Quantitative Assessment of Choroidal Parameters in Patients with Various Types of Diabetic Macular Oedema: A Single-Centre Cross-Sectional Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
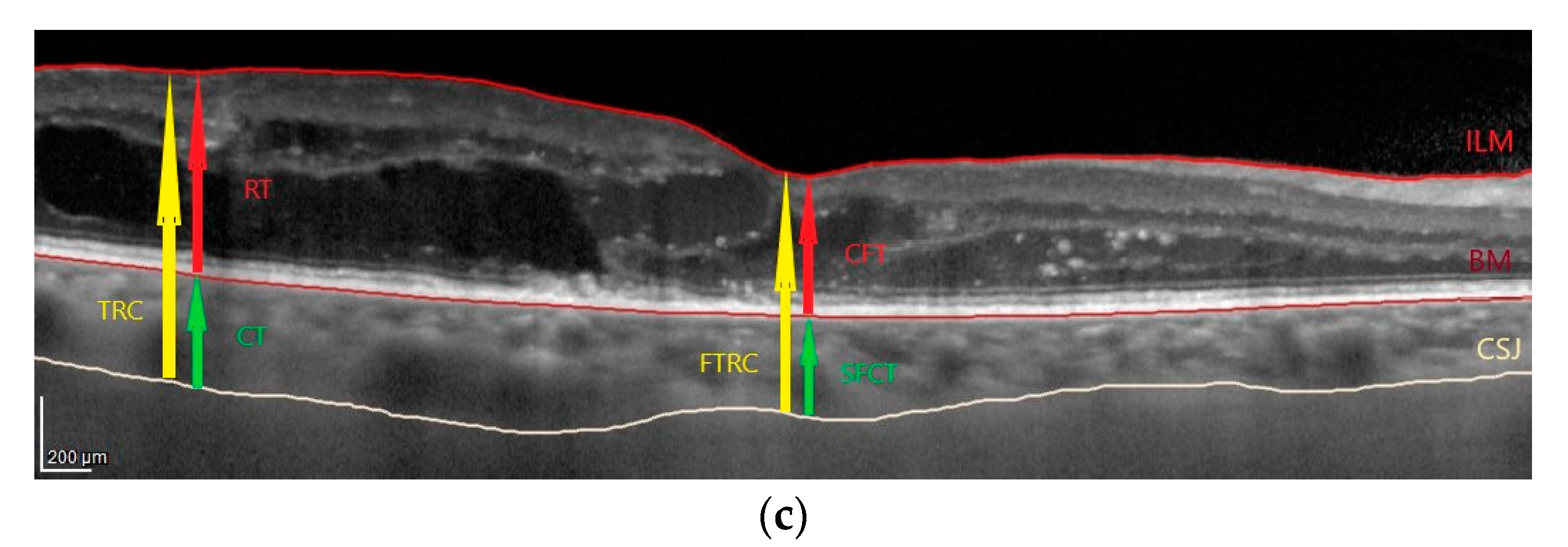
2.2. OCT Images Acquisition and Analysis
2.3. Fluorescein Angiograms Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
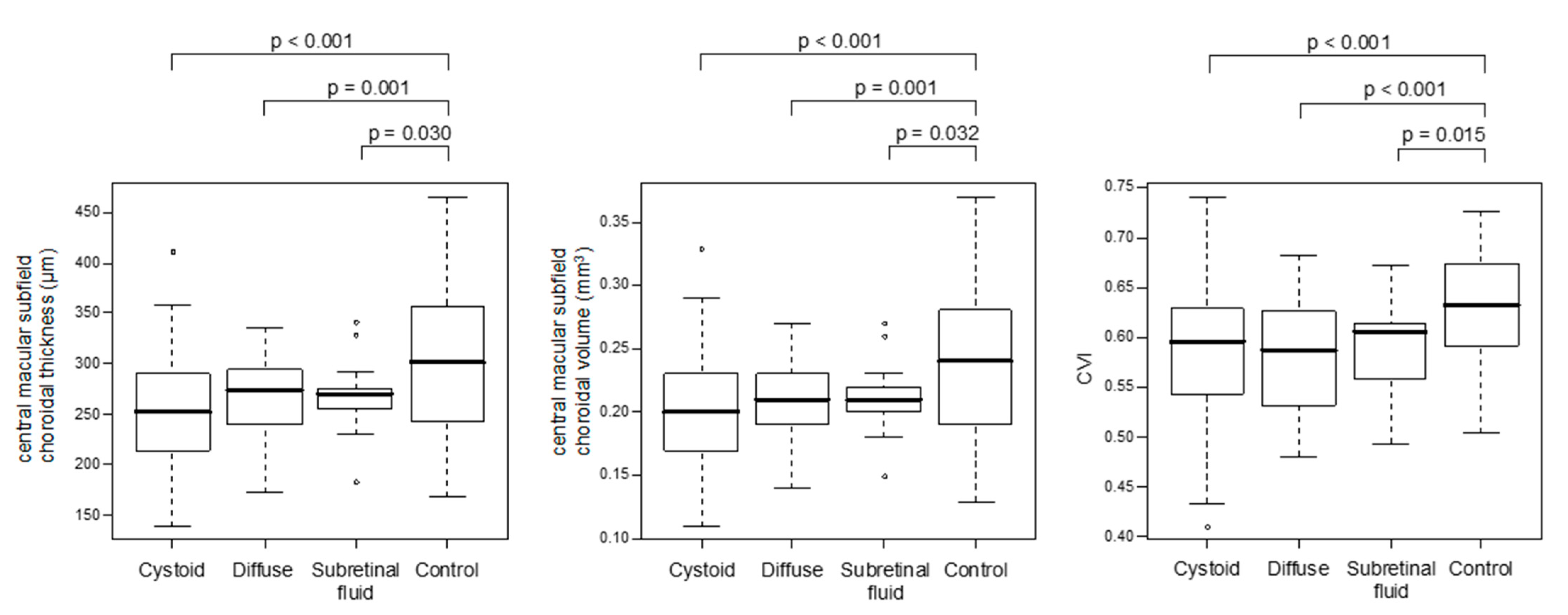
3.2. Choroidal Parameters in Eyes with Various Types of DME and Non-Diabetic Controls
3.3. Choroidal Parameters According to the Type of DME
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Krasnicki, P.; Dmuchowska, D.A.; Proniewska-Skretek, E.; Dobrzycki, S.; Mariak, Z. Ocular haemodynamics in patients with type 2 diabetes and coronary artery disease. Br. J. Ophthalmol. 2014, 98, 675–678. [Google Scholar] [CrossRef]
- Dmuchowska, D.A.; Krasnicki, P.; Mariak, Z. Can optical coherence tomography replace fluorescein angiography in detection of ischemic diabetic maculopathy? Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 731–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lutty, G.A. Diabetic choroidopathy. Vis. Res. 2017, 139, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Melancia, D.; Vicente, A.; Cunha, J.P.; Pinto, L.A.; Ferreira, J. Diabetic choroidopathy: A review of the current literature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1453–1461. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, D.; Tang, Z.; Ng, D.S.; Cheung, C.Y. Optical coherence tomography angiography in diabetic retinopathy: An updated review. Eye 2021, 35, 149–161. [Google Scholar] [CrossRef]
- Ferrara, D.; Waheed, N.K.; Duker, J.S. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog. Retin. Eye Res. 2016, 52, 130–155. [Google Scholar] [CrossRef]
- Murakami, T.; Uji, A.; Suzuma, K.; Dodo, Y.; Yoshitake, S.; Ghashut, R.; Yoza, R.; Fujimoto, M.; Yoshimura, N. In Vivo Choroidal Vascular Lesions in Diabetes on Swept-Source Optical Coherence Tomography. PLoS ONE 2016, 11, e0160317. [Google Scholar] [CrossRef]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of diabetic macular edema with optical coherence tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Allkabes, M.; Salsini, G.; Bonifazzi, C.; Perri, P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci. 2016, 162, 54–59. [Google Scholar] [CrossRef]
- Klaassen, I.; Van Noorden, C.J.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Omri, S.; Omri, B.; Savoldelli, M.; Jonet, L.; Thillaye-Goldenberg, B.; Thuret, G.; Gain, P.; Jeanny, J.C.; Crisanti, P.; Behar-Cohen, F.; et al. The outer limiting membrane (OLM) revisited: Clinical implications. Clin. Ophthalmol. 2010, 4, 183–195. [Google Scholar] [CrossRef]
