Acute Increase in Blood αCGRP at Maximal Exercise and Its Association to Cardiorespiratory Fitness, Carbohydrate Oxidation and Work Performed: An Exploratory Study in Young Men
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Anthropometric Measurements
2.3. Leisure Time Physical Activity (LPTA)
2.4. Graded Exercise Test
2.5. αCGRP Quantification
2.6. Statistical Analyses
2.7. Ethics
3. Results
3.1. Descriptive Results of the Whole Sample
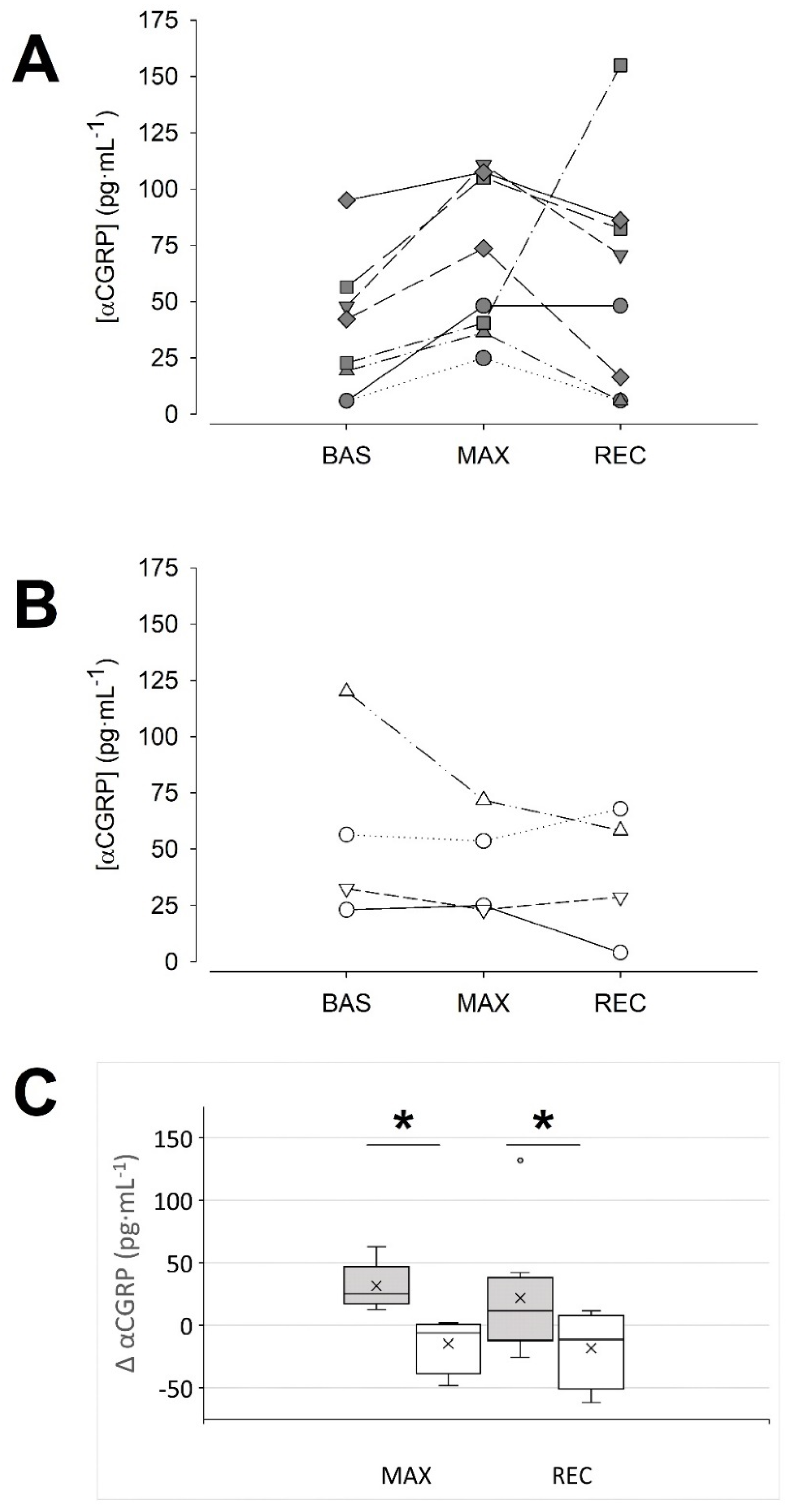
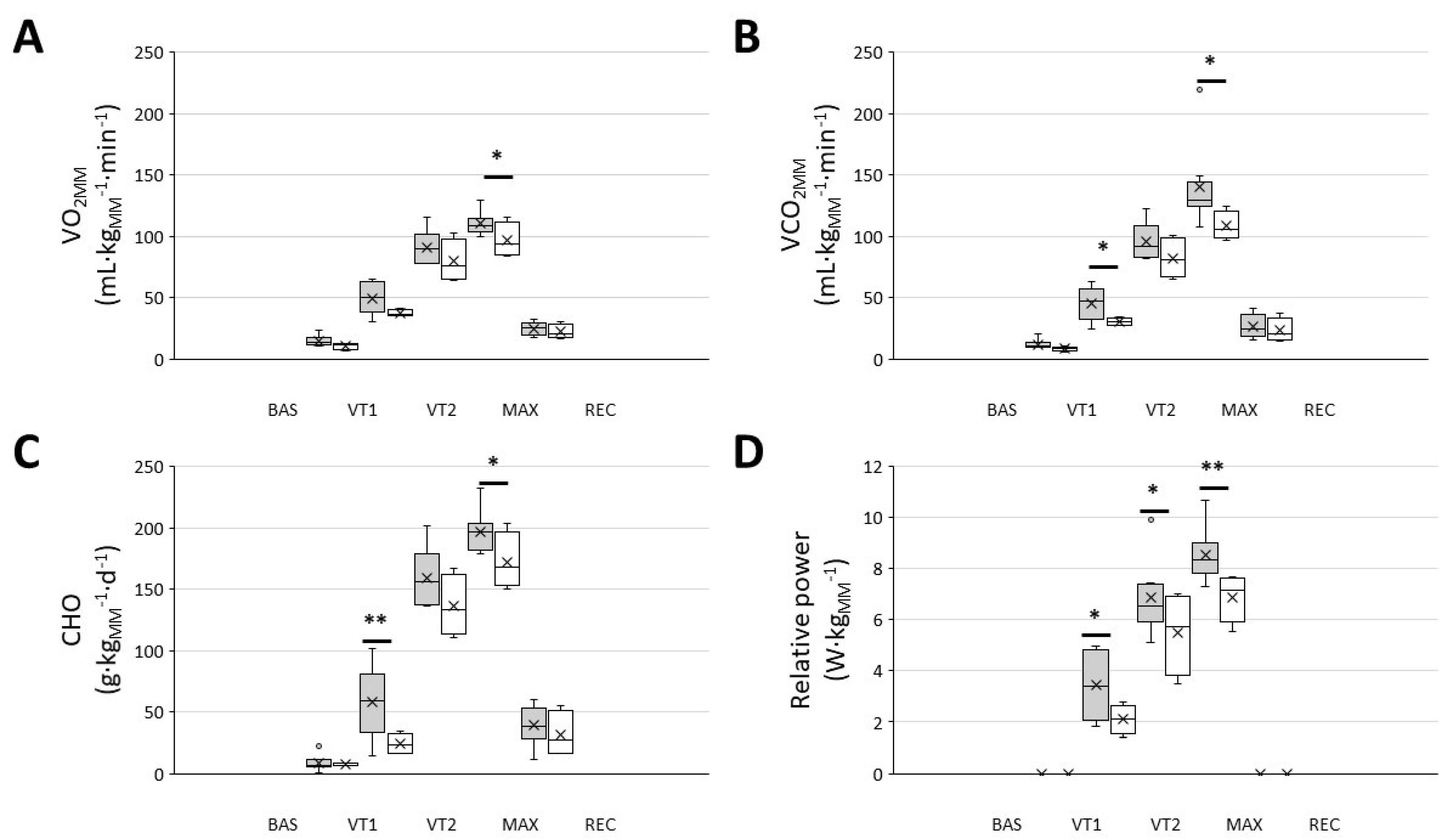
3.2. Post Hoc Comparison between ‘Responders’ and ‘Non-Responders’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amara, S.; Jonas, V.; Rosenfeld, M.G.; Ong, E.S.; Evans, R. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nat. Cell Biol. 1982, 298, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.-J.; Kodji, X.; Brain, S.D. Calcitonin Gene-Related Peptide: Physiology and Pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Brain, S.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nat. Cell Biol. 1985, 313, 54–56. [Google Scholar] [CrossRef] [PubMed]
- De Mey, J.G.; Vanhoutte, P.M. End O’ The Line Revisited: Moving on from nitric oxide to CGRP. Life Sci. 2014, 118, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Franco-Cereceda, A.; Lundberg, J.M. Calcitonin gene-related peptide (CGRP) and capsaicin-induced stimulation of heart contractile rate and force. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1985, 331, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; Scott, M.; Ittner, L.M.; Fischer, J.A.; Born, W.; Vogel, J. Calcitonin gene-related peptide-evoked sustained tachycardia in calcitonin receptor-like receptor transgenic mice is mediated by sympathetic activity. Am. J. Physiol. Circ. Physiol. 2007, 293, H2155–H2160. [Google Scholar] [CrossRef][Green Version]
- Schuler, B.; Rieger, G.; Gubser, M.; Arras, M.; Gianella, M.; Vogel, O.; Jirkof, P.; Cesarovic, N.; Klohs, J.; Jakob, P.; et al. Endogenous α-calcitonin-gene-related peptide promotes exercise-induced, physiological heart hypertrophy in mice. Acta Physiol. 2014, 211, 107–121. [Google Scholar] [CrossRef]
