Polyculture of Juvenile Dog Conch Laevistrombus canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Broodstock and Larval Rearing
2.2. Salinity Tolerance
2.3. Diet Experiments
2.3.1. Newly Metamorphosed Juveniles (1 mm)
2.3.2. Juveniles (10 mm)
2.4. Indoor Polyculture Experiments
2.5. Outdoor Polyculture Experiments
2.6. Statistical Analysis
3. Results
3.1. Salinity Tolerance
3.2. Diet Experiments
3.2.1. Newly Metamorphosed Juveniles (1 mm)
3.2.2. Juveniles (10 mm)
3.3. Indoor Polyculture Experiments
3.4. Outdoor Polyculture Experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cob, Z.C.; Arshad, A.; Bujang, J.S.; Abd Ghaffar, M. Description and evaluation of imposex in Strombus canarium Linnaeus, 1758 (Gastropoda, Strombidae): A potential bio-indicator of tributyltin pollution. Environ. Monit. Assess. 2011, 178, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Cob, Z.; Arshad, A.; Sidik, B.J.; Nurulhusna, W.; Mazlan, A. Feeding behaviour and stomach content analysis of Laevistrombus canarium (Linnaeus, 1758) from Merambong shoal, Johor. Malay. Nat. J. 2014, 66, 159–170. [Google Scholar]
- Husna, W.N.W.H.; Mazlan, A.G.; Cob, Z.C. Ontogenetic changes in feeding and food preferences of the dog conch Laevistrombus canarium Linnaeus 1758 (Mollusca: Gastropoda) from Merambong shoal, Malaysia. Chin. J. Oceanol. Limnol. 2017, 35, 1230–1238. [Google Scholar] [CrossRef]
- Hassan, W.N.H.W.; Sm, N.A.; Abd Ghaffar, M.; Cob, Z.C. Effects of temperature on food consumption of juveniles dog conch, Laevistrombus canarium (Linnaeus, 1758) in laboratory condition. J. Sustain. Sci. Manag. 2019, 14, 1–10. [Google Scholar]
- Supratman, O.; Syamsudin, T.S. Behavior and feeding habit of dog conch (Strombus turturella) in South Bangka Regency, Bangka Belitung islands province. El-Hayah J. Biol. 2016, 6, 15–21. [Google Scholar] [CrossRef]