- Marques, I.P.; Alves, D.; Santos, T.; Mendes, L.; Santos, A.R.; Lobo, C.; Durbin, M.; Cunha-Vaz, J. Multimodal Imaging of the Initial Stages of Diabetic Retinopathy: Different Disease Pathways in Different Patients. Diabetes 2019, 68, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Kase, S.; Takahashi, M.; Yokoi, M.; Isozaki, C.; Katsuta, S.; Kase, M. Alteration of layer thickness in the choroid of diabetic patients. Clin. Exp. Ophthalmol. 2018, 46, 926–933. [Google Scholar] [CrossRef]
- Gerendas, B.S.; Waldstein, S.M.; Simader, C.; Deak, G.; Hajnajeeb, B.; Zhang, L.; Bogunovic, H.; Abramoff, M.D.; Kundi, M.; Sonka, M.; et al. Three-dimensional automated choroidal volume assessment on standard spectral-domain optical coherence tomography and correlation with the level of diabetic macular edema. Am. J. Ophthalmol. 2014, 158, 1039–1048. [Google Scholar] [CrossRef]
- Eliwa, T.F.; Hegazy, O.S.; Mahmoud, S.S.; Almaamon, T. Choroidal Thickness Change in Patients With Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelpour, M.; Považay, B.; Hermann, B.; Hofer, B.; Kajic, V.; Hale, S.L.; North, R.V.; Drexler, W.; Sheen, N.J. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5311–5316. [Google Scholar] [CrossRef] [PubMed]
- Esmaeelpour, M.; Považay, B.; Hermann, B.; Hofer, B.; Kajic, V.; Hale, S.L.; North, R.V.; Drexler, W.; Sheen, N.J. Choroidal thinning in diabetes type 1 detected by 3-dimensional 1060 nm optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6803–6809. [Google Scholar] [CrossRef]
- Querques, G.; Lattanzio, R.; Querques, L.; Del Turco, C.; Forte, R.; Pierro, L.; Souied, E.H.; Bandello, F. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest. Ophthalmol. Vis. Sci. 2012, 53, 6017–6024. [Google Scholar] [CrossRef]
- Regatieri, C.V.; Branchini, L.; Carmody, J.; Fujimoto, J.G.; Duker, J.S. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 2012, 32, 563–568. [Google Scholar] [CrossRef]
- Adhi, M.; Brewer, E.; Waheed, N.K.; Duker, J.S. Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol. 2013, 131, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Unsal, E.; Eltutar, K.; Zirtiloğlu, S.; Dinçer, N.; Erkul, S.Ö.; Güngel, H. Choroidal thickness in patients with diabetic retinopathy. Clin. Ophthalmol. 2014, 8, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Rewbury, R.; Want, A.; Varughese, R.; Chong, V. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye 2016, 30, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Yamashita, M.; Ogata, N. Effects of intravitreal injection of ranibizumab on choroidal structure and blood flow in eyes with diabetic macular edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, J.T.; Jung, D.W.; Joe, S.G.; Yoon, Y.H. The relationship between foveal ischemia and spectral-domain optical coherence tomography findings in ischemic diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1080–1085. [Google Scholar] [CrossRef]
