MYOC Promotes the Differentiation of C2C12 Cells by Regulation of the TGF-β Signaling Pathways via CAV1
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Western Blotting
2.3. Immunofluorescence and Laser Confocal Microscopy
2.4. Construction of MYOC Overexpression Vector
2.5. Transfection of Overexpression Vector and siRNA
2.6. Co-Immunoprecipitation
2.7. Statistical Analysis
3. Results
3.1. Expression Pattern and Localization of MYOC during the Differentiation of C2C12 Cells
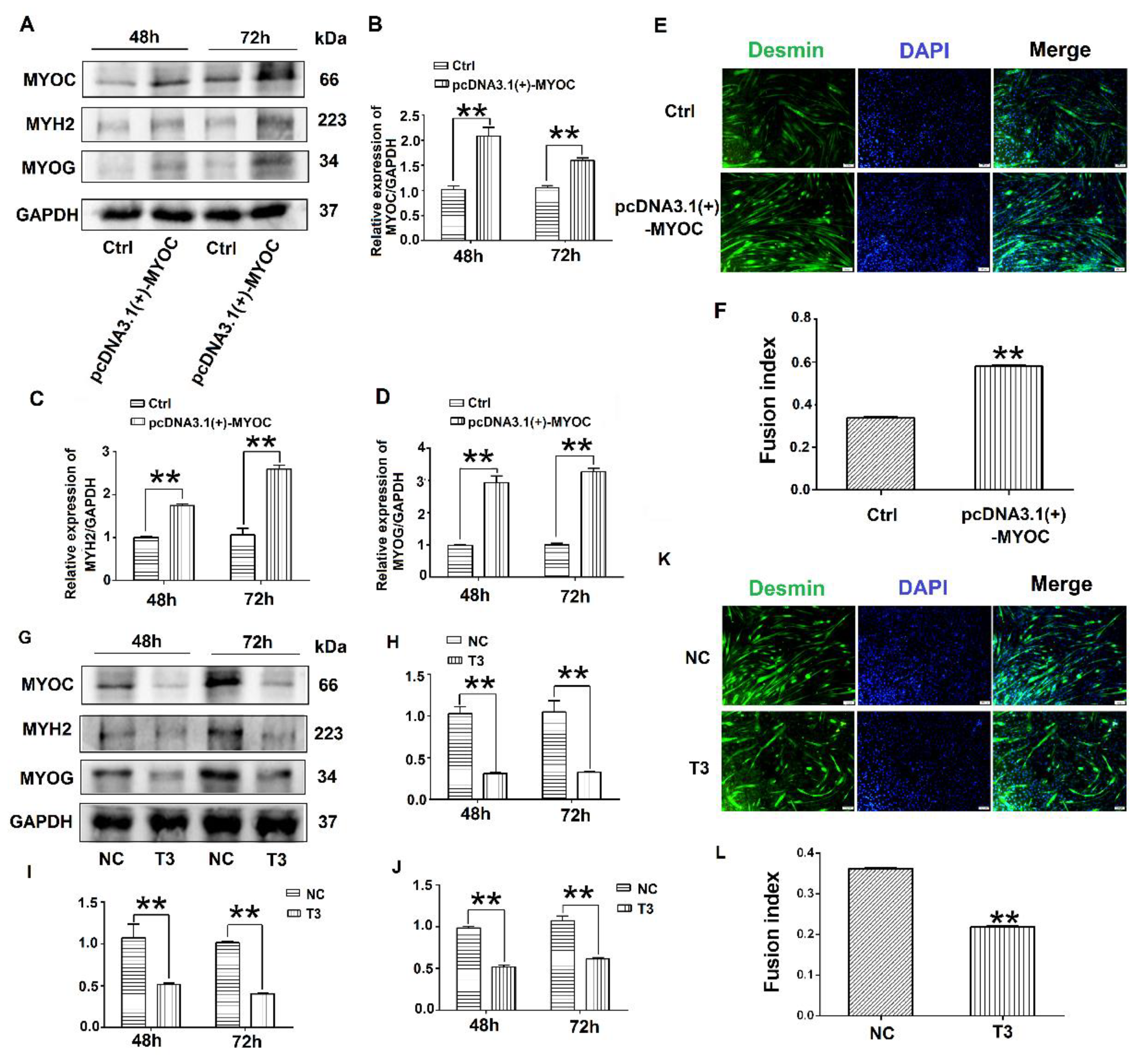
3.2. Effects of MYOC on the Differentiation of C2C12 Cells
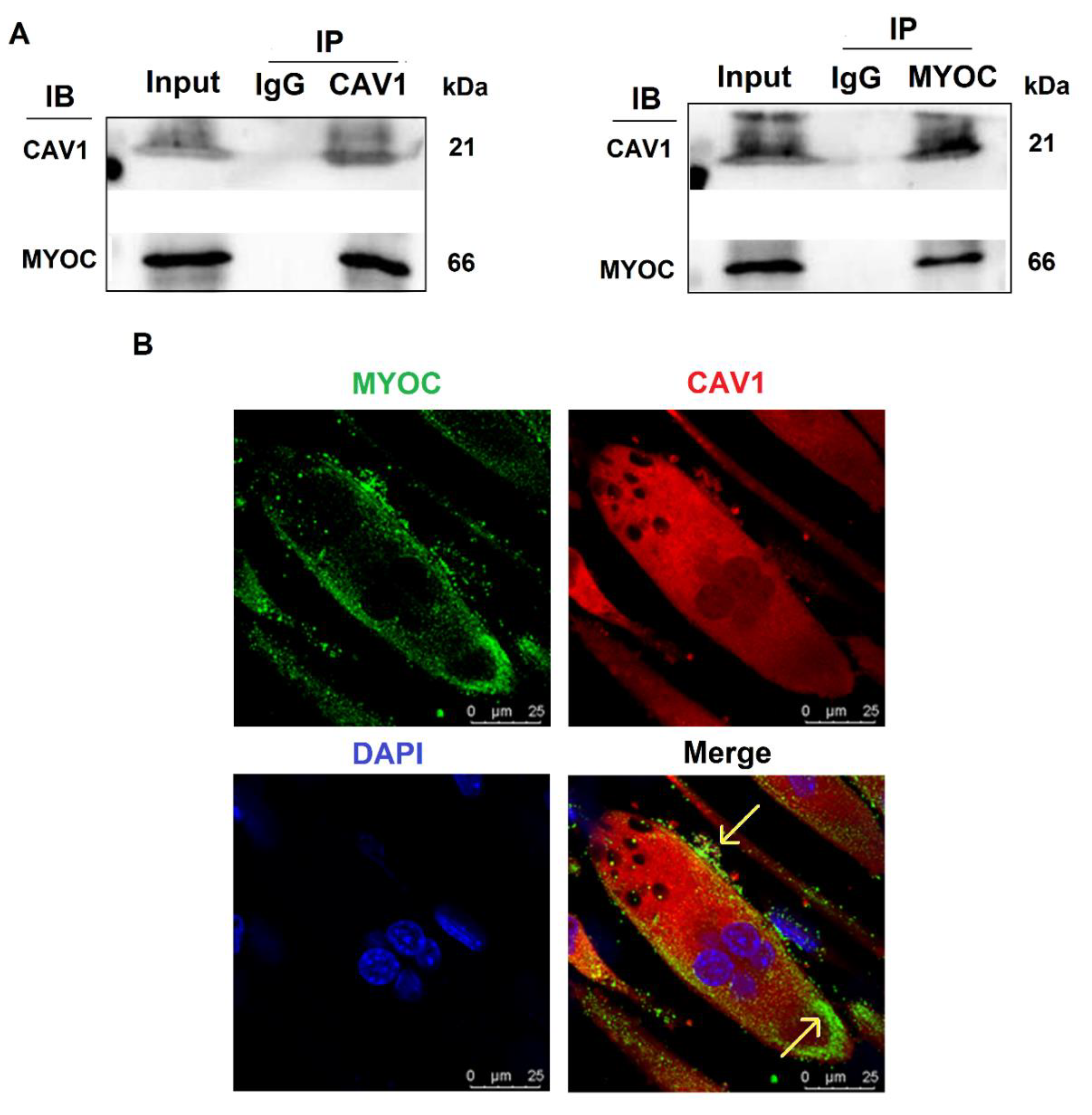
3.3. Interaction and Co-Localization of MYOC and CAV1
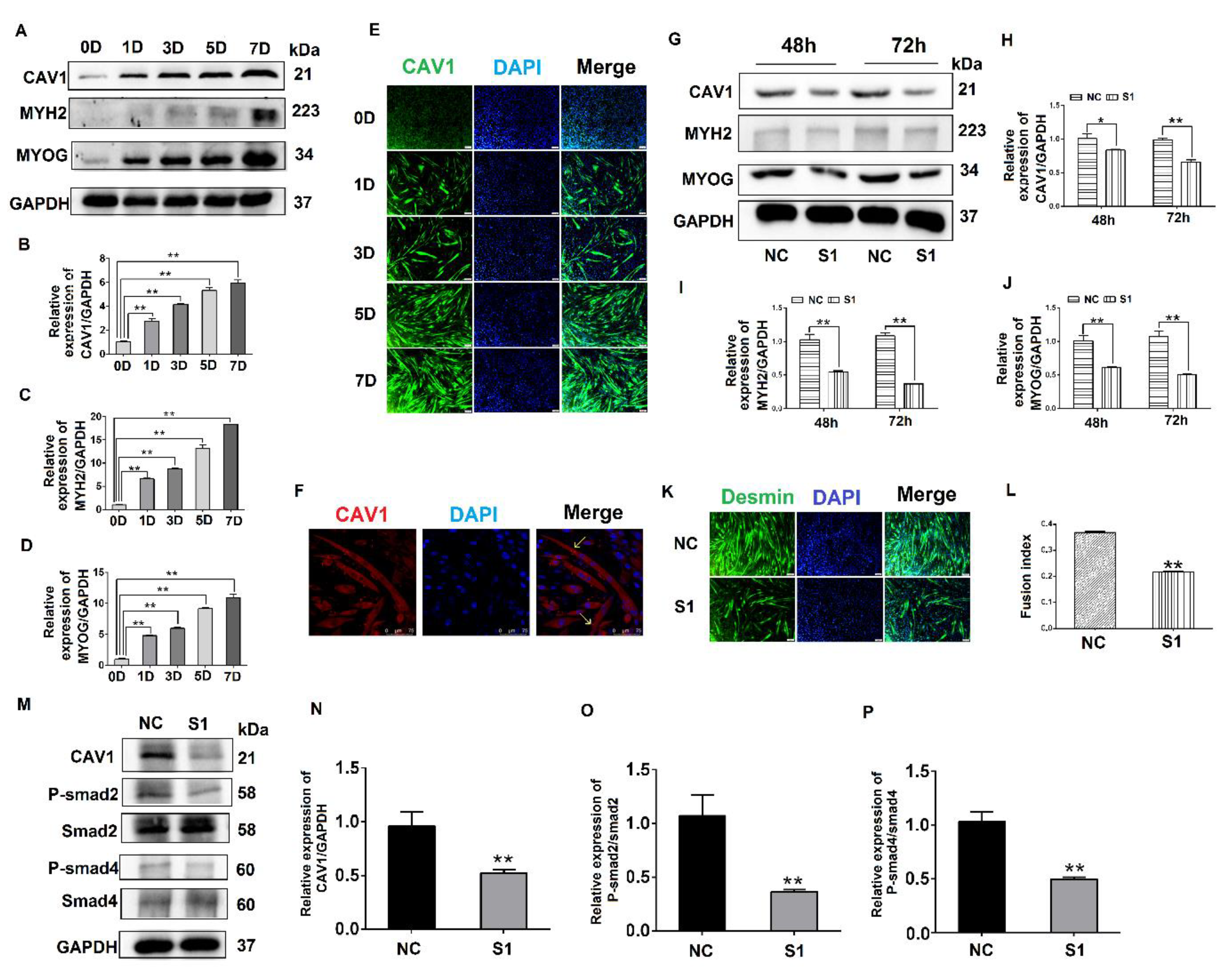
3.4. Effects of CAV1 on the Differentiation and Localization of C2C12 Cells
3.5. MYOC Exerts an Effect on the Differentiation of C2C12 Cells by Regulation of CAV1 That Affects the TGF-β Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahane, N.; Cinnamon, Y.; Kalcheim, C. The origin and fate of pioneer myotomal cells in the avian embryo. Mech. Dev. 1998, 74, 59–73. [Google Scholar] [CrossRef]
- Kahane, N.; Kalcheim, C. Identification of early postmitotic cells in distinct embryonic sites and their possible roles in morphogenesis. Cell Tissue Res. 1998, 294, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Choudhury, D.; Asmani, M.; Zhao, R.; Lei, P.; Andreadis, S.T. NANOG restores the impaired myogenic differentiation potential of skeletal myoblasts after multiple population doublings. Stem Cell Res. 2018, 26, 55–66. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Puig, L.S.; Smith, J.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef] [Green Version]
