Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fipronil, L-Arginine and L-Carnitine
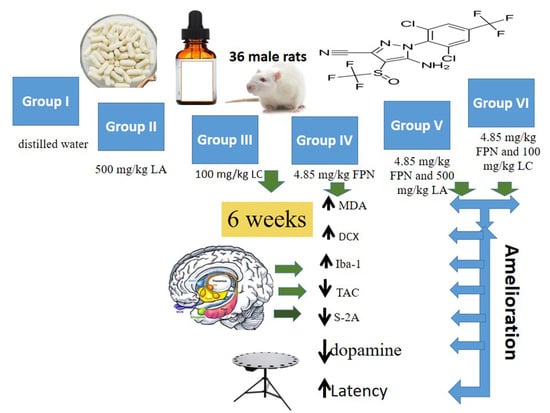
2.3. Experimental Design
- Group I, Control rats received only distilled water.
- Group III (L-carnitine, LC), Rats were gavaged 100 mg/kg LC [14].
- Group IV (Fipronil, FPN), Rats were treated with 4.85 mg/kg FPN (1/20 of FPN LD50). The dosage was chosen according to the available publications regarding oral LD50 of fipronil for rats [15].
- Group V (FPN + LA), Rats were gavaged 500 mg/kg LA (25% w/v in distilled water) and 4.85 mg/kg FPN (1/20 of FPN LD50), one hour apart.
- Group VI (FPN + LC), Rats were gavaged LC 100 mg/kg and 4.85 mg/kg FPN (1/20 of FPN LD50), one hour apart.
2.4. Body and Brain Weights
2.5. Barnes Maze (BM)
2.6. Blood and Samples Collection
2.7. Brain Homogenate
2.8. Total Anti-Oxidant Capacity (TAC) and Malondialdehyde (MDA)
2.9. Dopamine and Corticosterone Levels
2.10. Histopathology
2.11. Immunohistochemistry (IHC)
2.12. Statistical Analysis
3. Results
3.1. Body and Brain Weights
3.2. Barnes Maze
3.2.1. Acquisition Phase
3.2.2. Probe Trial
3.3. Total Anti-Oxidant Capacity and Malondialdehyde Levels
3.4. Dopamine and Corticosterone Levels
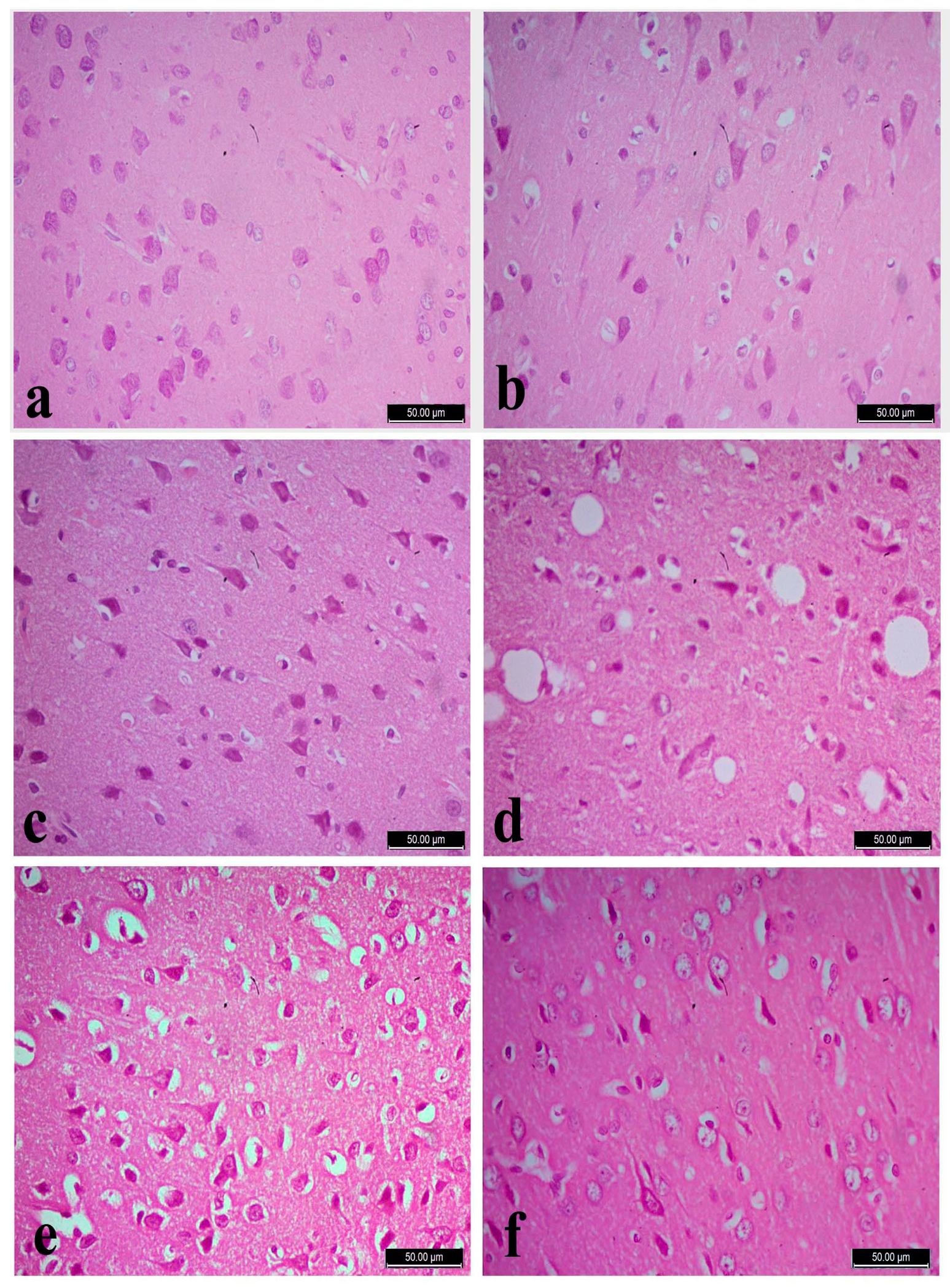
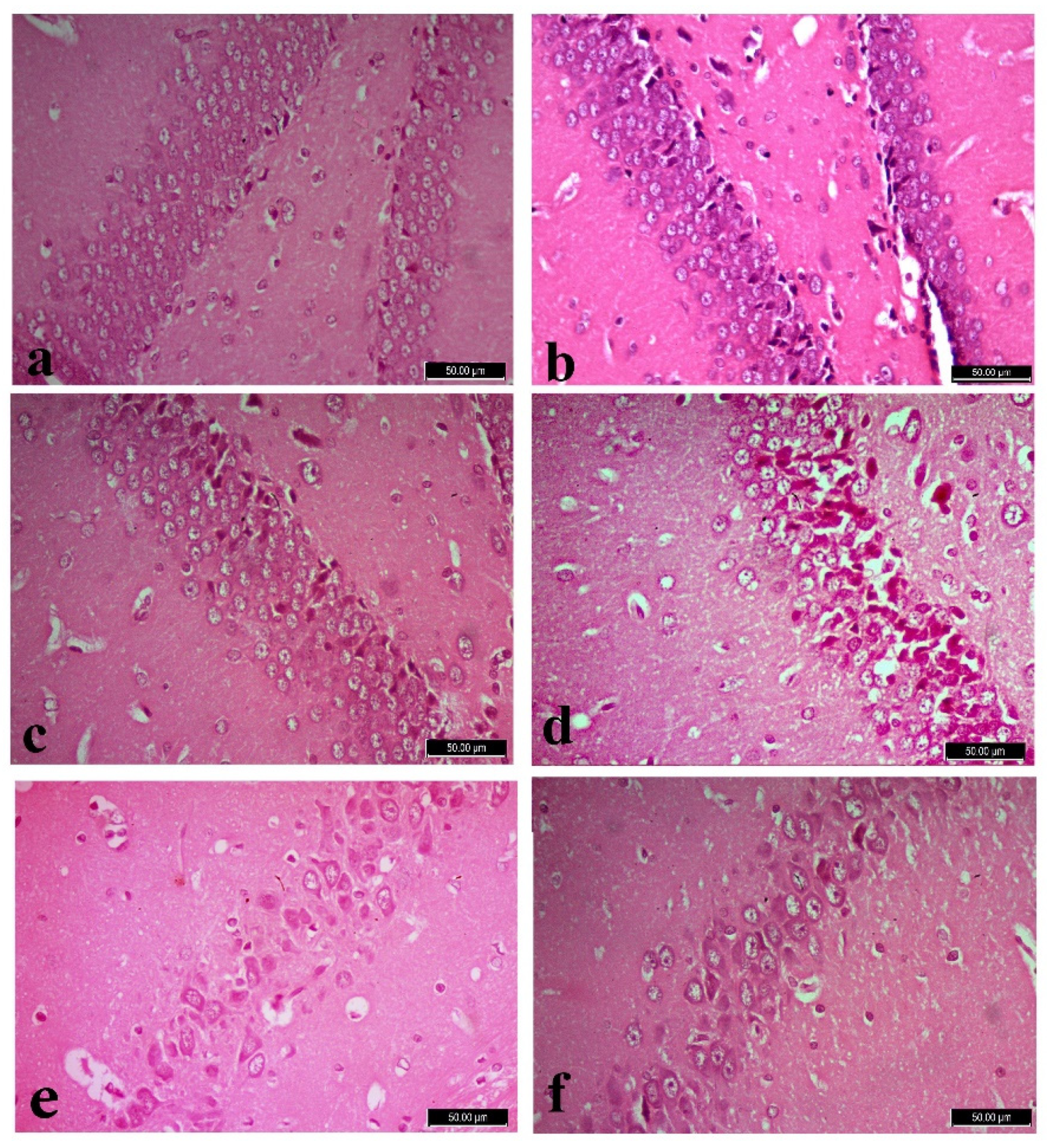
3.5. Histopathology
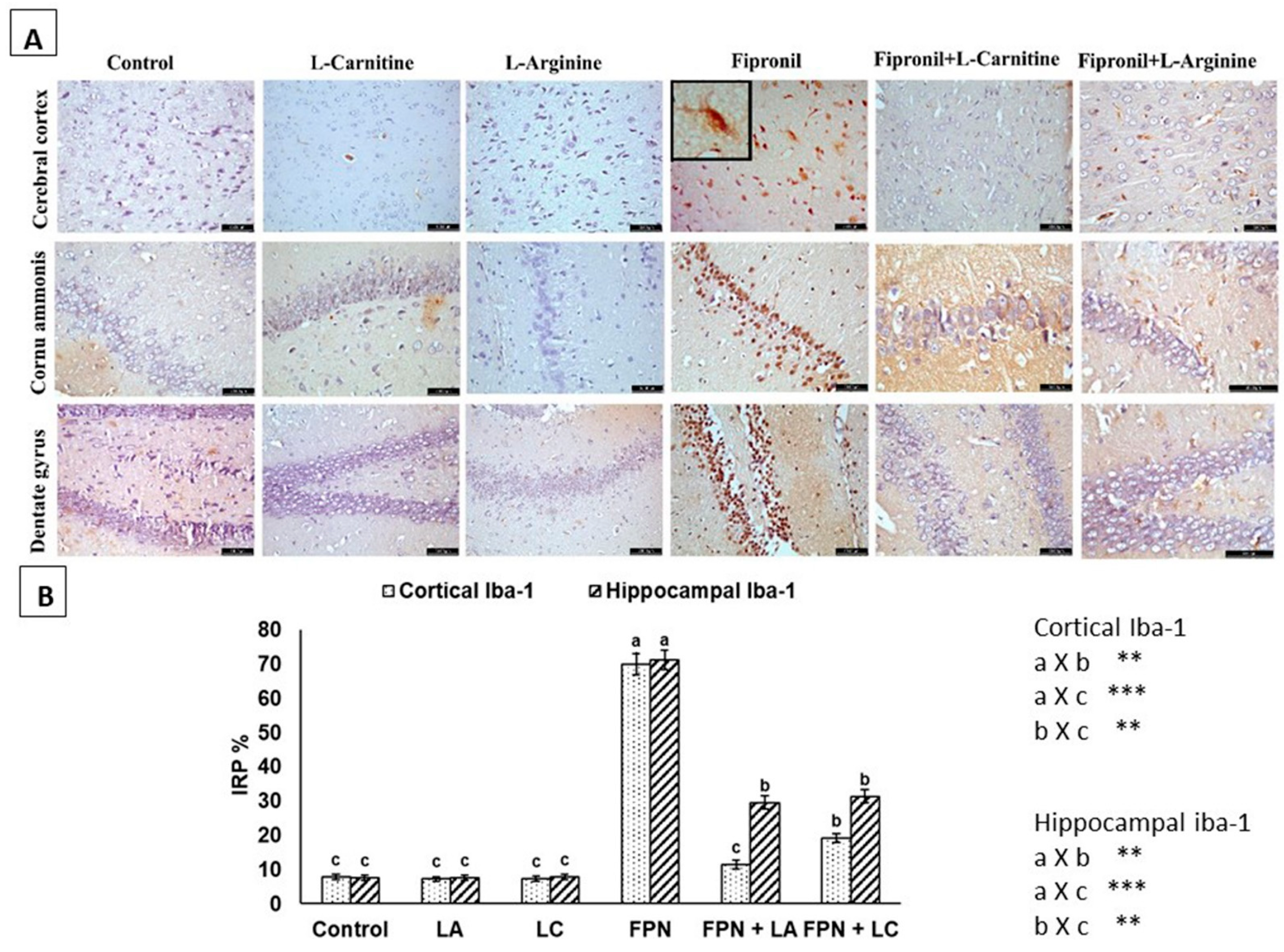
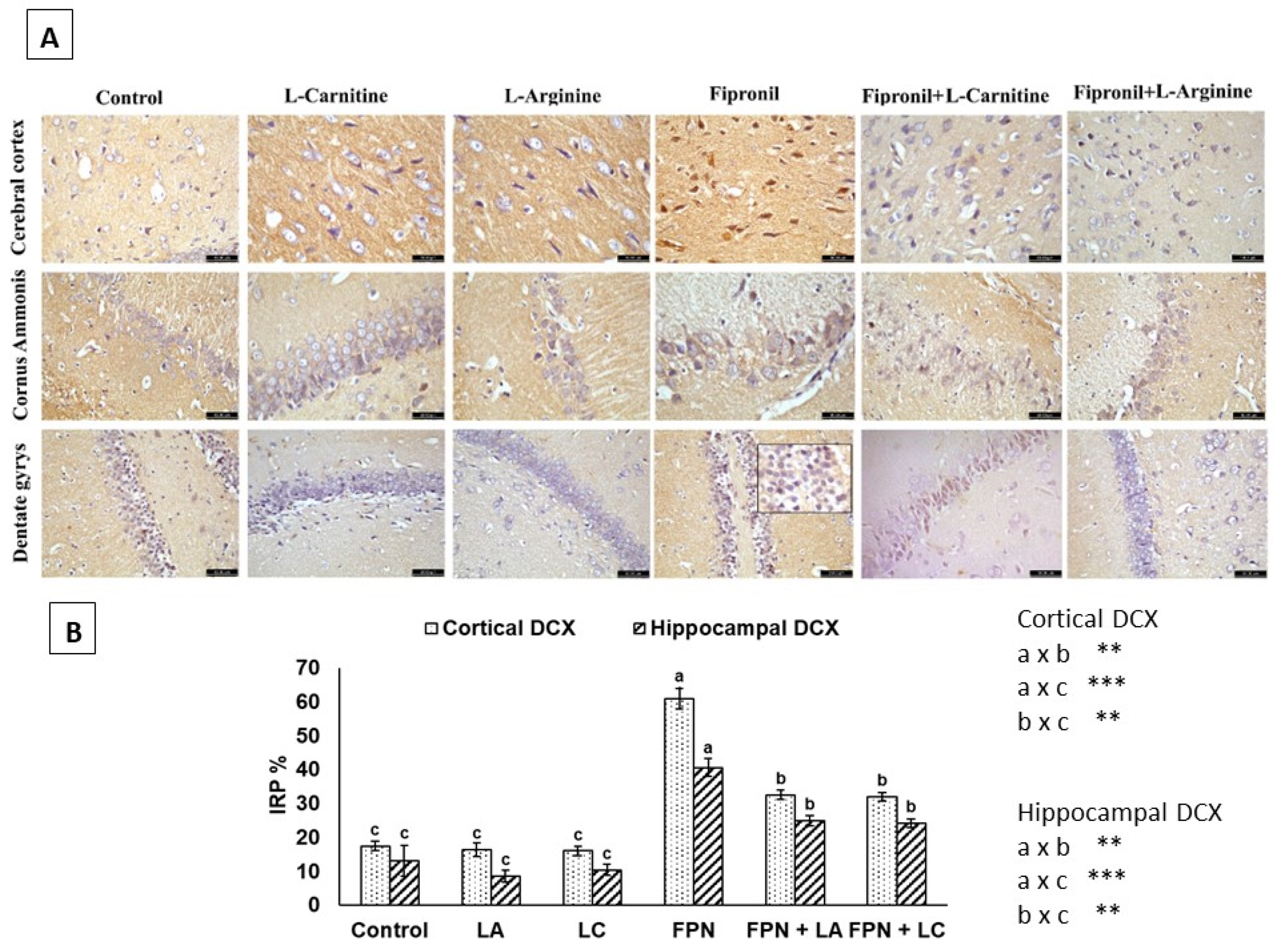
3.6. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamel, F.; Hoppin, J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004, 112, 950–958. [Google Scholar] [CrossRef]
- Khalaf, A.A.; Galal, M.K.; Ibrahim, M.A.; Allah, A.A.A.; Afify, M.M.; Refaat, R. The Terminalia laxiflora modulates the neurotoxicity induced by fipronil in male albino rats. Biosci. Rep. 2019, 39, BSR20181363. [Google Scholar] [CrossRef]
- Parrilla Vázquez, P.; Hakme, E.; Uclés, S.; Cutillas, V.; Martínez Galera, M.; Mughari, A.R.; Fernández-Alba, A.R. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1463, 20–31. [Google Scholar] [CrossRef]
- Gupta, R.C.; Anadón, A. Chapter 42—Fipronil. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 533–538. [Google Scholar]
- Zhao, X.; Yeh, J.Z.; Salgado, V.L.; Narahashi, T. Sulfone metabolite of fipronil blocks γ-aminobutyric acid- and glutamate-activated chloride channels in mammalian and insect neurons. J. Pharmacol. Exp. Ther. 2005, 314, 363–373. [Google Scholar] [CrossRef]
- Godinho, A.F.; de Oliveira Souza, A.C.; Carvalho, C.C.; Horta, D.F.; De Fraia, D.; Anselmo, F.; Chaguri, J.L.; Faria, C.A. Memory impairment due to fipronil pesticide exposure occurs at the GABAA receptor level, in rats. Physiol. Behav. 2016, 165, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Bharatiya, R.; Chagraoui, A.; De Deurwaerdere, S.; Argiolas, A.; Melis, M.R.; Sanna, F.; De Deurwaerdere, P. Chronic administration of fipronil heterogeneously alters the neurochemistry of monoaminergic systems in the rat brain. Int. J. Mol. Sci. 2020, 21, 5711. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2015, 30, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Stewart, M.K.; Barbul, A. Chapter 27—Cellular and physiological effects of arginine in seniors. In Nutrition and Functional Foods for Healthy Aging; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 317–336. [Google Scholar]