- Mishima, T.; Ito, Y.; Hosono, K.; Tamura, Y.; Uchida, Y.; Hirata, M.; Suzsuki, T.; Amano, H.; Kato, S.; Kurihara, Y.; et al. Calcitonin gene-related peptide facilitates revascularization during hindlimb ischemia in mice. Am. J. Physiol. Circ. Physiol. 2011, 300, H431–H439. [Google Scholar] [CrossRef] [PubMed]
- Leighton, B.; Cooper, G.J.S. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nat. Cell Biol. 1988, 335, 632–635. [Google Scholar] [CrossRef]
- Beaumont, K.; Pittner, R.A.; Moore, C.X.; Wolfe-Lopez, D.; Prickett, K.S.; Young, A.A.; Rink, T.J. Regulation of muscle glycogen metabolism by CGRP and amylin: CGRP receptors not involved. Br. J. Pharmacol. 1995, 115, 713–715. [Google Scholar] [CrossRef]
- Wang, M.-W.; Young, A.A.; Rink, T.J.; Cooper, G. 8-37 h-CGRP antagonizes actions of amylin on carbohydrate metabolism in vitro and in vivo. FEBS Lett. 1991, 291, 195–198. [Google Scholar] [CrossRef]
- Leighton, B.; Foot, E.A.; Cooper, G.; King, J.M. Calcitonin gene-related peptide-1 (CGRP-1) is a potent regulator of glycogen metabolism in rat skeletal muscle. FEBS Lett. 1989, 249, 357–361. [Google Scholar] [CrossRef]
- Rossetti, L.U.; Farrace, S.T.; Choi, S.B.; Giaccari, A.N.; Sloan, L.A.; Frontoni, S.I.; Katz, M.S. Multiple metabolic effects of CGRP in conscious rats: Role of glycogen synthase and phosphorylase. Am. J. Physiol. Endocrinol. Metab. 1993, 264, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Danaher, R.N.; Loomes, K.M.; Leonard, B.L.; Whiting, L.; Hay, D.; Xu, L.Y.; Kraegen, E.W.; Phillips, A.R.J.; Cooper, G. Evidence that α-Calcitonin Gene-Related Peptide Is a Neurohormone that Controls Systemic Lipid Availability and Utilization. Endocrinology 2007, 149, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Csillik, B.; Tajti, L.; Kovacs, T.; Kukla, E.; Rakic, P.; Knyihár-Csillik, E.; Young, R.D.; A Lawrence, P.; Duance, V.C.; Aigner, T.; et al. Distribution of calcitonin gene-related peptide in vertebrate neuromuscular junctions: Relationship to the acetylcholine receptor. J. Histochem. Cytochem. 1993, 41, 1547–1555. [Google Scholar] [CrossRef]