- Ramses, R.; Syamsi, F.; Notowinarto, N. Length-weight relationship, growth patterns and sex ratio of dog conch Strombus Canarium Linnaeus, 1758 in the waters of Kota Batam. Omni-Akuatika 2019, 15, 19–29. [Google Scholar] [CrossRef]
- Cob, Z.C.; Arshad, A.; Ghaffar, M.A.; Bujang, J.S.; Muda, W.W. Development and growth of larvae of the Dog Conch, Strombus canarium (Mollusca: Gastropoda), in the laboratory. Zool. Stud. 2008, 48, 1–11. [Google Scholar]
- Cob, Z.C.; Arshad, A.; Idris, M.H.; Bujang, J.S.; Ghaffar, M.A. Sexual polymorphism in a population of Strombus canarium Linnaeus, 1758 (Mollusca: Gastropoda) at Merambong Shoal, Malaysia. Zool. Stud. 2008, 47, 318–325. [Google Scholar]
- Hassan, W.N.H.H.W.; Nurul-Amin, S.; Abd Ghaffar, M.; Cob, Z.C. Food consumption and assimilation of the adult dog conch Laevistrombus canarium (Linnaeus 1758) at different temperatures. Malays. J. Sci. 2019, 38, 98–114. [Google Scholar] [CrossRef]
- Yokoyama, H. Growth and food source of the sea cucumber Apostichopus japonicus cultured below fish cages—Potential for integrated multi-trophic aquaculture. Aquaculture 2013, 372–375, 28–38. [Google Scholar] [CrossRef]
- Yokoyama, H. Suspended culture of the sea cucumber Apostichopus japonicus below a Pacific oyster raft—Potential for integrated multi-trophic aquaculture. Aquac. Res. 2015, 46, 825–832. [Google Scholar] [CrossRef]
- Steinberg, P.D. Feeding Preferences of Tegula Funebralis and Chemical Defenses of Marine Brown Algae. Ecol. Monogr. 1985, 55, 333–349. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, H.; Li, B.; Mao, Y.; Jiang, Z.; Zhang, J.; Fang, J. Suitability of two seaweeds, Gracilaria lemaneiformis and Sargassum pallidum, as feed for the abalone Haliotis discus hannai Ino. Aquaculture 2010, 300, 189–193. [Google Scholar] [CrossRef]