- Murach, K.A.; Dungan, C.M.; Kosmac, K.; Voigt, T.B.; Tourville, T.W.; Miller, M.S.; Bamman, M.M.; Peterson, C.A.; Toth, M.J. Fiber typing human skeletal muscle with fluorescent immunohistochemistry. J. Appl. Physiol. 2019, 127, 1632–1639. [Google Scholar] [CrossRef]
- Contreras, O.; Villarreal, M.; Brandan, E. Nilotinib impairs skeletal myogenesis by increasing myoblast proliferation. Skelet. Muscle 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Capetanaki, Y.; Milner, D.J.; Weitzer, G. Desmin in Muscle Formation and Maintenance: Knockouts and Consequences. Cell Struct. Funct. 1997, 22, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. C2C12 cell model: Its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J. Pharm. Pharmacol. 2020, 72, 1667–1693. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-Guzmán, C.; Contreras, O.; Brandan, E. Expression of CTGF/CCN2 in response to LPA is stimulated by fibrotic extracellular matrix via the integrin/FAK axis. Am. J. Physiol. Cell Physiol. 2018, 314, C415–C427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liang, X.; Shan, T.; Jiang, Q.; Deng, C.; Zheng, R.; Kuang, S. mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem. Biophys. Res. Commun. 2015, 463, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Vajjala, A.; McFarlane, C.; Wahli, W.; Sharma, M.; Kambadur, R. Lack of Smad3 signaling leads to impaired skeletal muscle regeneration. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E90–E102. [Google Scholar] [CrossRef] [Green Version]