- Van Der Kloot, W.; Benjamin, W.B.; Balezina, O.P. Calcitonin gene-related peptide acts presynaptically to increase quantal size and output at frog neuromuscular junctions. J. Physiol. 1998, 507, 689–695. [Google Scholar] [CrossRef]
- Fernandez, H.L.; A Hodges-Savola, C. Physiological regulation of G4 AChe in fast-twitch muscle: Effects of exercise and CGRP. J. Appl. Physiol. 1996, 80, 357–362. [Google Scholar] [CrossRef]
- Macdonald, W.A.; Nielsen, O.B.; Clausen, T. Effects of calcitonin gene-related peptide on rat soleus muscle excitability: Mechanisms and physiological significance. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1214–R1223. [Google Scholar] [CrossRef]
- Smillie, S.-J.; Brain, S. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 2011, 45, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Trasforini, G.; Margutti, A.; Portaluppi, F.; Menegatti, M.; Ambrosio, M.R.; Bagni, B.; Pansini, R.; Degli Uberti, E.C. Circadian Profile of Plasma Calcitonin Gene-Related Peptide in Healthy Man. J. Clin. Endocrinol. Metab. 1991, 73, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Schifter, S.; Breum, L.; Niclasen, B.; Vollmer-Larsen, A.; Rasmussen, H.; Graff-Larsen, O. Calcitonin Gene-Related Peptide During Exercise and Training. Horm. Metab. Res. 1995, 27, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Spetz, A.-C.E.; Ellefsen, K.; Theodorsson, E.; Lassvik, C.T.; Hammar, M.L. Calcitonin Gene-Related Peptide during Sweating in Young Healthy Women. Gynecol. Obstet. Investig. 2005, 60, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Tarperi, C.; Sanchis-Gomar, F.; Montagnana, M.; Danese, E.; Salvagno, G.L.; Gelati, M.; Skroce, K.; Schena, F.; Lippi, G. Effects of endurance exercise on serum concentration of calcitonin gene-related peptide (CGRP): A potential link between exercise intensity and headache. Clin. Chem. Lab. Med. 2020, 58, 1707–1712. [Google Scholar] [CrossRef]