- Angell, A.R.; Pirozzi, I.; de Nys, R.; Paul, N.A. Feeding Preferences and the Nutritional Value of Tropical Algae for the Abalone Haliotis asinina. PLoS ONE 2012, 7, e38857. [Google Scholar] [CrossRef]
- Iken, K. Feeding ecology of the Antarctic herbivorous gastropod Laevilacunaria antarctica Martens. J. Exp. Mar. Biol. Ecol. 1999, 236, 133–148. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Kashiyama, Y.; Ogawa, N.O.; Kitazato, H.; Ohkouchi, N. Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: Implications for aquatic food web studies. Mar. Ecol. Prog. Ser. 2007, 342, 85–90. [Google Scholar] [CrossRef]
- Cob, Z.; Bujang, J.; Ghaffar, M.; Arshed, A. Diversity and population structure characteristics of Strombus (Mesogastropod, Strombidae) in Johor Straits. Nat. Resour. Util. Environ. Preserv. Issues Chall. 2005, 2, 198–205. [Google Scholar]
- Abbott, R.T. The genus Strombus in the Indo-pacific. Indo-Pac. Mollusca 1960, 1, 33–146. [Google Scholar]
- Amini, S.; Pralampita, W. Pendugaan pertumbuhan beberapa parameter biologi gonggong (Strombus canarium) di perairan pantai Pulau Bintan-Riau. J. Pen. Perikan. Laut 1987, 41, 29–35. [Google Scholar]
- Erlambang, T. Some biology and ecology aspects of dog conch (Strombus canarium) based on a year round study in Riau province, Indonesia. J. Xiamen Fish. Coll. 1996, 18, 33–41. [Google Scholar]
- Fleurence, J.; Morançais, M.; Dumay, J.; Decottignies, P.; Turpin, V.; Munier, M.; Garcia-Bueno, N.; Jaouen, P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012, 27, 57–61. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Li, Y.; Liu, S.; Zhou, Y.; Zhang, L. Effects of different seaweed diets on growth, digestibility, and ammonia-nitrogen production of the sea cucumber Apostichopus japonicus (Selenka). Aquaculture 2012, 338–341, 304–308. [Google Scholar] [CrossRef]
- Xia, S.; Zhao, P.; Chen, K.; Li, Y.; Liu, S.; Zhang, L.; Yang, H. Feeding preferences of the sea cucumber Apostichopus japonicus (Selenka) on various seaweed diets. Aquaculture 2012, 344–349, 205–209. [Google Scholar] [CrossRef]