- Vujosevic, S.; Torresin, T.; Berton, M.; Bini, S.; Convento, E.; Midena, E. Diabetic Macular Edema With and Without Subfoveal Neuroretinal Detachment: Two Different Morphologic and Functional Entities. Am. J. Ophthalmol. 2017, 181, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Ding, J.; Sen, P.; Rousselot, A.; Chan, A.; Nivison-Smith, L.; Wei, X.; Mahajan, S.; Kim, R.; Mishra, C.; et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog. Retin. Eye Res. 2020, 77, 100829. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Uchino, E.; Kawano, H.; Yoshihara, N.; Terasaki, H.; Shirasawa, M.; Tomita, M.; Ishibashi, T. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am. J. Ophthalmol. 2015, 159, 1123–1131.e1. [Google Scholar] [CrossRef]
- Agrawal, R.; Salman, M.; Tan, K.A.; Karampelas, M.; Sim, D.A.; Keane, P.A.; Pavesio, C. Choroidal Vascularity Index (CVI)--A Novel Optical Coherence Tomography Parameter for Monitoring Patients with Panuveitis? PLoS ONE 2016, 11, e0146344. [Google Scholar] [CrossRef]
- Campos, A.; Campos, E.J.; Martins, J.; Ambrósio, A.F.; Silva, R. Viewing the choroid: Where we stand, challenges and contradictions in diabetic retinopathy and diabetic macular oedema. Acta. Ophthalmol. 2017, 95, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Vupparaboina, K.K.; Goud, A.; Dansingani, K.K.; Chhablani, J. Choroidal imaging biomarkers. Surv. Ophthalmol. 2019, 64, 312–333. [Google Scholar] [CrossRef] [PubMed]
- Iovino, C.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Govetto, A.; Vagge, A.; Di Zazzo, A.; Forlini, M.; Finocchio, L.; et al. Choroidal Vascularity Index: An In-Depth Analysis of This Novel Optical Coherence Tomography Parameter. J. Clin. Med. 2020, 9, 595. [Google Scholar] [CrossRef]
- Iovino, C.; Pellegrini, M.; Bernabei, F.; Borrelli, E.; Sacconi, R.; Govetto, A.; Vagge, A.; Di Zazzo, A.; Forlini, M.; Finocchio, L.; et al. Choroidal structural analysis in eyes with diabetic retinopathy and diabetic macular edema-A novel OCT based imaging biomarker. PLoS ONE 2018, 13, e0207435. [Google Scholar] [CrossRef]
- Kase, S.; Endo, H.; Takahashi, M.; Ito, Y.; Saito, M.; Yokoi, M.; Katsuta, S.; Sonoda, S.; Sakamoto, T.; Ishida, S.; et al. Alteration of choroidal vascular structure in diabetic macular edema. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 971–977. [Google Scholar] [CrossRef]