- Jonhagen, S. Calcitonin gene related peptide and neuropeptide Y in skeletal muscle after eccentric exercise: A microdialysis study. Br. J. Sports Med. 2006, 40, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Hasbak, P.; Lundby, C.; Olsen, N.V.; Schifter, S.; Kanstrup, I.-L. Calcitonin gene-related peptide and adrenomedullin release in humans: Effects of exercise and hypoxia. Regul. Pept. 2002, 108, 89–95. [Google Scholar] [CrossRef]
- Onuoha, G.N.; Nicholls, D.P.; Patterson, A.; Beringer, T. Neuropeptide secretion in exercise. Neuropeptides 1998, 32, 319–325. [Google Scholar] [CrossRef]
- Lind, H.; Brudin, L.; Lindholm, L.; Edvinsson, L. Different levels of sensory neuropeptides (calcitonin gene-related peptide and substance P) during and after exercise in man. Clin. Physiol. 1996, 16, 73–82. [Google Scholar] [CrossRef]
- Kjaer, M.; Mohr, T.; Dela, F.; Secher, N.; Galbo, H.; Olesen, H.L.; Sorensen, F.B.; Schifter, S. Leg uptake of calcitonin gene-related peptide during exercise in spinal cord injured humans. Clin. Physiol. 2001, 21, 32–38. [Google Scholar] [CrossRef]
- Alvero Cruz, J.E.; Cabañas, M.D.; Herrero, A.; Martínez, L.; Moreno, C.; Porta, J.; Sillero, M.; Sirvent, J.E. Protocolo de valoración de la composición corporal para el reconocimiento médico-deportivo. Documento de Consenso del Grupo Español de Cineantropometría de la Federación Española de Medicina del Deporte. Arch. Med. Deport. 2009, 26, 166–179. [Google Scholar]
- Durnin, J.V.G.A.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 Years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Pettitt, R.W.; Clark, I.E.; Ebner, S.M.; Sedgeman, D.T.; Murray, S.R. Gas Exchange Threshold and VO2max Testing for Athletes. J. Strength Cond. Res. 2013, 27, 549–555. [Google Scholar] [CrossRef]
- Astorino, T.A.; Robergs, R.A.; Ghiasvand, F.; Marks, D.; Burns, S. Incidence of the Oxygen Plateau at VO2max During Exercise Testing To Voli-tional Fatigue. J. Exerc. Physiol. 2000, 3, 1–12. [Google Scholar]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Gaskill, S.E.; Rice, T.; Bouchard, C.; Gagnon, J.