- Davis, M.; Cassar, V. Queen conch aquaculture: Hatchery and nursery phases. J. Shellfish Res. 2020, 39, 731–810. [Google Scholar] [CrossRef]
- Xiao, B.C.; Li, E.C.; Du, Z.Y.; Jiang, R.L.; Chen, L.Q.; Yu, N. Effects of temperature and salinity on metabolic rate of the Asiatic clam Corbicula fluminea (Müller, 1774). SpringerPlus 2014, 3, 455. [Google Scholar] [CrossRef]
- Moussa, R.M. The potential impacts of low and high salinities on salinity tolerance and condition index of the adult pearl oyster Pinctada imbricata radiata (Leach, 1814). J. Basic Appl. Zool. 2018, 79, 12. [Google Scholar] [CrossRef]
- Delorme, N.J.; Sewell, M.A. Temperature and salinity: Two climate change stressors affecting early development of the New Zealand sea urchin Evechinus chloroticus. Mar. Biol. 2014, 161, 1999–2009. [Google Scholar] [CrossRef]
- Sobral, P.; Widdows, J. Effects of elevated temperatures on the scope for growth and resistance to air exposure of the clam Ruditapes decussatus (L.), from southern Portugal. Sci. Mar. 1997, 61, 163–171. [Google Scholar]
- Resgalla, C., Jr.; Brasil, E.d.S.; Salomão, L.C. The effect of temperature and salinity on the physiological rates of the mussel Perna perna (Linnaeus 1758). Braz. Arch. Biol. Technol. 2007, 50, 543–556. [Google Scholar] [CrossRef]
- Dame, R.F.; Kenneth, M.J. Ecology of Marine Bivalves: An Ecosystem Approach, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Toral-Granda, V.; Lovatelli, A.; Vasconcellos, M. Sea cucumbers. In A Global Review of Fisheries and Trade; FAO Fisheries and Aquaculture Technical Paper. No. 516; FAO: Rome, Italy, 2008; p. 317. [Google Scholar]
- Katow, H.; Okumura, S.; Sakai, Y.; Shibuya, C. Sea cucumber farming in Japan. In Echinoderm Aquaculture; Wiley: Hoboken, NJ, USA, 2015; pp. 287–316. [Google Scholar]
