Third-Stage Dispersal Juveniles of Bursaphelenchus xylophilus Can Resist Low-Temperature Stress by Entering Cryptobiosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of B. xylophilus from Different Hosts
2.2. Survival of Bursaphelenchus Xylophilus Third-Stage Dispersal Juveniles in Pine Trees under Low-Temperature Stress
2.3. Assessment of the Ability to Resist Low-Temperature Stress by Osmotic Regulation after Cryptobiosis
2.4. Cryptobiosis by Dehydration of B. xylophilus Juveniles
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics of B. xylophilus in Different Pine Trees in Fushun during Winter
3.2. Survival Rates of B. xylophilus in P. tabuliformis after Low-Temperature Stress
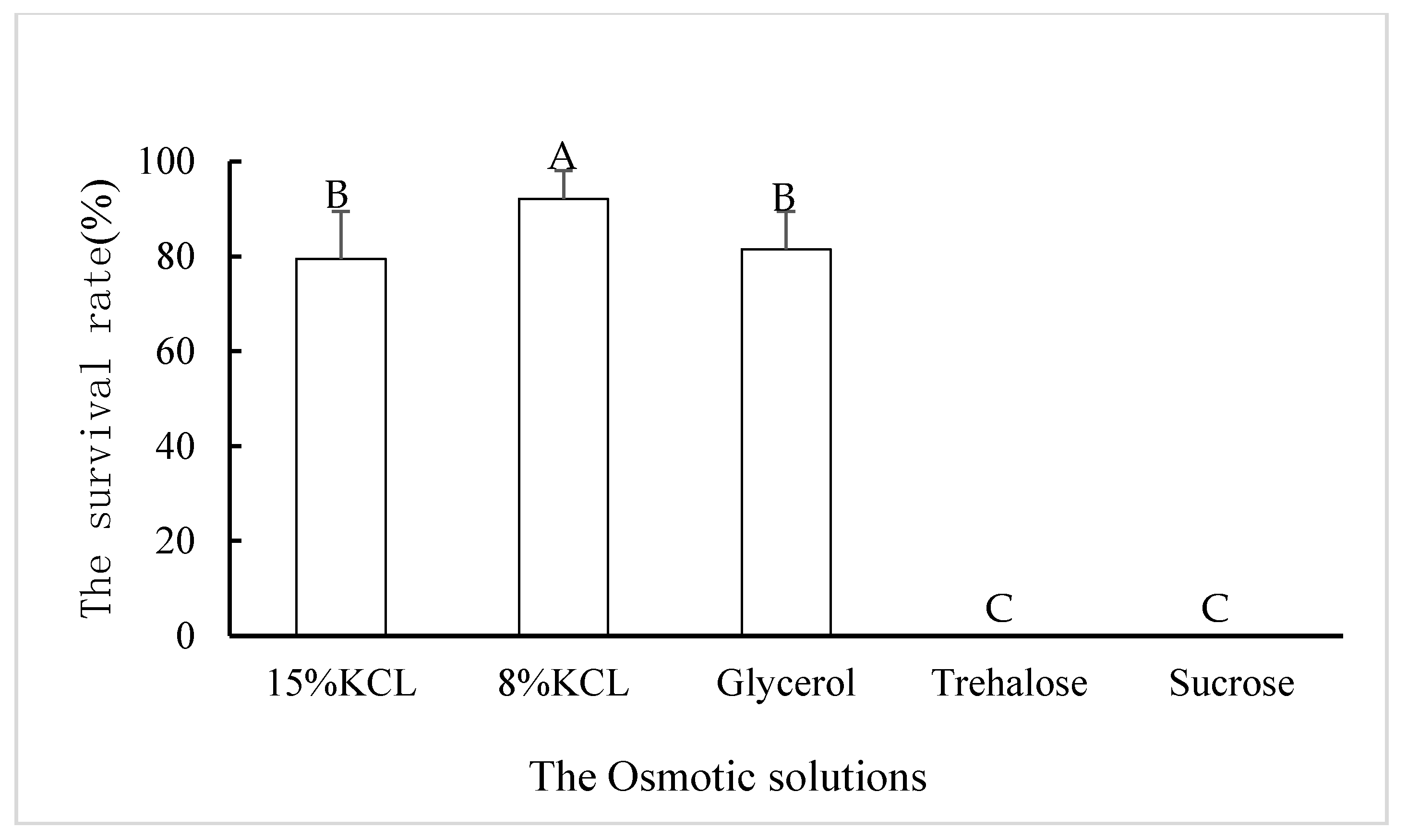
3.3. Survival Rate of Diffused the Third Stage Juveniles B. xylophilus under Low Temperature after Osmotic Regulation
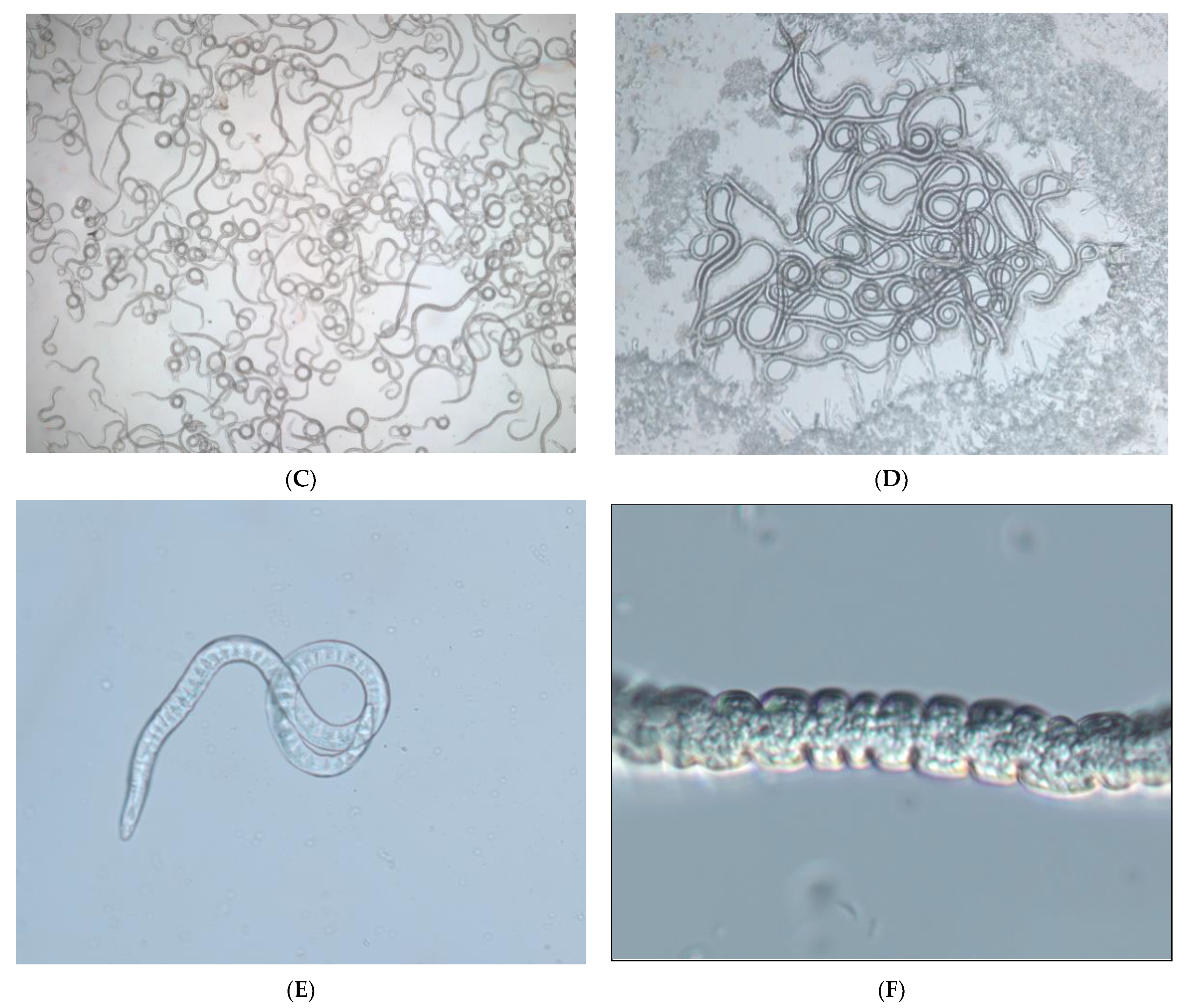
3.4. Cryptobiosis by Dehydration for B. xylophilus Third-Stage Dispersal Juveniles
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, H.; Wu, H.; Huang, R.; Wang, J.; Zhang, R.; Song, R. Separation and identification of Bursaphelenchus xylophilus from Pinus sylvestris var. mongolica in Fushun city. For. Pest Dis. 2020, 39, 6–10. [Google Scholar]
- Pan, L.; Li, Y.; Liu, Z.; Meng, F.; Chen, J.; Zhang, X. Isolation and identification of pine wood nematode in Pinus koraiensis in Fengcheng, Liaoning Province. For. Pest Dis. 2019, 38, 1–4. [Google Scholar]
- Liu, M.; Fu, X.; Yang, L.; Li, B.; Mi, L.; Zhang, H.; Ma, J.; Zhang, B.; Mi, N. Characteristics of main climatic elements from 1960 to 2013 in Fushun region. J. Meteorol. Environ. 2015, 31, 140–146. [Google Scholar]
- Zhu, Y. Studies on Anti-Drought and Resistence to Sphaeropsis Sapinea of Pine Seedlings Treated with Water-Remaining-Agents; Nanjing Forestry University: Nanjing, China, 2004. [Google Scholar]