- Virarkar, M.; Alappat, L.; Bradford, P.G.; Awad, A.B. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2013, 53, 1157–1167. [Google Scholar] [CrossRef]
- Maeda, T.; Wakasawa, T.; Shima, Y.; Tsuboi, I.; Aizawa, S.; Tamai, I. Role of polyamines derived from arginine in differentiation and proliferation of human blood cells. Biol. Pharm. Bull. 2006, 29, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.P.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, F.S.; da Silva, L.A.; Pochapski, J.A.; Raczenski, A.; da Silva, W.C.; Grassiolli, S.; Malfatti, C.R.M. Effects of L-arginine and creatine administration on spatial memory in rats subjected to a chronic variable stress model. Pharm. Biol. 2014, 52, 1033–1038. [Google Scholar] [CrossRef]
- Flatters, S.J.; Xiao, W.-H.; Bennett, G.J. Acetyl-L-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci. Lett. 2006, 397, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Tingle, C.C.D.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 2003, 176, 1–66. [Google Scholar]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74. [Google Scholar] [CrossRef] [PubMed]
- Culling, C.F.A.; Allison, R.T.; Barr, W.T. Cellular Pathology Technique, 4th ed.; Butterworth-Heinemann Ltd.: London, UK; Boston, MA, USA, 1985; pp. 155–163. [Google Scholar]
- Schacht, V.; Kern, J.S. Basics of immunohistochemistry. J. Investig. Dermatol. 2015, 135, e30. [Google Scholar] [CrossRef]
- Farrag, M.; Pukale, D.D.; Leipzig, N.D. Micro-computed tomography utility for estimation of intraparenchymal spinal cord cystic lesions in small animals. Neural Regen. Res. 2021, 16, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Sirerol-Piquer, M.; Gomez-Ramos, P.; Hernández, F.; Perez, M.; Morán, M.A.; Fuster-Matanzo, A.; Lucas, J.J.; Avila, J.; García-Verdugo, J.M. GSK3β overexpression induces neuronal death and a depletion of the neurogenic niches in the dentate gyrus. Hippocampus 2011, 21, 910–922. [Google Scholar] [CrossRef]
- EPA. EPA Guidelines for Responsible Pesticide Use; Environment Protection Authority: New South Wales, Australia, 2005. [Google Scholar]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. Int. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Campos, M.R.; Picanço, M.; Martins, J.; Tomaz, A.; Guedes, R.N. Insecticide selectivity and behavioral response of the earwig Doru luteipes. Crop Prot. 2011, 30, 1535–1540. [Google Scholar] [CrossRef]
- Gao, J.; Wang, F.; Jiang, W.; Miao, J.; Wang, P.; Zhou, Z.; Liu, D. A full evaluation of chiral phenylpyrazole pesticide flufiprole and the metabolites to non-target organism in paddy field. Environ. Pollut. 2020, 264, 114808. [Google Scholar] [CrossRef]
- Mossa, A.-T.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of natural insecticides: Toxic effects on experimental animals. BioMed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, T.; Duvarci, S. Hippocampal-prefrontal interactions in cognition, behavior and psychiatric disease. Front. Syst. Neurosci. 2016, 9. [Google Scholar] [CrossRef]
- Maren, S. 3.24—Emotional learning: Animals. In Learning and Memory: A Comprehensive Reference; Byrne, J.H., Ed.; Academic Press: Oxford, UK, 2008; pp. 475–502. [Google Scholar]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef]
- Taibi, N.; Kahloula, K.; Adli, D.; Arabi, W.; Brahmi, M.; Slimani, M. Effet thérapeutique de l’extrait aqueux de Pimpinella anisum L. chez les rats Wistar exposés de manière subchronique à l’imidaclopride. Étude neurocomportementale. Phytothérapie 2020. [Google Scholar] [CrossRef]