- Metaxas, A. The effect of salinity on larval survival and development in the sea urchin Echinometra lucunter. Invertebr. Reprod. Dev. 1998, 34, 323–330. [Google Scholar] [CrossRef]
- Montory, J.A.; Chaparro, O.R.; Pechenik, J.A.; Diederich, C.M.; Cubillos, V.M. Impact of short-term salinity stress on larval development of the marine gastropod Crepipatella fecunda (Calyptraeidae). J. Exp. Mar. Biol. Ecol. 2014, 458, 39–45. [Google Scholar] [CrossRef]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated multitrophic aquaculture applied to shrimp rearing in a biofloc system. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Robinson, G.; Slater, M.J.; Jones, C.L.W.; Stead, S.M. Role of sand as substrate and dietary component for juvenile sea cucumber Holothuria scabra. Aquaculture 2013, 392–395, 23–25. [Google Scholar] [CrossRef]
- Heflin, L.E.; Makowsky, R.; Taylor, J.C.; Williams, M.B.; Lawrence, A.L.; Watts, S.A. Production and economic optimization of dietary protein and carbohydrate in the culture of Juvenile Sea Urchin Lytechinus variegatus. Aquaculture 2016, 463, 51–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hannah, L.; Pearce, C.M.; Cross, S.F. Growth and survival of California sea cucumbers (Parastichopus californicus) cultivated with sablefish (Anoplopoma fimbria) at an integrated multi-trophic aquaculture site. Aquaculture 2013, 406–407, 34–42. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, Y.; Yang, H.; Hu, C. Bottom culture of the sea cucumber Apostichopus japonicus Selenka (Echinodermata: Holothuroidea) in a fish farm, southern China. Aquac. Res. 2014, 45, 1434–1441. [Google Scholar] [CrossRef]
- Chopin, T. Integrated multi-trophic aquaculture–ancient, adaptable concept focuses on ecological integration. Glob. Aquac. Advocate 2013, 16, 16–19. [Google Scholar]
