Does Coenzyme Q10 Supplementation Improve Testicular Function and Spermatogenesis in Male Mice with Chronic Kidney Disease?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Serum Analysis
2.3. Sperm Analysis
2.4. Testicular Histology
2.5. Analysis of Testicular CoQ10 and Testosterone Level
2.6. Antioxidant Capacity
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
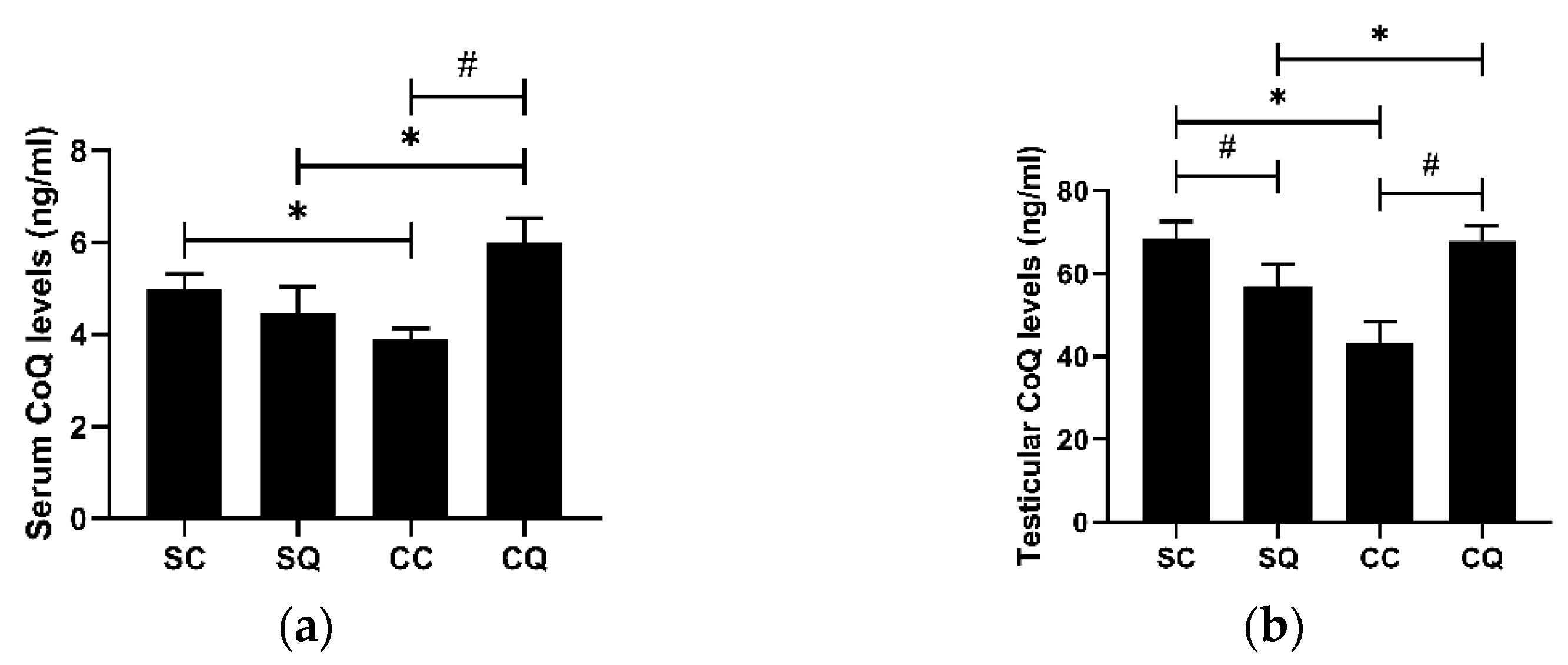
3.1. CoQ10 Supplementation Increased Both Serum and Testicular CoQ Levels in CKD Mice
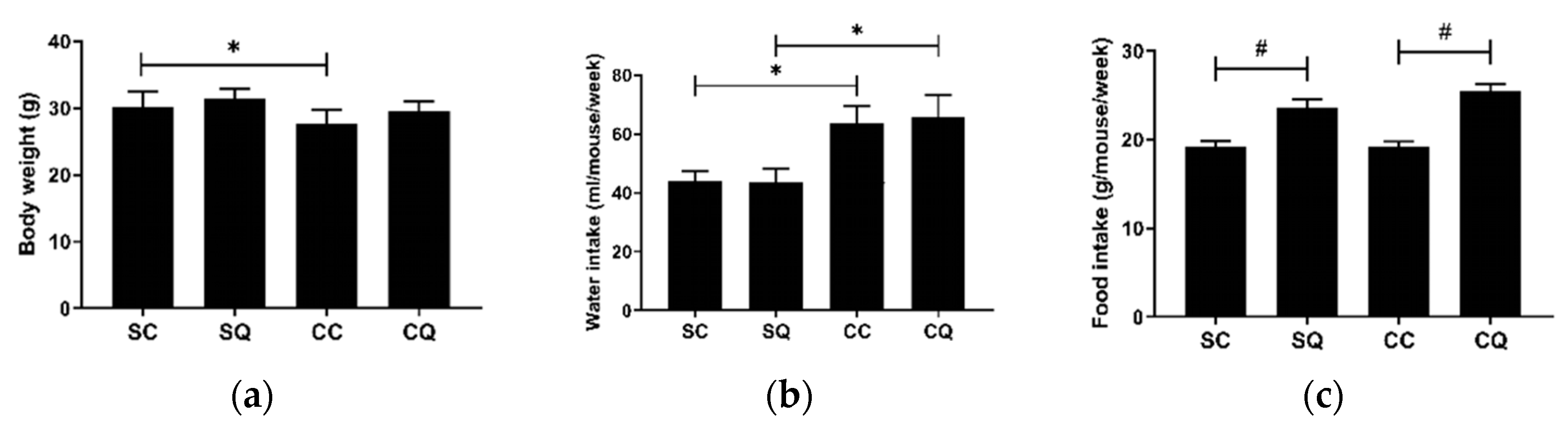
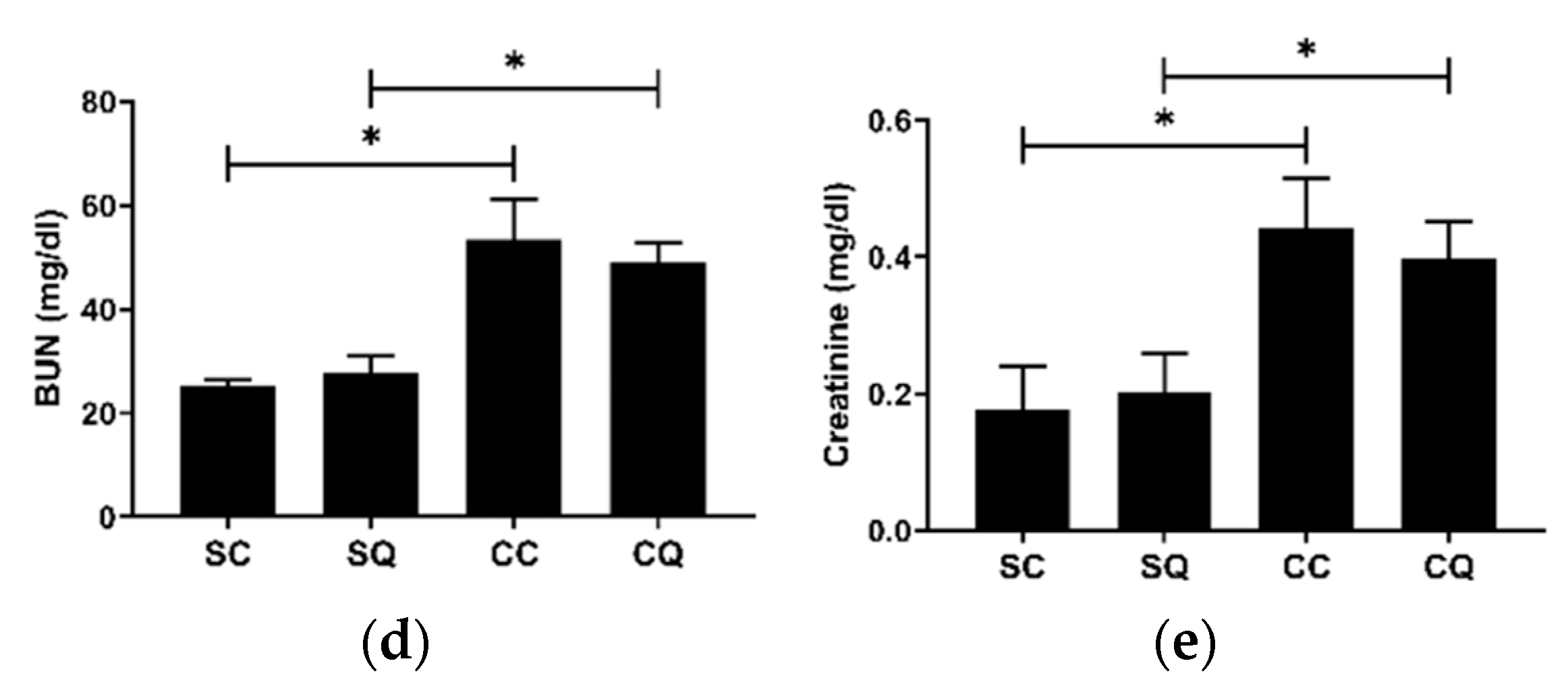
3.2. CoQ10 Supplementation Did Not Improve Renal Dysfunction in CKD Mice
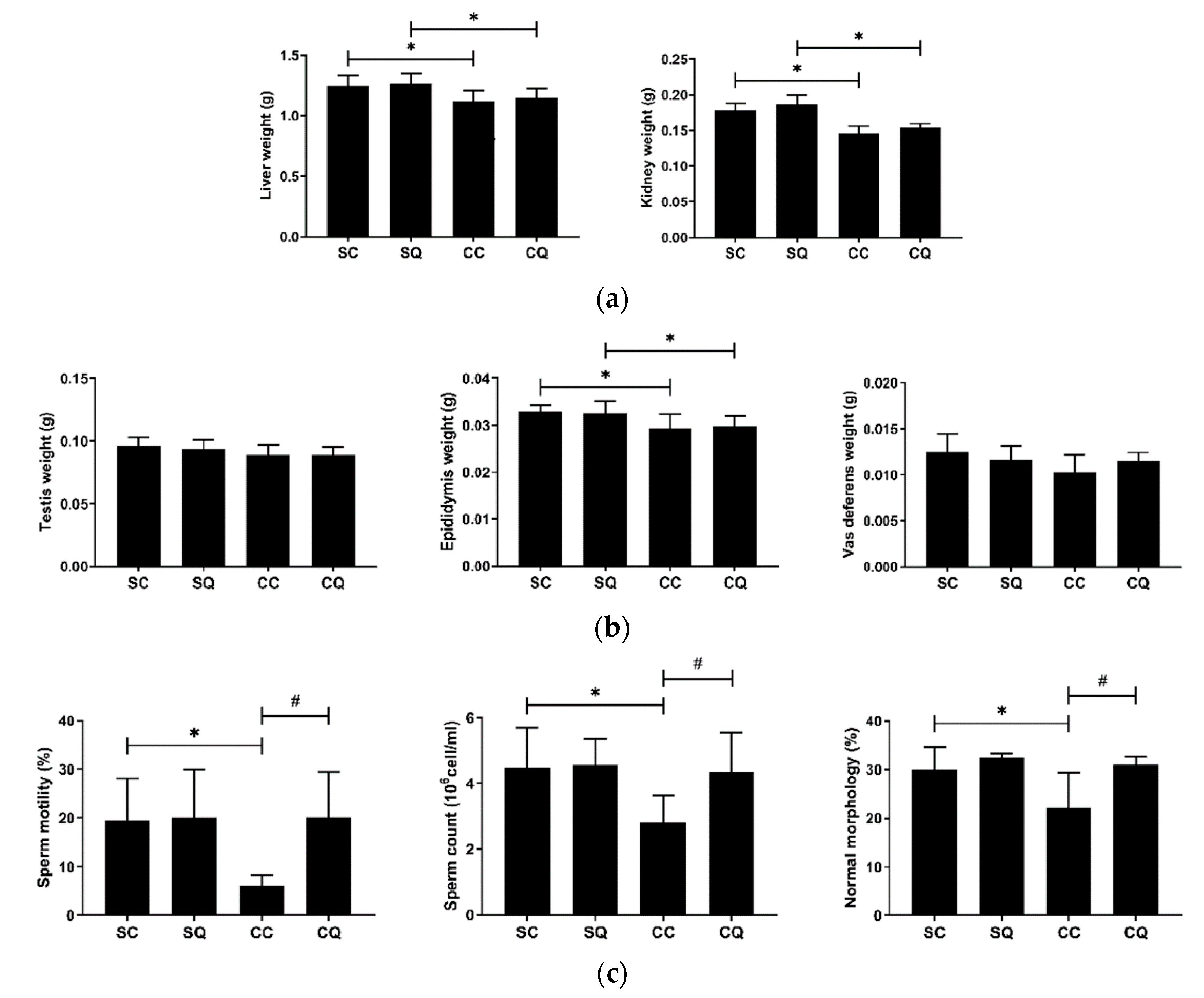
3.3. CoQ10 Supplementation Improved Semen Quality in CKD Mice
3.4. CoQ10 Supplementation Preserved Testicular Morphology in CKD Mice
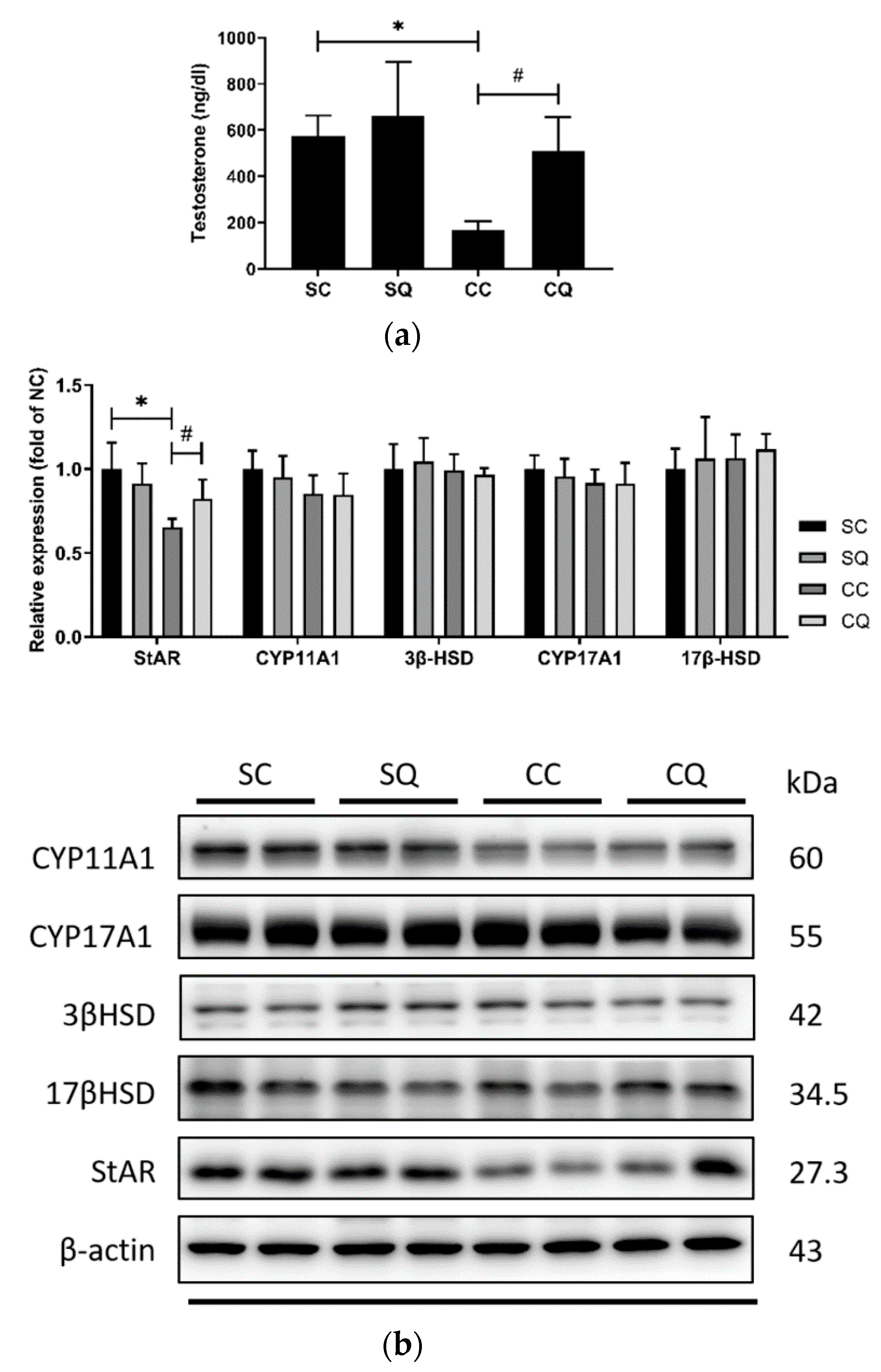
3.5. CoQ10 Supplementation Increased the Serum Testosterone Level and Partially Upregulated Protein Expressions of Testosterone Biosynthesis Enzymes in CKD Mice
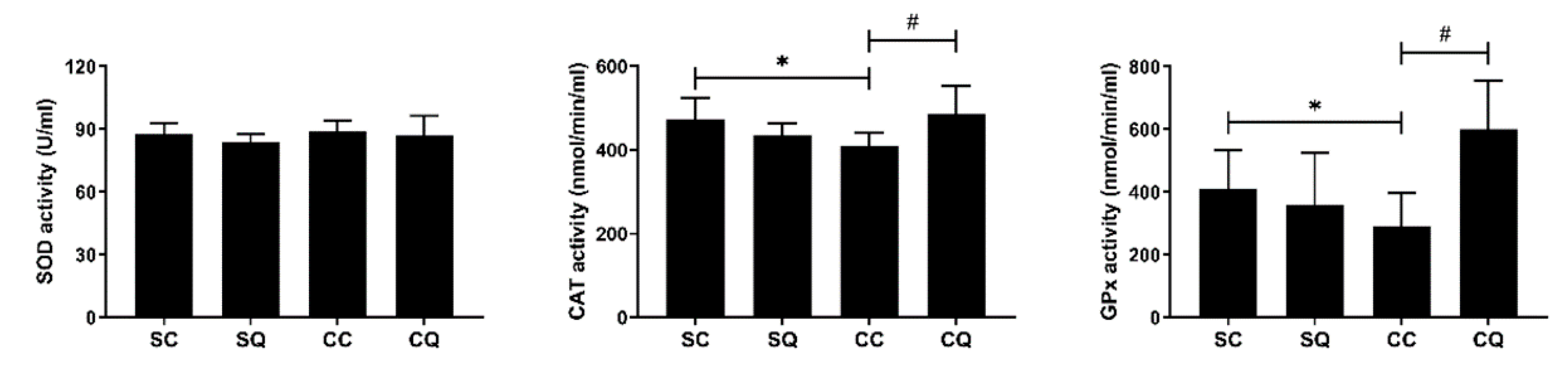
3.6. CoQ10 Supplementation Enhanced Testicular Antioxidant Activity in CKD Mice
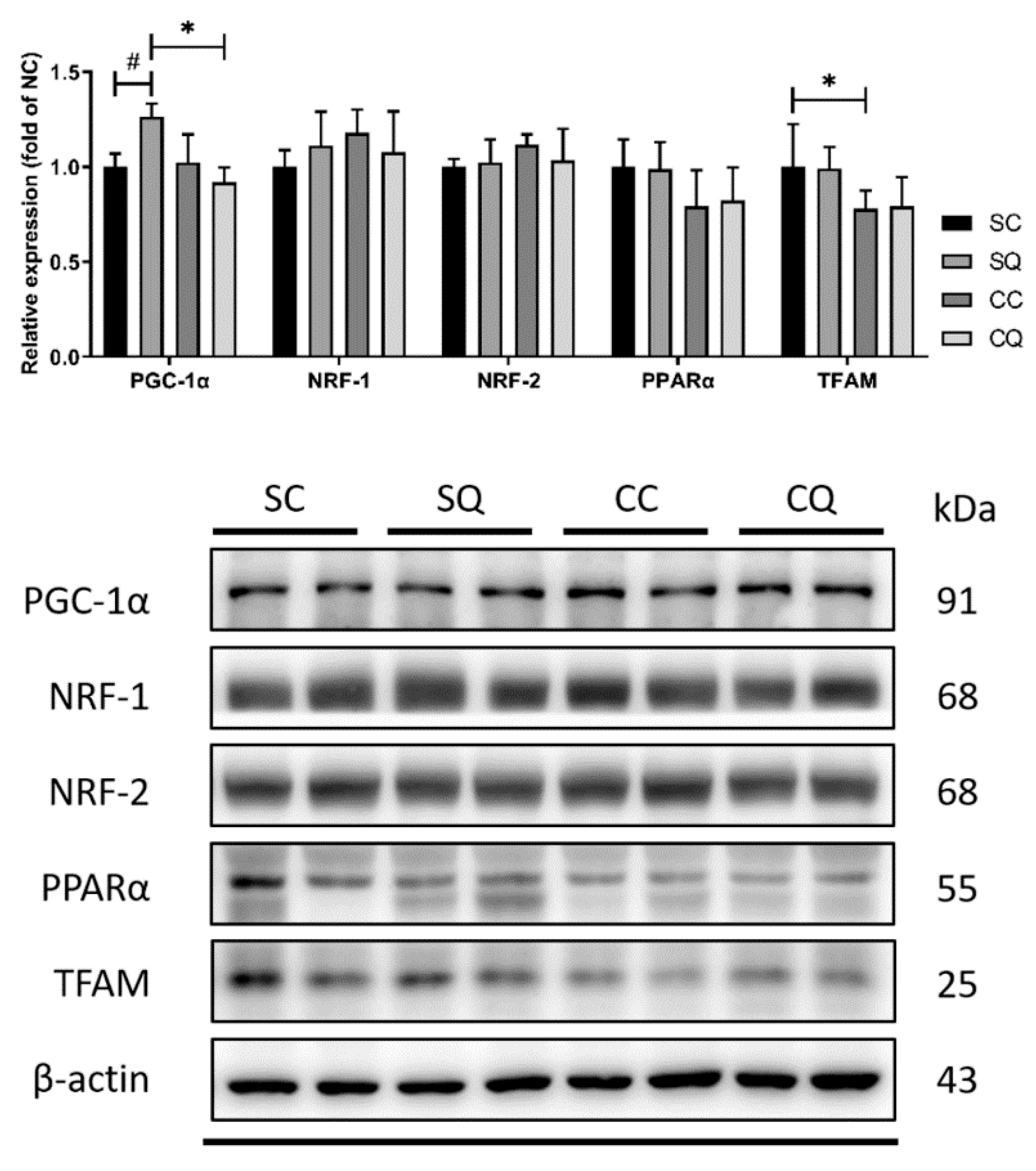
3.7. CoQ10 Supplementation Did Not Reverse Decrease in Protein Expression of Testicular Mitochondrial Biogenesis Markers in CKD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alemu, H.; Hailu, W.; Adane, A. Prevalence of chronic kidney disease and associated factors among patients with diabetes in northwest Ethiopia: A hospital-based cross-sectional study. Curr. Ther. Res. Clin. Exp. 2020, 92, 100578. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.; Afarideh, M.; Agarwal, S.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatric Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [Green Version]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. kidney Dis. Off. J. Natl. Kidney Found. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchta, A.; Pacanis, A.; Kortas-Stempak, B.; Cwiklińska, A.; Ziętkiewicz, M.; Renke, M.; Rutkowski, B. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press. Res. 2011, 34, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Alahmar, A.T. Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Palani, A.; Alahmar, A. Impact of oxidative stress on semen parameters in normozoospermic infertile men: A case–control study. Afr. J. Urol. 2020, 26, 50. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Alshahrani, S.; Durairajanayagam, D.; Sabanegh, E. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod. Biol. Endocrinol. RBE 2014, 12, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcelos, I.P.; Haas, R.H. CoQ10 and Aging. Biology 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhling, K.; Schumacher, A.; Eulenburg, C.Z.; Laakmann, E. Influence of oral vitamin and mineral supplementation on male infertility: A meta-analysis and systematic review. Reprod. Biomed. Online 2019, 39, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Gava, A.L.; Freitas, F.P.; Balarini, C.M.; Vasquez, E.C.; Meyrelles, S.S. Effects of 5/6 nephrectomy on renal function and blood pressure in mice. Int. J. Physiol. Pathophysiol. Pharm. 2012, 4, 167–173. [Google Scholar]
- Johnsen, S.G. Testicular biopsy score count—A method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Hormones 1970, 1, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.; Testai, L.; Colletti, A.; Cicero, A.F.G. Coenzyme Q(10): Clinical applications in cardiovascular diseases. Antioxidants 2020, 9, 341. [Google Scholar] [CrossRef] [Green Version]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free. Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Hargreaves, I.; Heaton, R.A.; Mantle, D. Disorders of human coenzyme Q10 metabolism: An overview. Int. J. Mol. Sci. 2020, 21, 6695. [Google Scholar] [CrossRef]
- Gazdíková, K.; Gvozdjáková, A.; Kucharská, J.; Spustová, V.; Braunová, Z.; Dzúrik, R. Oxidative stress and plasma concentrations of coenzyme Q10, alpha-tocopherol, and beta-carotene in patients with a mild to moderate decrease of kidney function. Nephron 2001, 88, 285. [Google Scholar] [CrossRef]
- Mehmetoglu, I.; Yerlikaya, F.H.; Kurban, S.; Erdem, S.S.; Tonbul, Z. Oxidative stress markers in hemodialysis and peritoneal dialysis patients, including coenzyme Q10 and ischemia-modified albumin. Int. J. Artif. Organs 