- Terçariol, P.R.G.; Godinho, A.F. Behavioral effects of acute exposure to the insecticide fipronil. Pestic. Biochem. Physiol. 2011, 99, 221–225. [Google Scholar] [CrossRef]
- Begega, A.; Cuesta, M.; Rubio, S.; Méndez, M.; Santín, L.J.; Arias, J.L. Functional networks involved in spatial learning strategies in middle-aged rats. Neurobiol. Learn. Mem. 2012, 97, 346–353. [Google Scholar] [CrossRef]
- Jacobson, L.; Zhang, R.; Elliffe, D.; Chen, K.F.; Mathai, S.; McCarthy, D.; Waldvogel, H.; Guan, J. Correlation of cellular changes and spatial memory during aging in rats. Exp. Gerontol. 2008, 43, 929–938. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Fole, A.; Martín, M.; Morales, L.; Victoria Cano, M.; Ruiz-Gayo, M.; Olmo, N.D. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol. Learn. Mem. 2013, 106, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Omoi, N.O.; Hayasaka, T.; Shinnkai, T.; Suzuki, S.; Abe, K.; Urano, S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann. N. Y. Acad. Sci. 2002, 959, 275–284. [Google Scholar] [CrossRef]
- Cravedi, J.P.; Delous, G.; Zalko, D.; Viguié, C.; Debrauwer, L. Disposition of fipronil in rats. Chemosphere 2013, 93, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef]
- Song, J.; Kim, J. Degeneration of dopaminergic neurons due to metabolic alterations and parkinson’s disease. Front. Aging Neurosci. 2016, 8. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Pawar, N.N.; Chandratre, G.A.; Telang, A.G.; Sharma, A.K. Fipronil induced oxidative stress in kidney and brain of mice: Protective effect of vitamin E and vitamin C. Pestic Biochem. Physiol. 2015, 118, 10–18. [Google Scholar] [CrossRef]
- AlBasher, G.; Abdel-Daim, M.M.; Almeer, R.; Ibrahim, K.A.; Hamza, R.Z.; Bungau, S.; Aleya, L. Synergistic antioxidant effects of resveratrol and curcumin against fipronil-triggered oxidative damage in male albino rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 6505–6514. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Fipronil sulfone induced higher cytotoxicity than fipronil in SH-SY5Y cells: Protection by antioxidants. Toxicol. Lett. 2016, 252, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant supplementation in the treatment of aging-associated diseases. Front Pharm. 2016, 7, 24. [Google Scholar] [CrossRef]
- Hosseini, M.; Anaeigoudari, A.; Beheshti, F.; Soukhtanloo, M.; Nosratabadi, R. Protective effect against brain tissues oxidative damage as a possible mechanism for beneficial effects of L-arginine on lipopolysaccharide induced memory impairment in rats. Drug Chem. Toxicol. 2018, 41, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.J.A.; Panneerselvam, C. Effect of L-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J. Gerontol. Ser. A 2002, 57, B134–B137. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef]