- Neori, A.; Shpige, M.; Guttman, L.; Israel, A. Development of Polyculture and Integrated Multi-Trophic Aquaculture (IMTA) in Israel: A Review. Isr. J. Aquac.-Bamidgeh 2017, 69, 20874. [Google Scholar]
- Fang, J.; Zhang, J.; Xiao, T.; Huang, D.; Liu, S. Integrated multi-trophic aquaculture (IMTA) in Sanggou Bay, China. Aquac. Environ. Interact. 2016, 8, 201–205. [Google Scholar] [CrossRef]
- Biswas, G.; Kumar, P.; Ghoshal, T.K.; Kailasam, M.; De, D.; Bera, A.; Mandal, B.; Sukumaran, K.; Vijayan, K.K. Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity and economic return in brackishwater ponds. Aquaculture 2020, 516, 734626. [Google Scholar] [CrossRef]
- Shpigel, M.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Neori, A.; Guttman, L. The sea urchin, Paracentrotus lividus, in an Integrated Multi-Trophic Aquaculture (IMTA) system with fish (Sparus aurata) and seaweed (Ulva lactuca): Nitrogen partitioning and proportional configurations. Aquaculture 2018, 490, 260–269. [Google Scholar] [CrossRef]



| Veligers | Salinity | 0 | 24 h | 48 h | 72 h | 96 h |
| 35 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 30 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 25 | 100 | 100 a | 100 a | 99 a | 98 a | |
| 20 | 100 | 80 b | 48 b | 19 b | 0 b | |
| 15 | 100 | 15 c | 0 c | 0 c | 0 b | |
| 10 | 100 | 10 d | 0 c | 0 c | 0 b | |
| Juvenile | salinity | 0 | 24 h | 48 h | Day 15 | Day 30 |
| 35 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 30 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 25 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 20 | 100 | 100 a | 100 a | 100 a | 100 a | |
| 15 | 100 | 100 a | 100 a | 72 b | 47 b | |
| 10 | 100 | 6 b | 0 b | 0 c | 0 c |
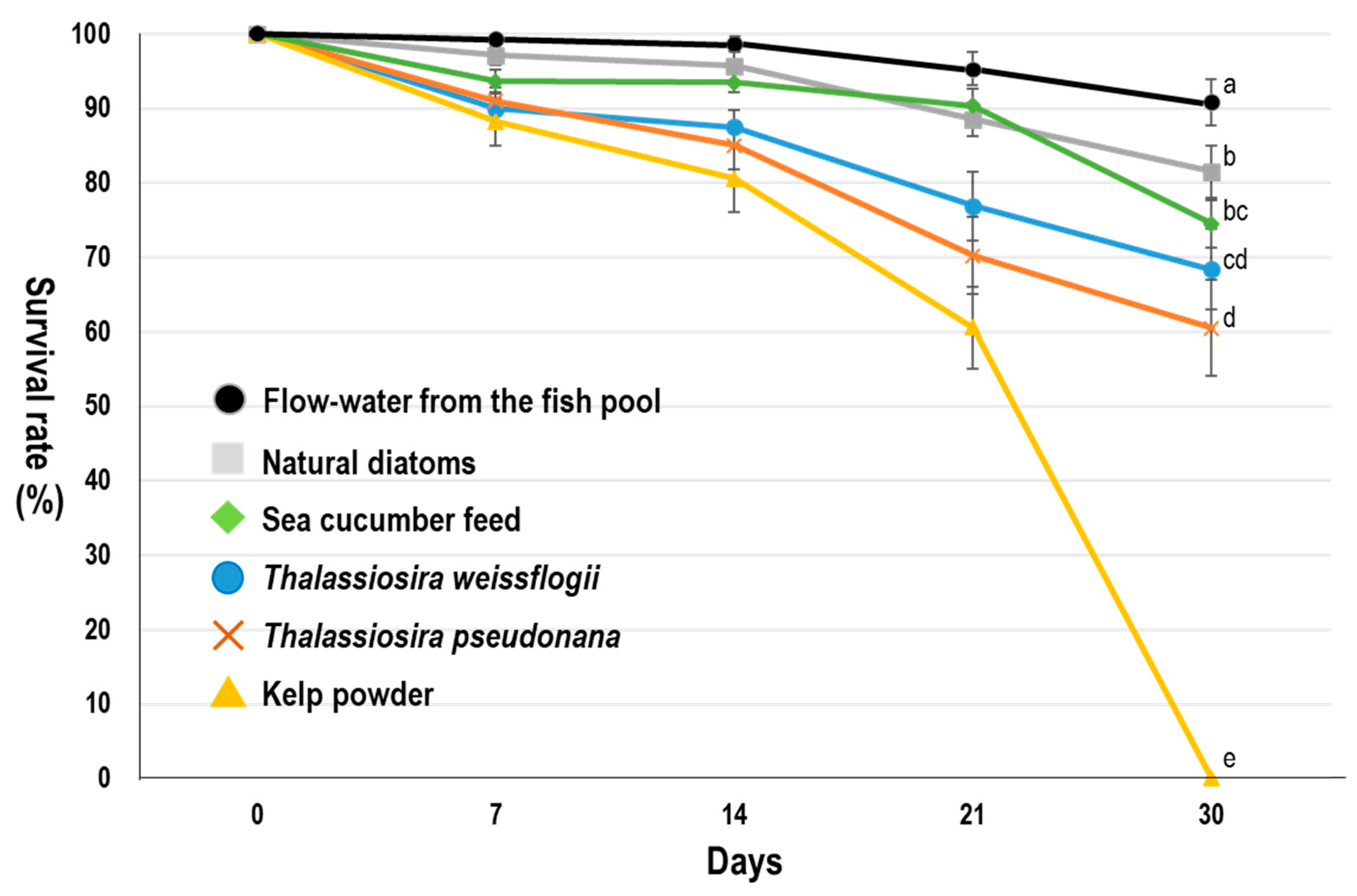
| Parameter | Flow-Water | Natural Diatoms | Sea Cucumber Feed | Kelp Powder | p |
|---|---|---|---|---|---|
| SL (mm) | 25.65 ± 4.75 a | 18.09 ± 2.10 b | 20.89 ± 3.79 c | 11.73 ± 2.35 d | *** |