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Yeung, C.K.; Billings, F.T.t.; Claessens, A.J.; Roshanravan, B.; Linke, L.; Sundell, M.B.; Ahmad, S.; Shao, B.; Shen, D.D.; Ikizler, T.A.; et al. Coenzyme Q10 dose-escalation study in hemodialysis patients: Safety, tolerability, and effect on oxidative stress. BMC Nephrol. 2015, 16, 183. [Google Scholar] [CrossRef] [Green Version]
- Lekli, I.; Das, S.; Das, S.; Mukherjee, S.; Bak, I.; Juhasz, B.; Bagchi, D.; Trimurtulu, G.; Krishnaraju, A.V.; Sengupta, K.; et al. Coenzyme Q9 provides cardioprotection after converting into coenzyme Q10. J. Agric. Food Chem. 2008, 56, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.-Z.; Zhong, X.; Li, J.-C.; Zhang, Y.-W.; Yan, Y.; Liao, Y.; Wen, D.; Diao, H.; Wang, L.; Shen, H.-C. An optimized 5/6 nephrectomy mouse model based on unilateral kidney ligation and its application in renal fibrosis research. Ren. Fail. 2019, 41, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Al-Sultan, A.I.; Refaie, S.M.; Yacoubi, M.T. Coenzyme Q10 treatment ameliorates acute cisplatin nephrotoxicity in mice. Toxicology 2010, 274, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.R.; Ibrahim, R.; El-Sayed, S. Protective effect of Coenzyme Q10 against gentamicin induced acute renal failure in mice. J. Biosci. Appl. Res. 2016, 2, 401–406. [Google Scholar] [CrossRef]