- Cherian, L.; Hlatky, R.; Robertson, C.S. Nitric oxide in traumatic brain injury. Brain Pathol. 2004, 14, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Kagan, V.E.; Ramsey, R.; Tsuchiya, M.; Khwaja, S.; Serbinova, E.A.; Packer, L. Antiradical effects in l-propionyl carnitine protection of the heart against ischemia-reperfusion injury: The possible role of iron chelation. Arch. Biochem. Biophys. 1992, 296, 394–401. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Saluk-Juszczak, J.; Wachowicz, B. L-Carnitine protects plasma components against oxidative alterations. Nutrition 2011, 27, 693–699. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Lyngsie, G.; Krumina, L.; Tunlid, A.; Persson, P. Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 2018, 8, 10834. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Douroudos, I.; Panagoutsos, S.; Pasadakis, P.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; Michailidis, Y.; Jamurtas, A.Z.; Mandalidis, D.; et al. Effects of L-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010, 42, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Lin, J.-S.; Lin, Y.-C.; Lin, P.-T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: A randomized, placebo-controlled trial. Nutr. J. 2014, 13, 79. [Google Scholar] [CrossRef]
- Augustyniak, A.; Skrzydlewska, E. The influence of L-carnitine suplementation on the antioxidative abilities of serum and the central nervous system of ethanol-induced rats. Metab. Brain Dis. 2010, 25, 381–389. [Google Scholar] [CrossRef]
- Neuman, S.L.; Lin, T.L.; Heste, P.Y. The effect of dietary carnitine on semen traits of white Leghorn roosters. Poult. Sci. 2002, 81, 495–503. [Google Scholar] [CrossRef]
- Bergin, D.H. L-Arginine Metabolism in Animal Models of Alzheimer’s Disease; University of Otago: Dunedin, New Zealand, 2015. [Google Scholar]
- Lohninger, S.; Strasser, A.; Bubna-Littitz, H. The effect of L-carnitine on T-maze learning ability in aged rats. Arch. Gerontol. Geriatr. 2001, 32, 245–253. [Google Scholar] [CrossRef]
- Shea, T.B. Effects of dietary supplementation with N-acetyl cysteine, acetyl-l-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. NeuroMolecular Med. 2007, 9, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Payet, N.; Battista, M.C. Steroidogenesis-adrenal cell signal transduction. Compr. Physiol. 2014, 4, 889–964. [Google Scholar] [CrossRef] [PubMed]
- Katsu, Y.; Iguchi, T. Subchapter 95D—Cortisol. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; p. 533-e95D-2. [Google Scholar]
- Prevatto, J.P.; Torres, R.C.; Diaz, B.L.; Silva, P.M.; Martins, M.A.; Carvalho, V.F. Antioxidant treatment induces hyperactivation of the HPA axis by upregulating ACTH receptor in the adrenal and downregulating glucocorticoid receptors in the pituitary. Oxid. Med. Cell. Longev. 2017, 2017, 4156361. [Google Scholar] [CrossRef] [PubMed]
- Finsterwald, C.; Alberini, C.M. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: From adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 2014, 112, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, Y.S.; Koh, H.C. Progressive loss of nigrostriatal dopaminergic neurons induced by inflammatory responses to fipronil. Toxicol. Lett. 2016, 258, 36–45. [Google Scholar] [CrossRef]
- Ventura, E.; Durant, R.; Jaussent, A.; Picot, M.C.; Morena, M.; Badiou, S.; Dupuy, A.M.; Jeandel, C.; Cristol, J.P. Homocysteine and inflammation as main determinants of oxidative stress in the elderly. Free Radic. Biol. Med. 2009, 46, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Stehr, C.M.; Linbo, T.L.; Incardona, J.P.; Scholz, N.L. The developmental neurotoxicity of fipronil: Notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol. Sci. 2006, 92, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Wong, A.H.C. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharm. Sin 2018, 39, 733–753. [Google Scholar] [CrossRef]