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Leon, A.S. Familial resemblance in ventilatory threshold: The HERITAGE Family Study. Med. Sci. Sports Exerc. 2001, 33, 1832–1840. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.; Nevill, M.; Meleagros, L.; Lakomy, H.K.A.; Hall, G.M.; Bloom, S.R.; Williams, C. The hormonal responses to repetitive brief maximal exercise in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 60, 144–148. [Google Scholar] [CrossRef]
- Smillie, S.-J.; King, R.; Kodji, X.; Outzen, E.; Pozsgai, G.; Fernandes, E.S.; Marshall, N.; De Winter, P.; Heads, R.J.; Dessapt-Baradez, C.; et al. An Ongoing Role of α-Calcitonin Gene–Related Peptide as Part of a Protective Network against Hypertension, Vascular Hypertrophy, and Oxidative Stress. Hypertension 2014, 63, 1056–1062. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 12) | ‘Responders’ (n = 8) | ‘Non-Responders’ (n = 4) | p | |
|---|---|---|---|---|
| Age (years) | 24.3 ± 0.9 | 23.9 ± 1.2 | 25.0 ± 1.6 | 0.608 a |
| Anthropometry | ||||
| BM (Kg) | 76.7 ± 2.1 | 75.6 ± 3.2 | 77.2 ± 1.8 | 0.897 |
| Height (cm) | 179.2 ± 1.9 | 180.3 ± 2.0 | 174.4 ± 3.9 | 0.080 |
| BMI (Kg·m−2) | 23.9 ± 0.6 | 23.1 ± 0.6 | 25.4 ± 1.0 | 0.154 |
| FM (%) | 14.1 ± 0.6 | 14.0 ± 1.6 | 14.2 ± 1.2 | 0.866 |
| MM (%) | 44.9 ± 0.8 | 44.4 ± 1.2 | 45.9 ± 0.9 | 0.461 a |
| LTPA (MET-min·week−1) | ||||
| Low intensity | 1178.4 ± 394.9 | 1049.8 ± 404.9 | 1435.5 ± 957.7 | 0.667 |
| Moderate intensity | 805.7 ± 302.5 | 552.7 ± 347.2 | 1311.7 ± 562.0 | 0.368 a |
| High intensity | 1381.8 ± 214.8 | 1392.1 ± 307.6 | 1361.2 ± 256.8 | 0.950 |
| Total | 3365.9 ± 613.3 | 2994.6 ± 843.3 | 4108.5 ± 736.9 | 0.418 |
| BASELINE | VT1 | VT2 | MAXIMAL | RECOVERY a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R (n = 8) | NR (n = 4) | d (CI95%) | R (n = 8) | NR (n = 4) | d (CI95%) | R (n = 8) | NR (n = 4) | d (CI95%) | R (n = 8) | NR (n = 4) | d (CI95%) | R (n = 8) | NR (n = 4) | d (CI95%) | |
| Time to (s) | - | - | - | 377.5 ± 63.7 | 177.5 ± 40.0 | 1.27 (0.09, 2.26) | 940.0 ± 72.5 | 761.2 ± 117.0 | 0.83 (−0.27, 1.81) | 1189.3 ± 52.0 | 992.5 ± 102.2 | 1.18 (0.02, 2.16) | 405.0 ± 67.0 | 431.2 ± 113.4 | −0.13 (−1.13, 0.89) |