- Winbanks, C.E.; Weeks, K.; Thomson, R.E.; Sepulveda, P.V.; Beyer, C.; Qian, H.; Chen, J.L.; Allen, J.M.; Lancaster, G.I.; Febbraio, M.A.; et al. Follistatin-mediated skeletal muscle hypertrophy is regulated by Smad3 and mTOR independently of myostatin. J. Cell Biol. 2012, 197, 997–1008. [Google Scholar] [CrossRef]
- Massague, J.; Cheifetz, S.; Endo, T.; Nadal-Ginard, B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA 1986, 83, 8206–8210. [Google Scholar] [CrossRef] [Green Version]
- Schabort, E.J.; Van Der Merwe, M.; Loos, B.; Moore, F.P.; Niesler, C.U. TGF-β’s delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp. Cell Res. 2009, 315, 373–384. [Google Scholar] [CrossRef]
- Haines, P.; Hant, F.N.; Lafyatis, R.; Trojanowska, M.; Bujor, A.M. Elevated expression of cav-1 in a subset of SSc fibroblasts contributes to constitutive Alk1/Smad1 activation. J. Cell. Mol. Med. 2012, 16, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Teeters, K.; Miyasato, S.; Choi, J.; Nakamatsu, G.; Richardson, J.A.; Starcher, B.; Davis, E.C.; Tam, E.K.; Saux, C.J.-L. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L1007–L1017. [Google Scholar] [CrossRef] [Green Version]