- Crowley, T.; Cryan, J.F.; Downer, E.J.; O’Leary, O.F. Inhibiting neuroinflammation: The role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav. Immun. 2016, 54, 260–277. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Bernal Fagiani, M.; Fluminhan, A.; de Azevedo Mello, F.; Yabuki, D.; Gonçalves, G.V.; Tsujigushi, L.K.; Pereira, L.G.; da Silva, K.A.; da Silva, S.B.B.; Santarem, C.L.; et al. l-arginine minimizes immunosuppression and prothrombin time and enhances the genotoxicity of 5-fluorouracil in rats. Nutrition 2019, 66, 94–100. [Google Scholar] [CrossRef]
- Lee, B.J.; Lin, J.S.; Lin, Y.C.; Lin, P.T. Antiinflammatory effects of L-carnitine supplementation (1000 mg/d) in coronary artery disease patients. Nutrition 2015, 31, 475–479. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef]
- Shen, K.Z.; Cox, B.A.; Johnson, S.W. L-Arginine potentiates GABA-mediated synaptic transmission by a nitric oxide-independent mechanism in rat dopamine neurons. Neuroscience 1997, 79, 649–658. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. L-carnitine and acetyl-L-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Ibrahim, W.; Ghada, F.S.; Abdallah, M. Therapeutic Effect of L-Carnitine on Acute Pancreatitis Induced by L-Arginine in Rats: Possible Role of Beclin Gene and Inducible Nitric Oxide Synthase. Med. J. Cairo Univ. 2019, 87, 1793–1803. [Google Scholar]
- Korish, A.A. Multiple antioxidants and L-arginine modulate inflammation and dyslipidemia in chronic renal failure rats. Ren. Fail. 2010, 32, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Parent, J.M. Epilepsy and Adult Neurogenesis. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.-Y.; Gil-Mohapel, J.; Christie, B.R.; So, K.-F. Physical exercise-induced adult neurogenesis: A good strategy to prevent cognitive decline in neurodegenerative diseases? BioMed Res. Int. 2014, 2014, 403120. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Regensburger, M.; Prots, I.; Winner, B. Adult hippocampal neurogenesis in parkinson’s disease: Impact on neuronal survival and plasticity. Neural Plast. 2014, 2014, 454696. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Ait-Bali, Y.; Ba-M’hamed, S.; Gambarotta, G.; Sassoè-Pognetto, M.; Giustetto, M.; Bennis, M. Pre- and postnatal exposure to glyphosate-based herbicide causes behavioral and cognitive impairments in adult mice: Evidence of cortical ad hippocampal dysfunction. Arch. Toxicol. 2020, 94, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-P.; Chen, W.-F.; Wang, D.-W. Prenatal organophosphates exposure alternates the cleavage plane orientation of apical neural progenitor in developing neocortex. PLoS ONE 2014, 9, e95343. [Google Scholar] [CrossRef]
- Herzine, A.; Laugeray, A.; Feat, J.; Menuet, A.; Quesniaux, V.; Richard, O.; Pichon, J.; Montécot-Dubourg, C.; Perche, O.; Mortaud, S. Perinatal exposure to glufosinate ammonium herbicide impairs neurogenesis and neuroblast migration through cytoskeleton destabilization. Front. Cell. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yan, M.; Wang, X.; Xiong, G.; Wu, C.; Wang, Z.; Zhou, Z.; Chang, X. Modification of Wnt signaling pathway on paraquat-induced inhibition of neural progenitor cell proliferation. Food Chem. Toxicol. 2018, 121, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Seth, B.; Yadav, A.; Agarwal, S.; Tiwari, S.K.; Chaturvedi, R.K. Inhibition of the transforming growth factor-β/SMAD cascade mitigates the anti-neurogenic effects of the carbamate pesticide carbofuran. J. Biol. Chem. 2017, 292, 19423–19440. [Google Scholar] [CrossRef]