- Gardner, T.W.; Sundstrom, J.M. A proposal for early and personalized treatment of diabetic retinopathy based on clinical pathophysiology and molecular phenotyping. Vis. Res. 2017, 139, 153–160. [Google Scholar] [CrossRef]
- Sidorczuk, P.; Pieklarz, B.; Konopinska, J.; Saeed, E.; Mariak, Z.; Dmuchowska, D. Foveal avascular zone does not correspond to choroidal characteristics in patients with diabetic retinopathy: A single-center cross-sectional analysis. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2893–2903. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Agrawal, R.; Gupta, P.; Tan, K.A.; Cheung, C.M.; Wong, T.Y.; Cheng, C.Y. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci. Rep. 2016, 6, 21090. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Sakamoto, T.; Yamashita, T.; Shirasawa, M.; Uchino, E.; Terasaki, H.; Tomita, M. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3893–3899. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of Diabetic Retinopathy from Fluorescein Angiograms: ETDRS Report Number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Kim, J.T.; Lee, D.H.; Joe, S.G.; Kim, J.G.; Yoon, Y.H. Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3378–3384. [Google Scholar] [CrossRef]
- Vujosevic, S.; Martini, F.; Cavarzeran, F.; Pilotto, E.; Midena, E. Macular and peripapillary choroidal thickness in diabetic patients. Retina 2012, 32, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Arf, S.; Muslubas, I.S.; Hocaoglu, M.; Ersoz, M.G.; Ozdemir, H.; Karacorlu, M. Spectral domain optical coherence tomography classification of diabetic macular edema: A new proposal to clinical practice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1165–1172. [Google Scholar] [CrossRef]
- Suciu, C.I.; Suciu, V.I.; Nicoara, S.D. Optical Coherence Tomography (Angiography) Biomarkers in the Assessment and Monitoring of Diabetic Macular Edema. J. Diabetes Res. 2020, 2020, 6655021. [Google Scholar] [CrossRef] [PubMed]
- Kase, S.; Endo, H.; Takahashi, M.; Saito, M.; Yokoi, M.; Ito, Y.; Katsuta, S.; Sonoda, S.; Sakamoto, T.; Ishida, S.; et al. Alteration of choroidal vascular structure in diabetic retinopathy. Br. J. Ophthalmol. 2020, 104, 417–421. [Google Scholar] [CrossRef]
- Kim, M.; Ha, M.J.; Choi, S.Y.; Park, Y.H. Choroidal vascularity index in type-2 diabetes analyzed by swept-source optical coherence tomography. Sci. Rep. 2018, 8, 70. [Google Scholar] [CrossRef]
- Nagaoka, T.; Kitaya, N.; Sugawara, R.; Yokota, H.; Mori, F.; Hikichi, T.; Fujio, N.; Yoshida, A. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br. J. Ophthalmol. 2004, 88, 1060–1063. [Google Scholar] [CrossRef]
- Goud, A.; Singh, S.R.; Sahoo, N.K.; Rasheed, M.A.; Vupparaboina, K.K.; Ankireddy, S.; Lupidi, M.; Chhablani, J. New Insights on Choroidal Vascularity: A Comprehensive Topographic Approach. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3563–3569. [Google Scholar] [CrossRef]