- Santibanez, J.F.; Blanco, F.-J.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; Del Pozo, M.A.; Bernabeu, C. Caveolin-1 interacts and cooperates with the transforming growth factor-β type I receptor ALK1 in endothelial caveolae. Cardiovasc. Res. 2007, 77, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, M.; Zhang, Z.; Xue, H.; Chen, X.; Ji, Y. Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork (Review). Int. J. Mol. Med. 2018, 43, 671–681. [Google Scholar] [CrossRef]
- Kwon, H.S.; Johnson, T.V.; Tomarev, S.I. Myocilin Stimulates Osteogenic Differentiation of Mesenchymal Stem Cells through Mitogen-activated Protein Kinase Signaling. J. Biol. Chem. 2013, 288, 16882–16894. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.M.; Dolman, A.J.; Guo, C.; Dolan, K.; Xiang, C.; Reda, S.; Li, B.; Prasanna, G. Mutant myocilin impacts sarcomere ultrastructure in mouse gastrocnemius muscle. PLoS ONE 2018, 13, e0206801. [Google Scholar] [CrossRef]
- Joe, M.K.; Kwon, H.S.; Cojocaru, R.; Tomarev, S.I. Myocilin Regulates Cell Proliferation and Survival. J. Biol. Chem. 2014, 289, 10155–10167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseychuk, O.; Akkiraju, H.; Dutta, J.; D’Angelo, A.; Bragdon, B.; Duncan, R.L.; Nohe, A. Inhibition of CK2 binding to BMPRIa induces C2C12 differentiation into osteoblasts and adipocytes. J. Cell Commun. Signal. 2013, 7, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozsa, F.; Shimizu, S.; Lichter, P.R.; Johnson, A.; Othman, M.I.; Scott, K.; Downs, C.A.; Nguyen, T.D.; Polansky, J.; Richards, J.E. GLC1A mutations point to regions of potential functional importance on the TIGR/MYOC protein. Mol. Vis. 1998, 4, 20. Available online: http://www.molvis.org/molvis/v4/p20 (accessed on 27 November 2020).
