The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Sample Maintenance
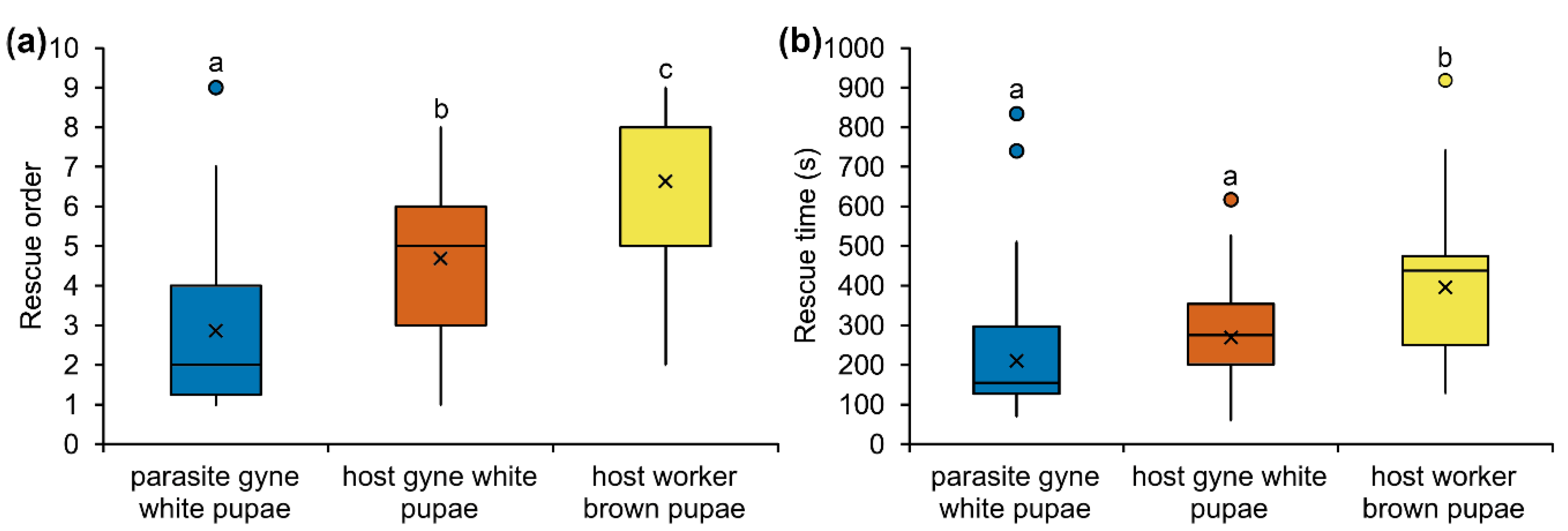
2.2. Brood Rescue Experiment
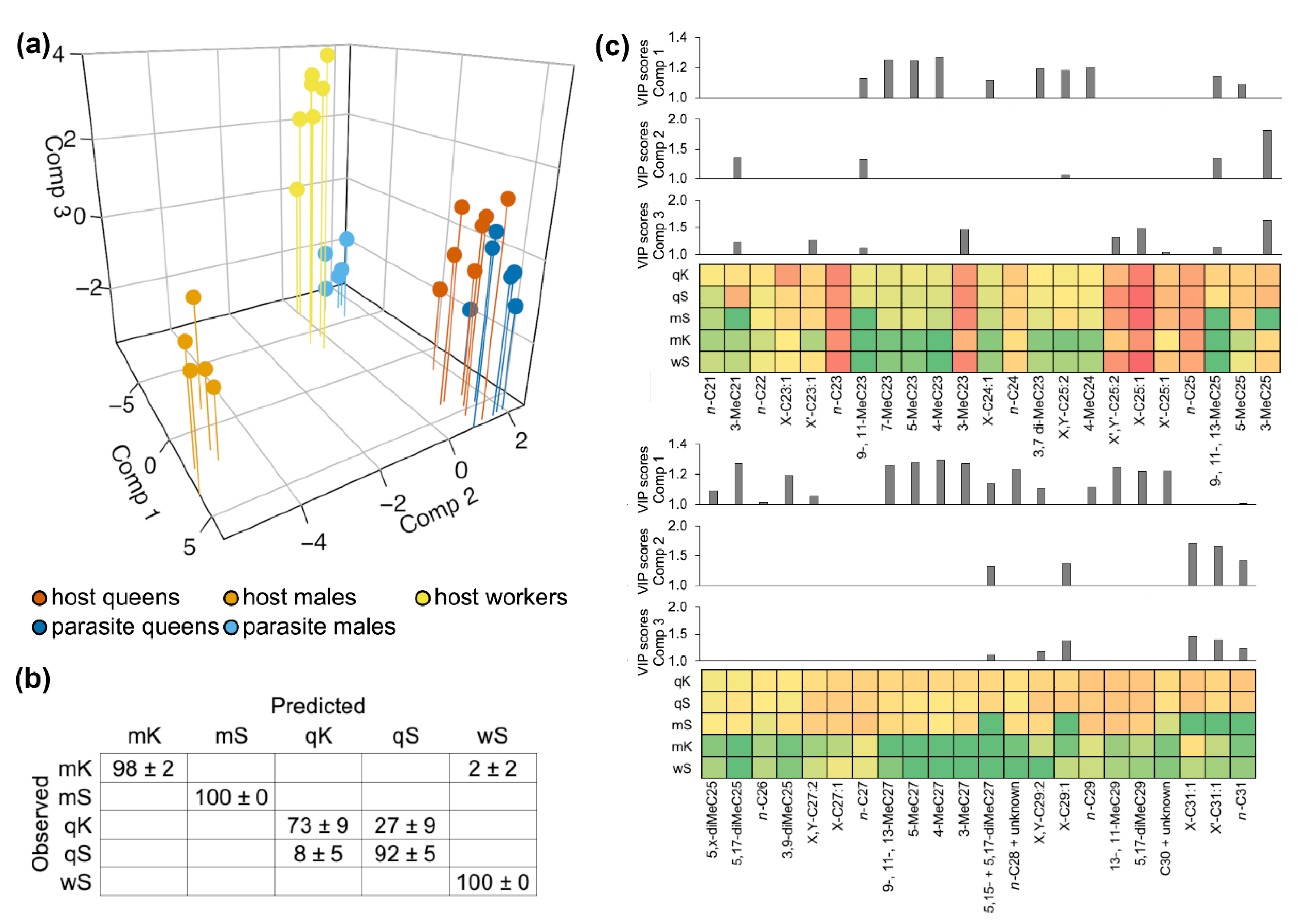
2.3. Chemical Analysis
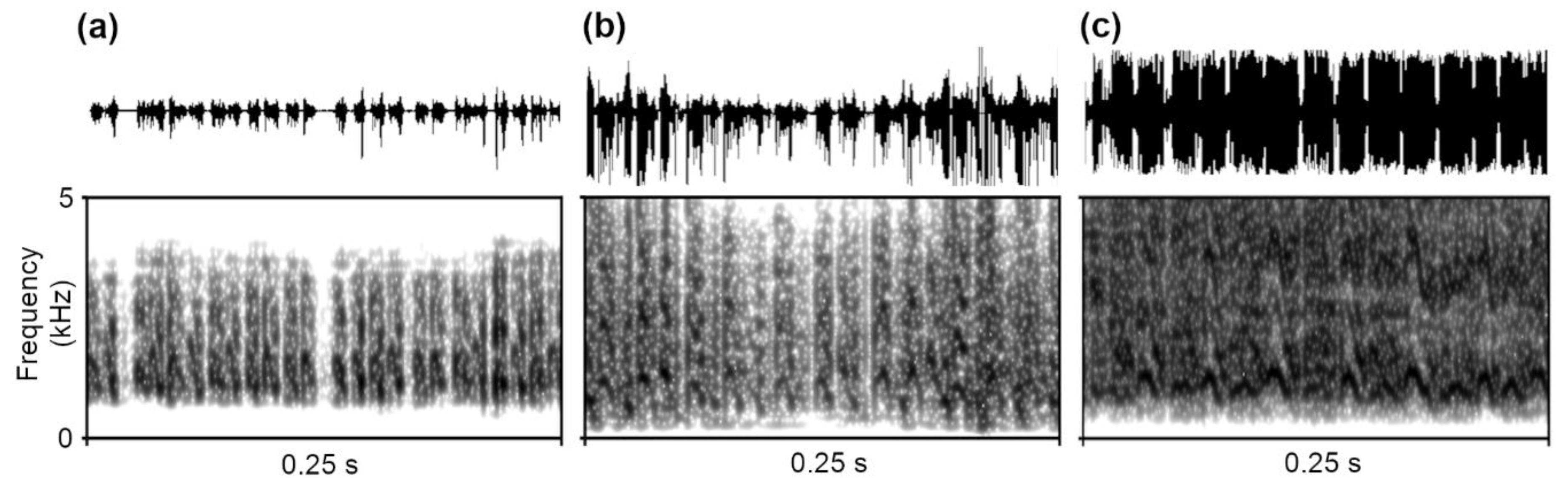
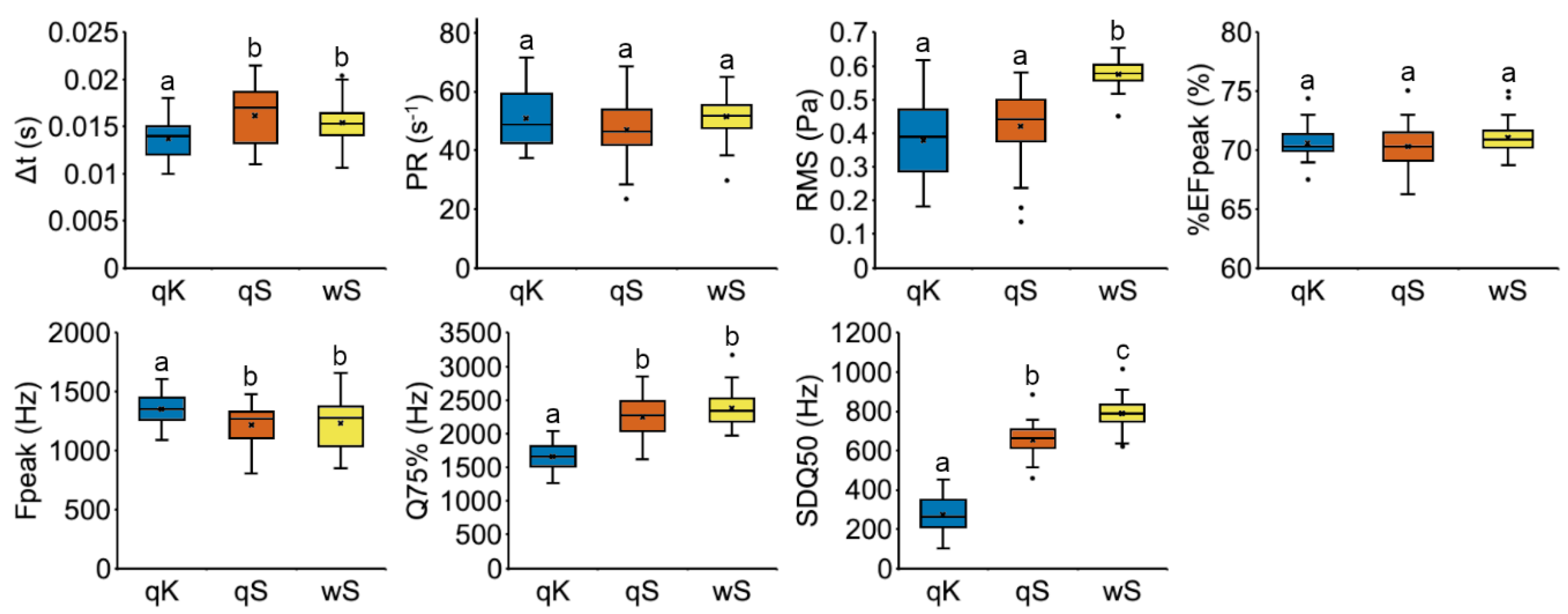