| SW (mm) | 12.49 ± 2.51 a | 8.76 ± 1.06 b | 10.17 ± 2.01 c | 5.51 ± 1.21 d | *** |
| SD (mm) | 9.91 ± 1.88 a | 6.99 ± 0.80 b | 8.06 ± 1.49 c | 4.55 ± 0.95 d | *** |
| Weight (g) | 1.01 ± 0.51 a | 0.46 ± 0.21 b | 0.82 ± 0.16 c | 0.15 ± 0.25 d | *** |
| Daily growth (g) | 0.02 | 0.01 | 0.01 | 0 | - |
| Survival rate (%) | 100 | 100 | 100 | 76 | - |
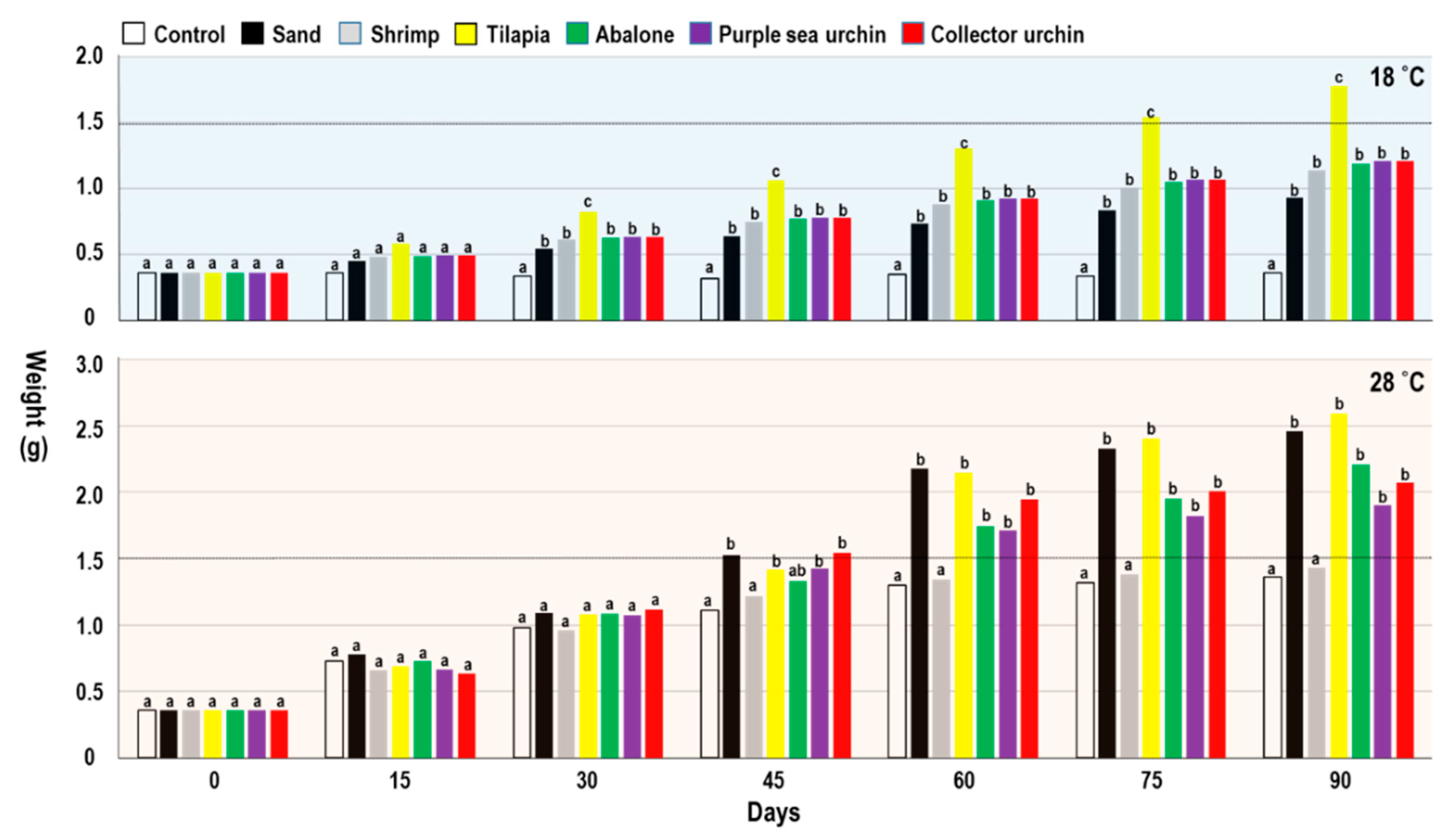
| 18 °C | Control | Sand | Shrimp | Tilapia | Abalone | PU | CU | p |
| SL (mm) | 18.17 ± 1.76 a | 18.00 ± 2.86 a | 18.77 ± 2.32 a | 22.54 ± 2.47 b | 20.09 ± 1.42 c | 20.48 ± 1.58 c | 20.11 ± 1.46 c | *** |
| SW (mm) | 8.85 ± 0.85 a | 9.10 ± 1.67 ab | 9.66 ± 1.26 c | 11.71 ± 1.22 d | 9.48 ± 0.65 bc | 10.03 ± 0.90 cd | 9.37 ± 0.91 cb | *** |
| SD (mm) | 6.81 ± 0.66 a | 7.41 ± 1.32 b | 8.08 ± 1.07 c | 9.06 ± 1.13 d | 7.40 ± 0.41 e | 7.48 ± 0.69 e | 7.78 ± 0.65 ce | *** |
| Weight (g) | 0.36 ± 0.1 a | 0.93 ± 0.4 b | 1.14 ± 0.42 c | 1.78 ± 0.32 d | 1.19 ± 0.12 c | 1.21 ± 0.28 c | 1.21 ± 0.34 c | *** |
| Daily growth (g) | 0.00 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | - |
| Survival rate (%) | 81 | 88 | 96 | 92 | 99 | 95 | 96 | - |
| 28°C | Control | Sand | Shrimp | Tilapia | Abalone | PU | CU | p |
| SL (mm) | 25.75 ± 2.49 a | 30.45 ± 4.01 b | 25.57 ± 3.52 a | 30.52 ± 4.11 b | 29.95 ± 2.76 bc | 28.37 ± 2.32 d | 28.97 ± 2.23 cd | *** |
| SW (mm) | 13.13 ± 1.27 a | 15.75 ± 2.24 b | 12.93 ± 1.80 a | 15.67 ± 2.16 bc | 15.74 ± 1.20 bc | 14.61 ± 1.32 d | 14.97 ± 1.21 cd | *** |
| SD (mm) | 10.46 ± 1.05 a | 12.5 ± 1.86 b | 10.28 ± 1.48 a | 12.51 ± 1.88 b | 12.35 ± 0.78 bc | 11.49 ± 1.06 d | 11.94 ± 1.01 bcd | *** |
| Weight (g) | 1.36 ± 0.36 a | 2.46 ± 0.59 b | 1.43 ± 0.61 a | 2.59 ± 0.73 b | 2.21 ± 0.53 c | 1.9 ± 0.44 d | 2.07 ± 0.58 cd | *** |
| Daily growth (g) | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | - |
| Survival rate (%) | 100 | 100 | 100 | 100 | 100 | 95 | 96 | - |
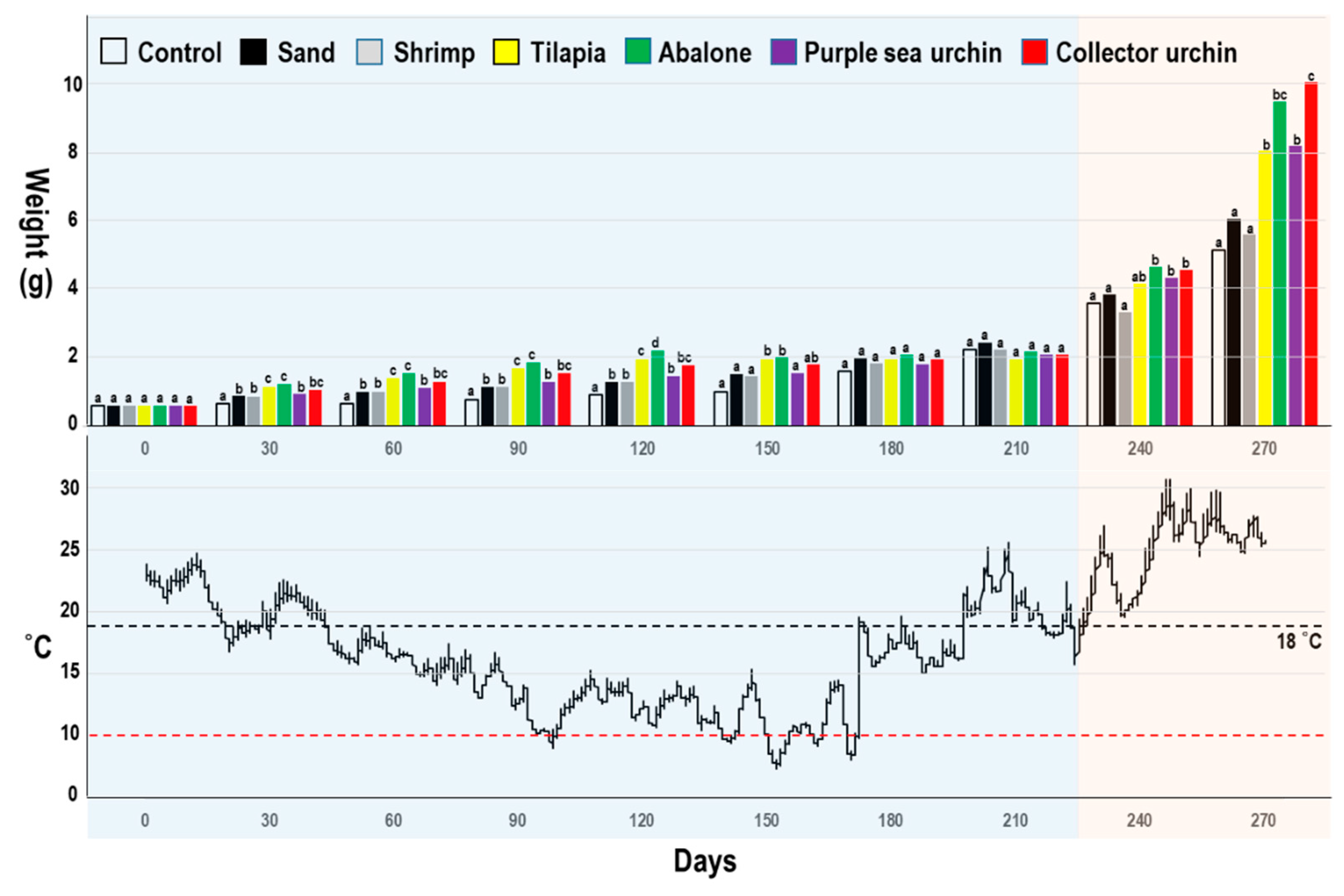
| Parameter | Control | Sand | Shrimp | Tilapia | Abalone | PU | CU | p |
|---|---|---|---|---|---|---|---|---|
| SL (mm) | 40.70 ± 6.05 a | 42.89 ± 4.23 b | 39.97 ± 3.93 a | 47.49 ± 3.95 c | 49.42 ± 3.38 cd | 48.35 ± 3.73 c | 50.90 ± 3.34 d | *** |
| SW (mm) | 20.24 ± 3.38 a | 22.07 ± 2.31 b | 20.61 ± 2.34 ab | 24.08 ± 3.05 c | 25.68 ± 3.27 d | 24.53 ± 3.01 cd | 27.28 ± 3.44 e | *** |
| SD (mm) | 16.45 ± 2.49 a | 17.75 ± 1.94 b | 16.75 ± 1.93 a | 19.50 ± 2.36 c | 20.31 ± 1.71 cd | 19.79 ± 1.64 c | 21.06 ± 1.72 d | *** |
| Weight (g) | 5.18 ± 2.57 a | 6.10 ± 2.31 a | 5.63 ± 1.82 a | 8.09 ± 2.50 b | 9.54 ± 2.39 c | 8.23 ± 1.92 b | 10.11 ± 2.28 c | *** |
| Daily growth (g) | 0.02 | 0.03 | 0.02 | 0.03 | 0.04 | 0.03 | 0.04 | - |
| Survival rate (%) | 76 | 92 | 61 | 86 | 84 | 82 | 88 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Ma, C.-H.; Lee, H.-T.; Hsu, T.-H. Polyculture of Juvenile Dog Conch Laevistrombus canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA). Biology 2021, 10, 812. https://doi.org/10.3390/biology10080812
Chang Y-C, Ma C-H, Lee H-T, Hsu T-H. Polyculture of Juvenile Dog Conch Laevistrombus canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA). Biology. 2021; 10(8):812. https://doi.org/10.3390/biology10080812
Chicago/Turabian StyleChang, Yung-Cheng, Chia-Huan Ma, Hung-Tai Lee, and Te-Hua Hsu. 2021. "Polyculture of Juvenile Dog Conch Laevistrombus canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA)" Biology 10, no. 8: 812. https://doi.org/10.3390/biology10080812
APA StyleChang, Y.-C., Ma, C.-H., Lee, H.-T., & Hsu, T.-H. (2021). Polyculture of Juvenile Dog Conch Laevistrombus canarium Reveals High Potentiality in Integrated Multitrophic Aquaculture (IMTA). Biology, 10(8), 812. https://doi.org/10.3390/biology10080812