| Pulmonary function | |||||||||||||||
| Respiratory frequency (ventilations·min−1) | 18.3 ± 1.5 | 16.5 ± 2.1 | 0.42 (−0.63, 1.40) | 22.5 ± 1.1 | 20.7 ± 1.2 | 0.63 (−0.44, 1.61) | 33.9 ± 2.7 | 28.8 ± 2.9 | 0.70 (−0.38, 1.68) | 48.5 ± 1.7 | 42.1 ± 3.4 | 1.18 (0.02, 2.16) | 22.20 ± 3.0 | 20.6 ± 3.0 | −1.06 (−2.04, 0.08) |
| Tidal volume (L) | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.40 (−0.65, 1.38) | 1.5± 0.1 | 1.4 ± 0.04 | 0.74 (−0.35, 1.71) | 2.3 ± 0.1 | 2.5 ± 0.1 | −0.57 (−1.55, 0.50) | 2.5 ± 0.1 | 2.76 ± 0.1 | −1.01 (−1.98, 0.12) | 1.6 ± 0.1 | 1.6 ± 0.2 | 0.15 (−0.87, 1.14) |
| Minute ventilation (L·min−1) | 12.9 ± 1.3 | 10.0 ± 0.8 | 0.88 (−0.23, 1.86) | 34.9± 2.5 | 28.6 ± 1.7 | 1.02 (−0.12, 1.99) | 78.5± 6.6 | 73.7 ± 9.6 | 0.25 (−0.78, 1.25) | 123.9± 6.1 | 115.7 ± 8.3 | 0.48 (−0.58, 1.46) | 35.4± 4.9 | 32.0 ± 6.2 | 0.26 (−0.77, 1.25) |
| Cardiovascular function | |||||||||||||||
| Heart rate (beats·min−1) | 73.3 ± 3.4 | 71.7 ± 8.1 | 0.13 (−0.88, 1.13) | 117.7 ± 5.9 | 106.5 ± 5.3 | 1.47 (0.025, 2.46) | 166.0 ± 2.4 | 160.0 ± 8.8 | 0.53 (−0.53, 1.51) | 179.8 ± 1.8 | 177.7 ± 5.4 | 0.29 (−0.75, 1.27) | 116.8 ± 3.9 | 114.2 ± 4.6 | 0.25 (−0.78, 1.24) |
| Stroke volume (mL) | 96.2 ± 9.9 | 75.7 ± 6.8 | 0.83 (−0.27, 1.81) | 133.2 ± 8.2 | 126.0 ± 7.1 | 0.35 (−0.69, 1.33) | 126.4 ± 5.7 | 120.6 ± 4.9 | 0.40 (−0.65, 1.38) | 125.5 ± 5.4 | 116.9 ± 4.1 | 0.63 (−0.45, 1.60) | 87.1 ± 6.7 | 81.5 ± 3.9 | 0.34 (−0.70, 1.33) |
| Cardiac output (L·min−1) | 6.9 ± 0.7 | 5.3 ± 0.6 | 0.85 (−0.26, 1.82) | 15.4 ± 0.6 | 13.3 ± 0.5 | 1.28 (0.10, 2.26) | 20.9 ± 0.9 | 19.3 ± 1.4 | 0.61 (−0.46, 1.59) | 22.5 ± 0.9 | 20.8 ± 1.2 | 0.66 (−0.42, 1.64) | 10.2 ± 0.8 | 9.3 ± 0.7 | 0.38 (−0.66, 1.37) |
| Systolic blood pressure (mmHg) | 133.5 ± 6.2 | 139.2 ± 5.8 | −0.33 (−10.34, 0.68) | 164.8 ± 9.5 b | 150.6 ± 6.5 c | 0.72 (−0.44, 1.73) | 174.1 ± 7.0 b | 141.0 ± 10.1 c | 1.91 (0.36, 3.03) | 179.4 ± 5.6 d | 172.2 ± 14.9 | 0.33 (−0.73, 1.34) | 140.6 ± 9.1 | 138.7 ± 4.6 | 0.08 (−0.93, 1.09) |