2.4. Recording of Vibroacoustic Signals
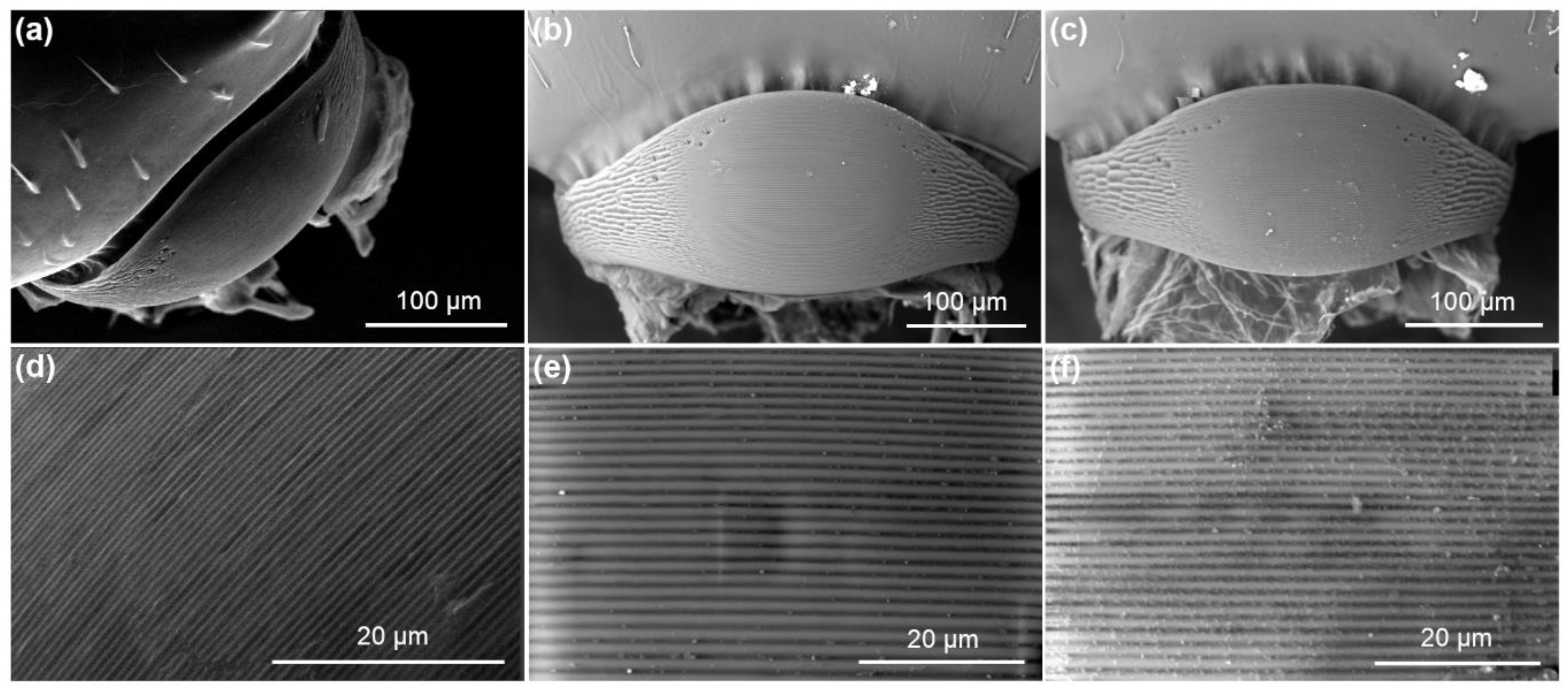
2.5. Scanning Electron Microscopy
2.6. Playback
2.7. Statistical Analysis
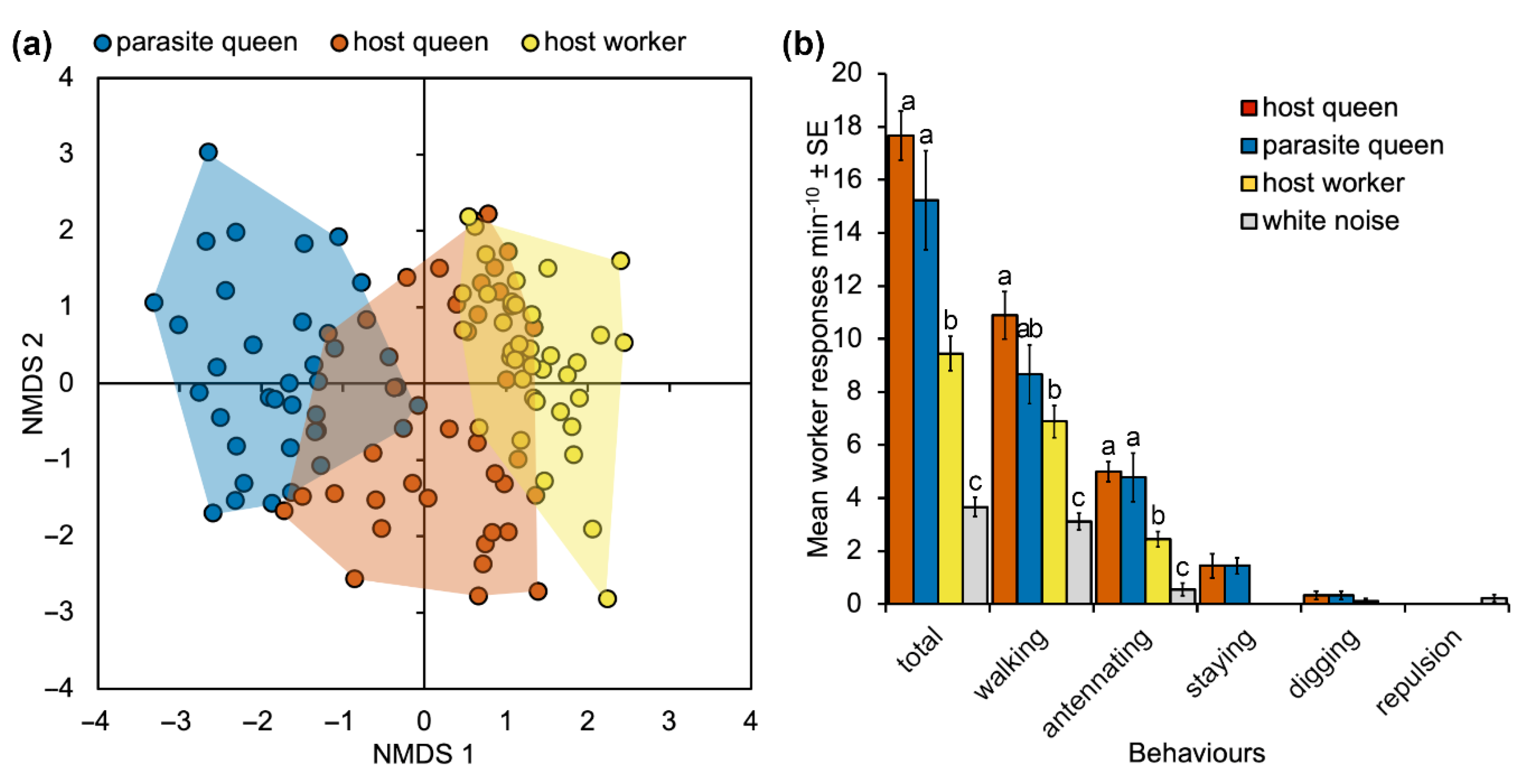
3. Results
3.1. Social Status of Parasite and Host Brood
3.2. Chemical Integration