- Agrawal, R.; Seen, S.; Vaishnavi, S.; Vupparaboina, K.K.; Goud, A.; Rasheed, M.A.; Chhablani, J. Choroidal Vascularity Index Using Swept-Source and Spectral-Domain Optical Coherence Tomography: A Comparative Study. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, e26–e32. [Google Scholar] [CrossRef]

| Overall | Type of DME | Controls | p | |||
|---|---|---|---|---|---|---|
| Cystoid | Diffuse | Subretinal Fluid | ||||
| Number of patients | 157 | 65 | 31 | 9 | 52 | |
| Number of eyes | 216 | 89 | 38 | 13 | 76 | |
| Age, years, mean ± SD | 58.98 ± 14.01 | 61.17 ± 10.41 | 60.28 ± 12.44 | 52.60 ± 16.46 | 55.73 ± 17.87 | 0.191 |
| Sex, female, n (%) | 82 (52.2) | 32 (49.2) | 16 (51.6) | 6 (66.7) | 28 (53.8) | 0.790 |
| Spherical equivalent, mean ± SD | 0.38 ± 1.16 | 0.24 ± 1.08 | 0.82 ± 1.05 | −0.06 ± 0.95 | 0.39 ± 1.28 | 0.198 |
| DR severity, n (%) | ||||||
| NPDR | 87 (40.3) | 56 (62.9) | 21 (55.3) | 10 (76.9) | - | 0.379 |
| PDR | 53 (24.5) | 33 (37.1) | 17 (44.7) | 3 (23.1) | - | |
| PRP, n (%) | ||||||
| no | 167 (77.3) | 59 (66.3) | 22 (57.9) | 10 (76.9) | - | 0.379 |
| yes | 49 (22.7) | 30 (33.7) | 16 (42.1) | 3 (23.1) | - | |
| Characteristic | Cystoid vs. Controls (Baseline) | Diffuse vs. Controls (Baseline) | Subretinal Fluid vs. Controls (Baseline) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | |
| Choroidal thickness (µm): | |||||||||
| outerT | −47.36 | 9.75 | <0.001 | −35.15 | 11.40 | 0.002 | −31.47 | 14.92 | 0.036 |
| innerT | −40.10 | 10.77 | <0.001 | −32.90 | 12.67 | 0.010 | −31.47 | 16.65 | 0.060 |
| central macular | −35.78 | 11.26 | 0.002 | −31.52 | 13.13 | 0.017 | −33.83 | 17.18 | 0.051 |
| innerN | −40.07 | 12.16 | 0.001 | −38.44 | 14.27 | 0.008 | −38.03 | 18.64 | 0.043 |
| outerN | −39.04 | 13.40 | 0.004 | −31.48 | 15.39 | 0.042 | −20.46 | 19.92 | 0.305 |
| outerS | −47.74 | 10.86 | <0.001 | −38.08 | 12.60 | 0.003 | −32.32 | 16.41 | 0.051 |
| innerS | −42.85 | 10.95 | <0.001 | −36.89 | 12.77 | 0.004 | −26.34 | 16.70 | 0.116 |
| innerI | −32.86 | 11.38 | 0.004 | −21.89 | 13.21 | 0.099 | −33.07 | 17.23 | 0.056 |
| outerI | −30.02 | 11.48 | 0.009 | −19.81 | 13.28 | 0.138 | −14.78 | 17.29 | 0.394 |
| SFCT | −33.13 | 11.83 | 0.006 | −25.31 | 14.00 | 0.072 | −28.05 | 18.42 | 0.129 |
| Choroidal volume (mm3): | |||||||||
| outerT | −0.25 | 0.05 | <0.001 | −0.19 | 0.06 | 0.002 | −0.17 | 0.08 | 0.038 |
| innerT | −0.07 | 0.02 | <0.001 | −0.05 | 0.02 | 0.011 | −0.05 | 0.03 | 0.059 |
| central macular | −0.03 | 0.01 | 0.002 | −0.03 | 0.01 | 0.015 | −0.03 | 0.01 | 0.054 |