| Diastolic blood pressure (mmHg) | 79.1 ± 3.2 | 78.7 ± 6.0 | 0.04 (−0.97, 1.04) | 74.3 ± 7.9 b | 60.3 ± 6.1 c | 0.83 (−0.34, 1.85) | 67.5 ± 5.1 b | 64.3 ± 8.9 c | 0.23 (−0.96, 1.37) | 67.5 ± 4.1 d | 67.0 ± 4.1 | −0.21 (−1.23, 0.84) | 61.2 ± 4.5 | 51.5 ± 4.7 | 0.83 (−0.28, 1.80) |
| Ventilatory exchange | |||||||||||||||
| VO2 (mL·min−1) | 507.0 ± 65.3 | 374.7 ± 49.0 | 0.81 (−0.29, 1.78) | 1641.5 ± 114.8 | 1311.3 ± 38.0 | 1.20 (0.03, 2.18) | 3069.2 ± 171.7 | 2802.2 ± 273.7 | 0.53 (−0.53, 1.51) | 3715.8 ± 156.9 | 3417.8 ± 199.3 | 0.69 (−0.39, 1.67) | 844.2 ± 94.3 | 777.7 ± 107.2 | 0.26 (−0.77, 1.25) |
| VCO2 (mL·min−1) | 409.3 ± 58.0 | 296.7 ± 31.2 | 0.80 (−0.30, 1.77) | 1483.2 ± 127.0 | 1075.4 ± 48.8 | 1.34 (0.14, 2.32) | 3226.6 ± 182.6 | 2902.2 ± 252.8 | 0.63 (−0.44, 1.61) | 4234.6 ± 179.0 | 3829.9 ± 158.2 | 0.88 (−0.23, 1.86) | 922.3 ± 137.3 | 822.5 ± 184.0 | 0.26 (−0.77, 1.25) |
| VO2 (mL·Kg−1·min−1) | 6.5 ± 0.6 | 4.8 ± 0.6 | 0.99 (−0.14, 1.97) | 21.9 ± 2.2 | 17.1 ± 0.9 | 0.98 (−0.15, 1.96) | 40.3 ± 1.6 | 36.7 ± 4.0 | 0.61 (−0.46, 1.59) | 48.8 ± 0.7 | 44.7 ± 3.2 | 1.02 (0−.11, 2.00) | 10.9 ± 0.9 | 10.1 ± 1.3 | 0.30 (−0.74, 1.29) |
| VCO2 (mL·Kg−1·min−1) | 5.21 ± 0.6 | 3.8 ± 0.3 | 0.97 (−0.16, 1.94) | 19.7 ± 2.1 | 13.9 ± 0.9 * | 1.11 (−0.04, 2.09) | 42.1 ± 1.8 | 37.6 ± 3.5 | 0.78 (−0.32, 1.75) | 55.3 ± 1.1 | 49.7 ± 2.7 * | 1.45 (0.24, 2.44) | 11.9 ± 1.5 | 10.6 ± 2.3 | 0.27 (−0.76, 1.26) |
| RER | 0.8 ± 0.2 | 0.80 ± 0.04 | 0 (−1.01, 1.01) | 0.89 ± 0.02 | 0.81 ± 0.01 | 1.71 (0.44, 2.72) | 1.05 ± 0.01 | 1.04 ± 0.02 | 0.44 (−0.61, 1.42) | 1.14 ± 0.01 | 1.12 ± 0.01 | 0.46 (−0.60, 1.44) | 1.06 ± 0.06 | 1.02 ± 0.09 | 0.24 (−0.79, 1.23) |
| Metabolism | |||||||||||||||
| Energy expenditure (Kcal·min−1) | 2.4 ± 0.3 | 1.7 ± 0.2 | 0.81 (−0.29, 1.79) | 8.1 ± 0.6 | 6.2 ± 0.2 | 1.24 (0.07, 2.22) | 15.5 ± 0.8 | 14.2 ± 1.3 | 0.56 (−0.51, 1.54) | 19.2 ± 0.8 | 17.6 ± 0.9 | 0.75 (−0.34, 1.73) | 4.3 ± 0.5 | 3.9 ± 0.6 | 0.27 (−0.77, 1.26) |