- Klempin, F.; Kronenberg, G.; Cheung, G.; Kettenmann, H.; Kempermann, G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS ONE 2011, 6, e25760. [Google Scholar] [CrossRef]
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Yin, J.; Duan, J.; Cui, Z.; Ren, W.; Li, T.; Yin, Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015, 5, 15479–15486. [Google Scholar] [CrossRef]
- Williams, J.M.; Duckworth, C.A.; Watson, A.J.; Frey, M.R.; Miguel, J.C.; Burkitt, M.D.; Sutton, R.; Hughes, K.R.; Hall, L.J.; Caamaño, J.H.; et al. A mouse model of pathological small intestinal epithelial cell apoptosis and shedding induced by systemic administration of lipopolysaccharide. Dis. Model. Mech. 2013, 6, 1388–1399. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Liu, B.; Wang, Y.; Shao, J.; Wang, J.; Xia, K.; Liang, C.; Fang, W.; Zhou, C.; et al. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017, 8, e3113. [Google Scholar] [CrossRef]
- Sayed, A.A.; El-Desouky, M.A.; Ibrahim, K.A. Garlic and allopurinol attenuate hepatic apoptosis induced by fipronil in male albino rats. Regul. Toxicol. Pharm. 2019, 107, 104400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; You, Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front. Cell. Neurosci. 2018, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Briones, B.A.; Gould, E. Chapter 7—Adult neurogenesis and stress. In Stress: Physiology, Biochemistry, and Pathology; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 79–92. [Google Scholar]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp. Neurol. 2012, 233, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, E.; Sachana, M.; Flaskos, J.; Harris, W.; Hargreaves, A.J.; Woldehiwet, Z. Fipronil interferes with the differentiation of mouse N2a neuroblastoma cells. Toxicol. Lett. 2011, 201, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, T.L.; MacKillop, E.A.; Ryde, I.T.; Seidler, F.J.; Slotkin, T.A. Is fipronil safer than chlorpyrifos? Comparative developmental neurotoxicity modeled in PC12 cells. Brain Res. Bull. 2009, 78, 313–322. [Google Scholar] [CrossRef]
- Yin, J. Study on the progress of neural mechanism of positive emotions. Transl. Neurosci. 2019, 10, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Meneses, A.; Liy-Salmeron, G. Serotonin and emotion, learning and memory. Rev. Neurosci. 2012, 23, 543–553. [Google Scholar] [CrossRef]
- Jones, D.C.; Miller, G.W. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem. Pharmacol. 2008, 76, 569–581. [Google Scholar] [CrossRef]
- Aldridge, J.E.; Meyer, A.; Seidler, F.J.; Slotkin, T.A. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health Perspect. 2005, 113, 1027–1031. [Google Scholar] [CrossRef]
- Bharatiya, R.; Bratzu, J.; Lobina, C.; Corda, G.; Cocco, C.; De Deurwaerdere, P.; Argiolas, A.; Melis, M.R.; Sanna, F. The pesticide fipronil injected into the substantia nigra of male rats decreases striatal dopamine content: A neurochemical, immunohistochemical and behavioral study. Behav. Brain Res. 2020, 384, 112562. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Pita, R.; García-Uzcátegui, Y.; Díaz, M.J.; Martínez-Larrañaga, M.R. Decrease of 5-HT levels after fipronil treatment. Toxicol. Sci. 2004, 78, 228. [Google Scholar]