| innerN | −0.05 | 0.02 | 0.009 | −0.006 | 0.02 | 0.007 | −0.005 | 0.03 | 0.074 |
| outerN | −0.22 | 0.07 | 0.002 | −0.17 | 0.08 | 0.033 | −0.10 | 0.10 | 0.329 |
| outerS | −0.26 | 0.06 | <0.001 | −0.21 | 0.06 | 0.001 | −0.18 | 0.08 | 0.039 |
| innerS | −0.07 | 0.02 | <0.001 | −0.06 | 0.02 | 0.001 | −0.04 | 0.03 | 0.136 |
| innerI | −0.05 | 0.02 | 0.004 | −0.03 | 0.02 | 0.105 | −0.05 | 0.03 | 0.056 |
| outerI | −0.17 | 0.06 | 0.007 | −0.11 | 0.07 | 0.105 | −0.10 | 0.09 | 0.247 |
| total | −1.16 | 0.30 | <0.001 | −0.89 | 0.34 | 0.008 | −0.76 | 0.43 | 0.079 |
| Other choroidal parameters: | |||||||||
| CVI | −0.03 | 0.01 | 0.009 | −0.04 | 0.01 | 0.003 | −0.04 | 0.02 | 0.027 |
| LA (mm2) | −0.17 | 0.07 | 0.017 | −0.10 | 0.08 | 0.227 | 0.04 | 0.11 | 0.703 |
| SA (mm2) | 0.0001 | 0.04 | 0.998 | 0.11 | 0.05 | 0.034 | 0.17 | 0.07 | 0.011 |
| TCA (mm2) | −0.17 | 0.10 | 0.099 | 0.005 | 0.12 | 0.965 | 0.21 | 0.16 | 0.176 |
| Choroidal Parameters | Cystoid vs. Diffuse | Cystoid vs. Subretinal Fluid | Diffuse vs. Subretinal Fluid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | |
| CVI | −0.008 | 0.01 | 0.456 | 0.01 | 0.02 | 0.502 | 0.002 | 0.02 | 0.920 |
| LA (mm2) | 0.11 | 0.06 | 0.099 | −0.20 | 0.10 | 0.047 | −0.12 | 0.10 | 0.228 |
| SA (mm2) | 0.12 | 0.04 | 0.008 | −0.17 | 0.07 | 0.011 | −0.07 | 0.07 | 0.352 |
| TCA (mm2) | 0.21 | 0.09 | 0.021 | −0.40 | 0.15 | 0.011 | −0.20 | 0.15 | 0.192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmuchowska, D.A.; Sidorczuk, P.; Pieklarz, B.; Konopińska, J.; Mariak, Z.; Obuchowska, I. Quantitative Assessment of Choroidal Parameters in Patients with Various Types of Diabetic Macular Oedema: A Single-Centre Cross-Sectional Analysis. Biology 2021, 10, 725. https://doi.org/10.3390/biology10080725
Dmuchowska DA, Sidorczuk P, Pieklarz B, Konopińska J, Mariak Z, Obuchowska I. Quantitative Assessment of Choroidal Parameters in Patients with Various Types of Diabetic Macular Oedema: A Single-Centre Cross-Sectional Analysis. Biology. 2021; 10(8):725. https://doi.org/10.3390/biology10080725
Chicago/Turabian StyleDmuchowska, Diana Anna, Patryk Sidorczuk, Barbara Pieklarz, Joanna Konopińska, Zofia Mariak, and Iwona Obuchowska. 2021. "Quantitative Assessment of Choroidal Parameters in Patients with Various Types of Diabetic Macular Oedema: A Single-Centre Cross-Sectional Analysis" Biology 10, no. 8: 725. https://doi.org/10.3390/biology10080725
APA StyleDmuchowska, D. A., Sidorczuk, P., Pieklarz, B., Konopińska, J., Mariak, Z., & Obuchowska, I. (2021). Quantitative Assessment of Choroidal Parameters in Patients with Various Types of Diabetic Macular Oedema: A Single-Centre Cross-Sectional Analysis. Biology, 10(8), 725. https://doi.org/10.3390/biology10080725