3.3. Vibroacoustic Integration
3.3.1. Vibroacoustic Signals
3.3.2. Playback Assays
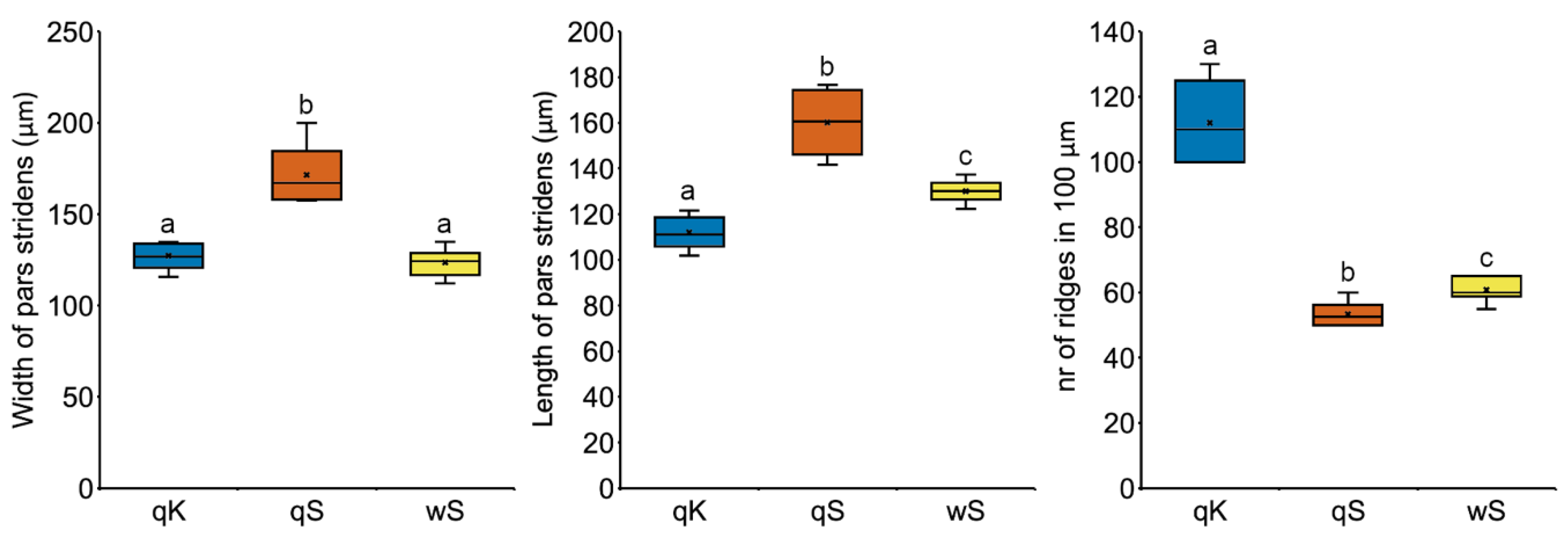
3.3.3. Stridulatory Organs (SO)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Myrmica scabrinodis | Myrmica karavajevi | |||||