- Chen, Q. Trehalose Metabolism Participates in Pine Wood Nematode Third-Stage Dispersal Juvenile Low Temperature Resistance; Northeast Forestry University: Harbin, China, 2020. [Google Scholar]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.-M.; Fahmy, K.; Kurzchalia, T.V. Trehalose Renders the Dauer Larva of Caenorhabditis elegans, Resistant to Extreme Desiccation. Curr. Biol. 2012, 21, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Madin, K.A.C.; Crowe, J.H. Anhydrobiosis in nematodes: Carbohydrate and lipid metabolism during dehydration. J. Exp. Zool. 2010, 193, 335–342. [Google Scholar] [CrossRef]
- Shatilovich, A.V.; Tchesunov, A.V.; Neretina, T.V.; Grabarnik, I.P.; Gubin, S.V.; Vishnivetskaya, T.A.; Onstott, T.C.; Rivkina, E.M. Viable Nematodes from Late Pleistocene Permafrost of the Kolyma River Lowland. Dokl. Biol. Sci. 2018, 480, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.H.; Cooper, A.F. Cryptobiosis. Sci. Am. 1971, 225, 30–36. [Google Scholar] [CrossRef]
- Browne, J.; Tunnacliffe, A.; Burnell, A. Anhydrobiosis: Plant desiccation gene found in a nematode. Nature 2002, 416, 38. [Google Scholar] [CrossRef] [PubMed]
- Wharton, D.A. Anhydrobiosis. Curr. Biol. 2015, 25, 1107–1125. [Google Scholar] [CrossRef]
- Lamitina, T.; Huang, C.G.; Strange, K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 12173–12178. [Google Scholar] [CrossRef]
- Wharton, D.A.; Petrone, L.; Duncan, A.; McQuillan, A.J. A surface lipid may control the permeability slump associated with entry into anhydrobiosis in the plant parasitic nematode Ditylenchus dipsaci. J. Exp. Biol. 2008, 211, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. Pathology of the Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Ann. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Mamiya, Y.; Enda, N. Transmission of Bursaphelenchus lignicolus (nematoda: Aphelenchoididae) by Monochamus alternatus (coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Pan, L.; Cui, R.; Li, Y.; Feng, Y.; Zhang, X. Investigation of Pinewood Nematodes in Pinus tabuliformis Carr. under Low-Temperature Conditions in Fushun, China. Forests 2020, 11, 993. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, S.; Wei, W.; Hao, H.; Zhang, B.; Butcher, R.A.; Sun, J. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr. Biol. 2013, 23, 2038–2043. [Google Scholar] [CrossRef] [PubMed]
- Huang, R. The Study on Cold Tolerance among Geographic Populations of Pine Wood Nematode, B. xylophilus in China; Beijing Forestry University: Beijing, China, 2015. [Google Scholar]
- Tunnacliffe, A.; Hincha, D.K.; Leprince, O.; Macherel, D. LEA Proteins: Versatility of Form and Function. Top. Curr. Genet. 2010, 21, 91–108. [Google Scholar]
- Shannon, A.J.; Browne, J.A.; Boyd, J.; Fitzpatrick, D.A.; Burnell, A.M. The anhydrobiotic potential and molecular phylogenetics of species and strains of Panagrolaimus (Nematoda, Panagrolaimidae). J. Exp. Biol. 2005, 208, 2433–2445. [Google Scholar] [CrossRef]
- Wharton, D.A.; Barclay, S. Anhydrobiosis in the free-living antarctic nematode Panagrolaimus davidi (Nematoda: Rhabditida). Fundam. Appl. Nematol. 1993, 16, 17–22. [Google Scholar]
- Schottenfeld-Roames, J.; Ghabrial, A.S. Osmotic regulation of seamless tube growth. Nat. Cell Biol. 2013, 15, 137–139. [Google Scholar] [CrossRef][Green Version]
- Lai, Y.; Yang, Z.; Wang, X.; Tang, Y.; Zhang, Y. Distribution and Function of the Ovarian Nematode Contortylenchus genitalicola (Tylenchida: Allantonematidae) on Monochamus alternatus (Coleoptera: Cerambycidae) in China. For. Res. 2018, 31, 51. [Google Scholar]