- Gao, H.-M.; Zhou, H.; Hong, J.-S. Oxidative stress, neuroinflammation, and neurodegeneration. In Neuroinflammation and Neurodegeneration; Peterson, P.K., Toborek, M., Eds.; Springer: New York, NY, USA, 2014; pp. 81–104. [Google Scholar]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative stress and neuroinflammation potentiate each other to promote progression of dopamine neurodegeneration. Oxid. Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N.; Magdy, Y. Targeting oxidative stress, cytokines and serotonin interactions via indoleamine 2, 3 dioxygenase by coenzyme Q10: Role in suppressing depressive like behavior in rats. J. Neuroimmune Pharmacol. 2017, 12, 277–291. [Google Scholar] [CrossRef]
- Shajib, M.S.; Khan, W. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2014, 213, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.A.; Rhodes, J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013, 27, 22–32. [Google Scholar] [CrossRef]
- Strasser, A.; McCarron, R.M.; Ishii, H.; Stanimirovic, D.; Spatz, M. L-arginine induces dopamine release from the striatum in vivo. Neuroreport 1994, 5, 2298–2300. [Google Scholar] [CrossRef]
- Volz, T.J.; Schenk, J.O. L-arginine increases dopamine transporter activity in rat striatum via a nitric oxide synthase-dependent mechanism. Synapse 2004, 54, 173–182. [Google Scholar] [CrossRef]
- Lorrain, D.S.; Hull, E.M. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport 1993, 5, 87–89. [Google Scholar] [CrossRef]
- Lechin, F.; van der Dijs, B.; Baez, S.; Hernandez, G.; Orozco, B.; Rodriguez, S. The effects of oral arginine on neuroautonomic parameters in healthy subjects. J. Appl. Res. 2006, 6, 201. [Google Scholar]
- Juliet, P.A.; Balasubramaniam, D.; Balasubramaniam, N.; Panneerselvam, C. Carnitine: A neuromodulator in aged rats. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. L-carnitine acts as a neuroprotecor against aspartame injury in Wistar albino rat. J. Basic Appl. Zool. 2020, 81, 28. [Google Scholar] [CrossRef]
- El-Ansary, A.; Shaker, G.; Siddiqi, N.J.; Al-Ayadhi, L.Y. Possible ameliorative effects of antioxidants on propionic acid/clindamycin—induced neurotoxicity in Syrian hamsters. Gut Pathog. 2013, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T. Glutamate, at the interface between amino acid and carbohydrate metabolism. J. Nutr. 2000, 130, 988s–990s. [Google Scholar] [CrossRef]
- Manchia, M.; Comai, S.; Pinna, M.; Pinna, F.; Fanos, V.; Denovan-Wright, E.; Carpiniello, B. Chapter Four—Biomarkers in aggression. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 93, pp. 169–237. [Google Scholar]
- Monfort, P.; Muñoz, M.D.; Kosenko, E.; Llansola, M.; Sánchez-Pérez, A.; Cauli, O.; Felipo, V. Sequential activation of soluble guanylate cyclase, protein kinase G and cGMP-degrading phosphodiesterase is necessary for proper induction of long-term potentiation in CA1 of hippocampus. Alterations in hyperammonemia. Neurochem. Int. 2004, 45, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, N.; Dachtler, J.; Fox, K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front. Cell. Neurosci. 2013, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Head, E.; Kuratsune, H.; Cotman, C.W.; Ames, B.N. Comparison of the effects of L-carnitine and acetyl-L-carnitine on carnitine levels, ambulatory activity, and oxidative stress biomarkers in the brain of old rats. Ann. N. Y. Acad. Sci. 2004, 1033, 117–131. [Google Scholar] [CrossRef]