- Dumanski, S.M.; Ahmed, S.B. Fertility and reproductive care in chronic kidney disease. J. Nephrol. 2019, 32, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Eckersten, D.; Tsatsanis, C.; Giwercman, A.; Bruun, L.; Pihlsgård, M.; Christensson, A. MicroRNA-155 and anti-müllerian hormone: New potential markers of subfertility in men with chronic kidney disease. Nephron Extra 2017, 7, 33–41. [Google Scholar] [CrossRef]
- Eckersten, D.; Giwercman, A.; Pihlsgård, M.; Bruun, L.; Christensson, A. Impact of kidney transplantation on reproductive hormone levels in males: A longitudinal study. Nephron 2018, 138, 192–201. [Google Scholar] [CrossRef]
- Lehtihet, M.; Hylander, B. Semen quality in men with chronic kidney disease and its correlation with chronic kidney disease stages. Andrologia 2015, 47, 1103–1108. [Google Scholar] [CrossRef]
- Jones, R.E.; Lopez, K.H. Human Reproductive Biology; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Xu, L.G.; Xu, H.M.; Zhu, X.F.; Jin, L.M.; Xu, B.; Wu, Y.; Lu, N.Q. Examination of the semen quality of patients with uraemia and renal transplant recipients in comparison with a control group. Andrologia 2009, 41, 235–240. [Google Scholar] [CrossRef]
- Lafuente, R.; González-Comadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Alahmar, A.T. The impact of two doses of coenzyme Q10 on semen parameters and antioxidant status in men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2019, 46, 112–118. [Google Scholar] [CrossRef]
