimental F 2 hybrids elucidates origin of morphological diversity of the apomictic Ranunculus auricomus complex. Taxon 2018, 67, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 3 September 2020).
- Wickham, H.; François, R.; Lionel, H.; Müller, K. Dplyr: A Grammar of Data Manipulation. 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 12 November 2020).
- Wickham, H. Tidyr: Tidy Messy Data. 2021. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 12 November 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 12 November 2020).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility mong packages for zero-inflated generalized linear mixed modeling. R. J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M. Stress tolerance of photosystem ii in vivo: Antagonistic effects of water, heat, and photoinhibition stresses. Plant Physiol. 1992, 100, 424–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ježilová, E.; Nožková-Hlaváčková, V.; Duchoslav, M. Photosynthetic characteristics of three ploidy levels of Allium oleraceum L. (Amaryllidaceae) differing in ecological amplitude. Plant Cell Environ. 2015, 30, 212–224. [Google Scholar] [CrossRef]
- Achenbach, L.; Lambertini, C.; Brix, H. Phenotypic traits of Phragmites australis clones are not related to ploidy level and distribution range. AoB Plants 2012, 2012, pls017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Q.; Zhang, X.; Gao, X.; Wang, L.; Jia, G. Effects of ploidy level on the cellular, photochemical and photosynthetic characteristics in Lilium FO hybrids. Plant Physiol. Biochem. 2018, 133, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Leonardos, E.D.; Ma, X.; Lanoue, J.; Grodzinski, B. Leaf and whole-plant gas exchange and water-use efficiency of chrysanthemums under HPS and LEDs during the vegetative and flower-induction stages. Can. J. Plant Sci. 2019, 99, 639–653. [Google Scholar] [CrossRef]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; Gawroński, P.; Karpiński, S. Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 6679–6695. [Google Scholar] [CrossRef] [Green Version]
- Weaver, G.; van Iersel, M.W. Longer photoperiods with adaptive lighting control can improve growth of greenhouse-grown ‘Little Gem’ lettuce (Lactuca sativa). Hortscience 2020, 55, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Li, X.; Mendanha dos Santos, T.; Rosenqvist, E.; Ottosen, C.-O. Combined high light and heat stress induced complex response in tomato with better leaf cooling after heat priming. Plant Physiol. Biochem. 2020, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Govindjee. The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: Definitions, timelines, viewpoints, open questions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–44. ISBN 978-94-017-9031-4. [Google Scholar]
- Ribeiro, R.V.; Machado, E.C.; de Oliveira, R.F. Temperature response of photosynthesis and its interaction with light intensity in sweet orange leaf discs under non-photorespiratory condition. Ciênc. Agrotec. 2006, 30, 670–678. [Google Scholar] [CrossRef]
- Rakić, T.; Gajić, G.; Lazarević, M.; Stevanović, B. Effects of different light intensities, CO2 concentrations, temperatures and drought stress on photosynthetic activity in two paleoendemic resurrection plant species Ramonda serbica and R. nathaliae. Environ. Exp. Bot. 2015, 109, 63–72. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Paun, O.; Greilhuber, J.; Temsch, E.M.; Hörandl, E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae). Mol. Ecol. 2006, 15, 897–910. [Google Scholar] [CrossRef]
- Schinkel, C.C.F.; Syngelaki, E.; Kirchheimer, B.; Dullinger, S.; Klatt, S.; Hörandl, E. Epigenetic patterns and geographical parthenogenesis in the alpine plant species Ranunculus kuepferi (Ranunculaceae). Int. J. Mol. Sci. 2020, 21, 3318. [Google Scholar] [CrossRef] [PubMed]
| Ploidy | Origin |
|---|---|
| 2x | Synthetic F2 hybrid crosses of the sexual taxa R. carpaticola × R. notabilis that occur in forest sites in Central Europe [38]. |
| 4x | Open habitat-adapted meadow type grown from seeds of plants that were originally collected near Schönau, Mühlkreis, Austria (48°22′46.00″ N 14°44′46.00″ E, wet meadow) by L. Hodač and K. Spitzer (LH002). |
| 6x | Hexaploid plants (6x) were grown from seeds of natural hybrids of R. carpaticola × R. cassubicifolius from Slovakia (original clone 29 from a forest margin and clone 35 from a meadow [25,38]). Originally, both clones were combined to obtain sufficient replicate numbers. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulum, F.B.; Hadacek, F.; Hörandl, E. Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae). Biology 2021, 10, 811. https://doi.org/10.3390/biology10080811
Ulum FB, Hadacek F, Hörandl E. Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae). Biology. 2021; 10(8):811. https://doi.org/10.3390/biology10080811
Chicago/Turabian StyleUlum, Fuad Bahrul, Franz Hadacek, and Elvira Hörandl. 2021. "Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae)" Biology 10, no. 8: 811. https://doi.org/10.3390/biology10080811
APA StyleUlum, F. B., Hadacek, F., & Hörandl, E. (2021). Polyploidy Improves Photosynthesis Regulation within the Ranunculus auricomus Complex (Ranunculaceae). Biology, 10(8), 811. https://doi.org/10.3390/biology10080811