| Energy from carbohydrates (%) | 34.4 ± 6.9 | 36.6 ± 12.7 | −0.10 (−1.10, 0.92) | 65.9 ± 7.0 | 39.5 ± 5.7 * | 1.47 (0.25, 2.47) | 100 | 98.7 ± 1.2 | 0.91 (−0.20, 1.89) | 100 | 100 | - | 87.0 ± 7.7 | 77.7 ± 12.9 | 0.40 (−0.64, 1.39) |
| Work | |||||||||||||||
| Power (W) | - | - | - | 112.5 ± 12.5 | 75.0 ± 10.2 * | 1.19 (0.02, 2.17) | 231.2 ± 16.8 | 193.7 ± 27.7 * | 0.75 (−0.34, 1.72) | 287.5 ± 11.5 | 243.7 ± 15.7 * | 1.35 (0.16, 2.34) | - | - | - |
| METs | 1.8 ± 0.1 | 1.4 ± 0.1 | 0.99 (−0.14, 1.97) | 6.2 ± 0.5 | 4.9 ± 0.2 | 0.98 (−0.15, 1.96) | 11.5 ± 0.4 | 10.4 ± 1.1 | 0.61 (−0.46, 1.59) | 13.9 ± 0.2 | 12.7 ± 0.9 | 1.02 (−0.11, 2.00) | 3.1 ± 0.2 | 2.9 ± 0.3 | 0.30 (−0.74, 1.29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aracil-Marco, A.; Sarabia, J.M.; Pastor, D.; Guillén, S.; López-Grueso, R.; Gallar, J.; Moya-Ramón, M. Acute Increase in Blood αCGRP at Maximal Exercise and Its Association to Cardiorespiratory Fitness, Carbohydrate Oxidation and Work Performed: An Exploratory Study in Young Men. Biology 2021, 10, 783. https://doi.org/10.3390/biology10080783
Aracil-Marco A, Sarabia JM, Pastor D, Guillén S, López-Grueso R, Gallar J, Moya-Ramón M. Acute Increase in Blood αCGRP at Maximal Exercise and Its Association to Cardiorespiratory Fitness, Carbohydrate Oxidation and Work Performed: An Exploratory Study in Young Men. Biology. 2021; 10(8):783. https://doi.org/10.3390/biology10080783
Chicago/Turabian StyleAracil-Marco, Adolfo, José Manuel Sarabia, Diego Pastor, Silvia Guillén, Raúl López-Grueso, Juana Gallar, and Manuel Moya-Ramón. 2021. "Acute Increase in Blood αCGRP at Maximal Exercise and Its Association to Cardiorespiratory Fitness, Carbohydrate Oxidation and Work Performed: An Exploratory Study in Young Men" Biology 10, no. 8: 783. https://doi.org/10.3390/biology10080783
APA StyleAracil-Marco, A., Sarabia, J. M., Pastor, D., Guillén, S., López-Grueso, R., Gallar, J., & Moya-Ramón, M. (2021). Acute Increase in Blood αCGRP at Maximal Exercise and Its Association to Cardiorespiratory Fitness, Carbohydrate Oxidation and Work Performed: An Exploratory Study in Young Men. Biology, 10(8), 783. https://doi.org/10.3390/biology10080783