- Khurana, K.K.; Navaneethan, S.D.; Arrigain, S.; Schold, J.D.; Nally, J.V.; Shoskes, D.A. Serum testosterone levels and mortality in men with CKD stages 3-4. Am. J. kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.R.; Stetson, C.L.; Slominski, A.T.; Pruitt, K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine 2016, 51, 7–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.M.; Mahipal, S.V.; Subhashini, J.; Reddy, M.C.; Roy, K.R.; Reddy, G.V.; Reddy, P.R.; Reddanna, P. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod. Toxicol. 2006, 22, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Diemer, T.; Allen, J.A.; Hales, K.H.; Hales, D.B. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology 2003, 144, 2882–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayanagi, R.; Takeshige, K.; Minakami, S. NADH- and NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem. J. 1980, 192, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safarinejad, M.R. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J. Urol. 2009, 182, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A. Effect of coenzyme Q(10) supplementation on testosterone. Biomolecules 2018, 8, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Maheshwari, R.; Balaraman, R.; Sen, A.K.; Shukla, D.; Seth, A. Effect of concomitant administration of coenzyme Q10 with sitagliptin on experimentally induced diabetic nephropathy in rats. Ren. Fail. 2017, 39, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Festa, R.; Giacchi, E.; Raimondo, S.; Tiano, L.; Zuccarelli, P.; Silvestrini, A.; Meucci, E.; Littarru, G.P.; Mancini, A. Coenzyme Q10 supplementation in infertile men with low-grade varicocele: An open, uncontrolled pilot study. Andrologia 2013, 46. [Google Scholar] [CrossRef]