- Maldonado, C.; Vázquez, M.; Fagiolino, P. Potential therapeutic role of carnitine and acetylcarnitine in neurological disorders. Curr. Pharm. Des. 2020, 26, 1277–1285. [Google Scholar] [CrossRef]



| Control | LA | LC | FPN | FPN + LA | FPN + LC | p Value | |
|---|---|---|---|---|---|---|---|
| Initial body weight (g) | 184.00 a ± 0.85 | 179.20 a ± 2.21 | 179.30 a ± 1.87 | 182.70 a ± 2.48 | 179.30 a ± 1.87 | 182.30 a ± 2.9 | >0.05 |
| Final body weight (g) | 271.70 ab ± 11.74 | 305.30 a ± 11.73 | 290.00 a ± 13.04 | 243.50 b ± 13.04 | 288.00 a ± 13.04 | 294.70 a ± 13.04 | * |
| Brain weight (g) | 4.12 a ±0.51 | 4.17 a ±0.75 | 4.13 a ±0.44 | 4.01 a ±0.24 | 4.06 a ±0.15 | 4.04 a ±0.11 | >0.05 |
| Control | LA | LC | FPN | FPN + LA | FPN + LC | p Value | ||
|---|---|---|---|---|---|---|---|---|
| TAC (U/mg) | Cortical | 89.48 a ± 13.16 | 90.13 a ± 11.21 | 88.67 a ± 9.29 | 33.56 b ± 13.73 | 62.56 ab ± 23.39 | 63.30 ab ± 19.56 | Control × FPN ** LA × FPN *** LC × FPN ** |
| Hippocampal | 93.76 a ± 7.20 | 96.75 a ± 6.81 | 97.55 a ± 4.49 | 39.73 b ± 13.69 | 52.90 b ± 7.55 | 54.47 b ± 4.59 | *** | |
| MDA (U/mg) | Cortical | 1.17 c ± 0.18 | 1.07 c ± 0.11 | 1.01 c ± 0.03 | 1.80 a ± 0.08 | 1.38 b ± 0.17 | 1.38 b ± 0.10 | Control × FPN *** LA, LC × FPN *** LA × FPN + LA * LA × FPN + LC * LC × FPN + LA, FPN + LC ** FPN × FPN + LA, FPN + LC ** |
| Hippocampal | 0.97 b ± 0.09 | 1.02 b ± 0.06 | 1.03 b ± 0.06 | 1.81 a ± 0.09 | 1.40 c ± 0.12 | 1.38 c ± 0.04 | *** | |
| Control | LA | LC | FPN | FPN + LA | FPN + LC | p Value | |
|---|---|---|---|---|---|---|---|
| Corticosterone (pg/mL) | 98.83 a ± 2.51 | 96.52 a ± 1.98 | 101.78 a ± 2.39 | 141.31 b ± 4.41 | 116.12 c ± 6.25 | 112.65 ac ± 2.79 | FPN × control, LA, LC *** FPN × FPN + LA, FPN + LC * FPN + LA × control, LA * |
| Dopamine (ng/g tissue) | 29.39 a ± 0.87 | 31.76 a ± 0.75 | 32.02 a ± 0.89 | 13.54 b ± 2.12 | 20.73 c ± 2.03 | 21.00 c ± 1.83 | FPN × control, LA, LC *** LA, LC × FPN + LA, FPN + LC *** FPN × FPN + LA, FPN + LC * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, Y.K.; Ali, A.A.; Abdelrazek, H.M.A.; Aldayel, T.S.; Abdel-Daim, M.M.; El-Menyawy, M.A.I. Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine. Biology 2021, 10, 682. https://doi.org/10.3390/biology10070682
Mahmoud YK, Ali AA, Abdelrazek HMA, Aldayel TS, Abdel-Daim MM, El-Menyawy MAI. Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine. Biology. 2021; 10(7):682. https://doi.org/10.3390/biology10070682
Chicago/Turabian StyleMahmoud, Yasmina K., Ahmed A. Ali, Heba M. A. Abdelrazek, Tahany Saleh Aldayel, Mohamed M. Abdel-Daim, and Menna Allah I. El-Menyawy. 2021. "Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine" Biology 10, no. 7: 682. https://doi.org/10.3390/biology10070682
APA StyleMahmoud, Y. K., Ali, A. A., Abdelrazek, H. M. A., Aldayel, T. S., Abdel-Daim, M. M., & El-Menyawy, M. A. I. (2021). Neurotoxic Effect of Fipronil in Male Wistar Rats: Ameliorative Effect of L-Arginine and L-Carnitine. Biology, 10(7), 682. https://doi.org/10.3390/biology10070682