- Taguchi, M.; Kanno, H.; Kubota, R.; Miwa, S.; Shishiba, Y.; Ozawa, Y. Molecular Cloning and Expression Profile of Rat Myocilin. Mol. Genet. Metab. 2000, 70, 75–80. [Google Scholar] [CrossRef]
- Swiderski, R.E.; Ying, L.; Cassell, M.D.; Alward, W.L.; Stone, E.M.; Sheffield, V.C. Expression pattern and in situ localization of the mouse homologue of the human MYOC (GLC1A) gene in adult brain. Mol. Brain Res. 1999, 68, 64–72. [Google Scholar] [CrossRef]
- Gruber, H.; Ingram, J.; Hanley, E.N., Jr. Cellular immunohistochemical localization of the matricellular protein myocilin in the intervertebral disc. Biotech. Histochem. 2006, 81, 119–124. [Google Scholar] [CrossRef]
- Milewska, M.D.; Domoradzki, T.; Majewska, A.; Błaszczyk, M.; Gajewska, M.; Hulanicka, M.; Ciecierska, A.; Grzelkowska-Kowalczyk, K. Interleukin-8 enhances myocilin expression, Akt-FoxO3 signaling and myogenic differentiation in rat skeletal muscle cells. J. Cell. Physiol. 2019, 234, 19675–19690. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.K.; Kee, C.; Tomarev, S.I. Myocilin Interacts with Syntrophins and Is Member of Dystrophin-associated Protein Complex. J. Biol. Chem. 2012, 287, 13216–13227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoli, P.; Godbole, A.; Romanowski, M.J.; Clark, K.; Meredith, E.; Saenz-Vash, V.; Wang, Y.K.; Lewicki, N.; Nguyen, A.A.; Lynch, J.M. Full-length myocilin protein is purified from mammalian cells as a dimer. Protein Expr. Purif. 2018, 147, 38–48. [Google Scholar] [CrossRef]
- Boscher, C.; Nabi, I.R. CAVEOLIN-1: Role in Cell Signaling. Adv. Exp. Med. Biol. 2012, 729, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.; Zhang, G.; You, Y.; Tuan, R.S. Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 3773–3787. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Liu, Y.; Kaul, A.; Peipe, I.; Dooley, S. Caveolin-1 abrogates TGF-β mediated hepatocyte apoptosis. Cell Death Dis. 2013, 4, e466. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Li, S.; Hu, X.-Y.; Tong, H.-L.; Li, S.-F.; Yan, Y.-Q. TCEA3 promotes differentiation of C2C12 cells via an Annexin A1-mediated transforming growth factor-β signaling pathway. J. Cell. Physiol. 2019, 234, 10554–10565. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, S.; Wen, X.; Tong, H.; Li, S.; Yan, Y. MYOC Promotes the Differentiation of C2C12 Cells by Regulation of the TGF-β Signaling Pathways via CAV1. Biology 2021, 10, 686. https://doi.org/10.3390/biology10070686
Zhang Y, Li S, Wen X, Tong H, Li S, Yan Y. MYOC Promotes the Differentiation of C2C12 Cells by Regulation of the TGF-β Signaling Pathways via CAV1. Biology. 2021; 10(7):686. https://doi.org/10.3390/biology10070686
Chicago/Turabian StyleZhang, Yuhan, Shuang Li, Xin Wen, Huili Tong, Shufeng Li, and Yunqin Yan. 2021. "MYOC Promotes the Differentiation of C2C12 Cells by Regulation of the TGF-β Signaling Pathways via CAV1" Biology 10, no. 7: 686. https://doi.org/10.3390/biology10070686
APA StyleZhang, Y., Li, S., Wen, X., Tong, H., Li, S., & Yan, Y. (2021). MYOC Promotes the Differentiation of C2C12 Cells by Regulation of the TGF-β Signaling Pathways via CAV1. Biology, 10(7), 686. https://doi.org/10.3390/biology10070686