- Fallah, M.; Askari, G.; Soleimani, A.; Feizi, A.; Asemi, Z. Clinical trial of the effects of coenzyme q10 supplementation on biomarkers of inflammation and oxidative stress in diabetic hemodialysis patients. Int. J. Prev. Med. 2019, 10, 12. [Google Scholar] [CrossRef]
- Alahmar, A.; Calogero, A.; Sengupta, P.; Dutta, S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J. Men’s Health 2020, 38. [Google Scholar] [CrossRef] [Green Version]
- Bakhshayeshkaram, M.; Lankarani, K.B.; Mirhosseini, N.; Tabrizi, R.; Akbari, M.; Dabbaghmanesh, M.H.; Asemi, Z. The effects of coenzyme Q10 supplementation on metabolic profiles of patients with chronic kidney disease: A systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des. 2018, 24, 3710–3723. [Google Scholar] [CrossRef]
- Small, D.M.; Coombes, J.S.; Bennett, N.; Johnson, D.W.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A new therapeutic target in chronic kidney disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamboa, J.L.; Billings IV, F.T.; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts II, L.J.; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4, e12780. [Google Scholar] [CrossRef]
- Cordero, M.; Alcocer-Gómez, E.; Miguel, M.; Culic, O.; Carrion, A.; Alvarez-Suarez, J.; Bullon, P.; Battino, M.; Fernández-Rodríguez, A.; Sánchez-Alcázar, J. Can coenzyme Q 10 improve clinical and molecular parameters in fibromyalgia? Antioxid. Redox Signal. 2013, 19, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Ghanayem, B.I.; Bai, R.; Kissling, G.E.; Travlos, G.; Hoffler, U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol. Reprod. 2010, 82, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, C.-W.; Hsu, Y.-J.; Tseng, X.-T.; Chang, T.-C.; Tsao, C.-H.; Liu, C.-Y. Does Coenzyme Q10 Supplementation Improve Testicular Function and Spermatogenesis in Male Mice with Chronic Kidney Disease? Biology 2021, 10, 786. https://doi.org/10.3390/biology10080786
Tsao C-W, Hsu Y-J, Tseng X-T, Chang T-C, Tsao C-H, Liu C-Y. Does Coenzyme Q10 Supplementation Improve Testicular Function and Spermatogenesis in Male Mice with Chronic Kidney Disease? Biology. 2021; 10(8):786. https://doi.org/10.3390/biology10080786
Chicago/Turabian StyleTsao, Chih-Wei, Yu-Juei Hsu, Xiang-Ting Tseng, Ting-Chia Chang, Chang-Huei Tsao, and Chin-Yu Liu. 2021. "Does Coenzyme Q10 Supplementation Improve Testicular Function and Spermatogenesis in Male Mice with Chronic Kidney Disease?" Biology 10, no. 8: 786. https://doi.org/10.3390/biology10080786
APA StyleTsao, C.-W., Hsu, Y.-J., Tseng, X.-T., Chang, T.-C., Tsao, C.-H., & Liu, C.-Y. (2021). Does Coenzyme Q10 Supplementation Improve Testicular Function and Spermatogenesis in Male Mice with Chronic Kidney Disease? Biology, 10(8), 786. https://doi.org/10.3390/biology10080786