- Khan, L.A.; Zhang, H.; Abraham, N.; Sun, L.; Fleming, J.T.; Buechner, M.; Hall, D.H.; Gobel, V. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol. 2013, 15, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X. High risk of invasion and expansion of pine wood nematode in middle temperate zone of China. J. Temp. For. Res. 2018, 1, 3–6. [Google Scholar]
- Mcgill, L.M.; Shannon, A.J.; Pisani, D.; Felix, M.-A.; Ramlov, H.; Dix, I.; Wharton, D.A.; Burnell, A.M. Anhydrobiosis and Freezing-Tolerance: Adaptations That Facilitate the Establishment of Panagrolaimus Nematodes in Polar Habitats. PLoS ONE 2015, 10, e0116084. [Google Scholar] [CrossRef]
- Pan, L.; Li, Y.; Liu, Z. Isolation and identification of pine wood nematodes from Korean pine in Fengcheng city, Liaoning Province. For. Pest Dis. 2019, 38, 1–4. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Cui, R.; Li, Y.; Zhang, W.; Bai, J.; Li, J.; Zhang, X. Third-Stage Dispersal Juveniles of Bursaphelenchus xylophilus Can Resist Low-Temperature Stress by Entering Cryptobiosis. Biology 2021, 10, 785. https://doi.org/10.3390/biology10080785
Pan L, Cui R, Li Y, Zhang W, Bai J, Li J, Zhang X. Third-Stage Dispersal Juveniles of Bursaphelenchus xylophilus Can Resist Low-Temperature Stress by Entering Cryptobiosis. Biology. 2021; 10(8):785. https://doi.org/10.3390/biology10080785
Chicago/Turabian StylePan, Long, Rong Cui, Yongxia Li, Wei Zhang, Jianwei Bai, Juewen Li, and Xingyao Zhang. 2021. "Third-Stage Dispersal Juveniles of Bursaphelenchus xylophilus Can Resist Low-Temperature Stress by Entering Cryptobiosis" Biology 10, no. 8: 785. https://doi.org/10.3390/biology10080785
APA StylePan, L., Cui, R., Li, Y., Zhang, W., Bai, J., Li, J., & Zhang, X. (2021). Third-Stage Dispersal Juveniles of Bursaphelenchus xylophilus Can Resist Low-Temperature Stress by Entering Cryptobiosis. Biology, 10(8), 785. https://doi.org/10.3390/biology10080785