|---|---|---|---|---|---|---|
| nr | CHC | Queens | Workers | Males | Queens | Males |
| 1 | n-C21 | 0.14 ± 0.11 | 0.12 ± 0.08 | 0.06 ± 0.01 | 0.42 ± 0.15 | 0.12 ± 0.04 |
| 2 | 3-MeC21 | 5.64 ± 5.22 | 0.11 ± 0.13 | --- | 0.71 ± 0.16 | 0.14 ± 0.09 |
| 3 | n-C22 | 0.28 ± 0.1 | 0.3 ± 0.08 | 0.35 ± 0.11 | 0.64 ± 0.18 | 0.32 ± 0.09 |
| 4 | X-C23:1 | 0.72 ± 0.19 | 0.55 ± 0.33 | 0.86 ± 0.3 | 7.28 ± 1.72 | 1.1 ± 0.15 |
| 5 | X’-C23:1 | 0.85 ± 0.26 | 0.67 ± 0.34 | 0.87 ± 0.14 | 1.02 ± 0.14 | 0.16 ± 0.1 |
| 6 | n-C23 | 8.4 ± 2.63 | 19.43 ± 7.17 | 11.76 ± 1.11 | 15.81 ± 5.31 | 30.93 ± 4.91 |
| 7 | 9-, 11-MeC23 | 0.2 ± 0.08 | --- | --- | 0.3 ± 0.06 | --- |
| 8 | 7-MeC23 | 0.19 ± 0.08 | 0.02 ± 0.01 | 0.22 ± 0.1 | 0.28 ± 0.07 | 0.02 ± 0.01 |
| 9 | 5-MeC23 | 0.17 ± 0.11 | 0.02 ± 0.01 | 0.25 ± 0.15 | 0.31 ± 0.06 | 0.02 ± 0.01 |
| 10 | 4-MeC23 | 0.17 ± 0.08 | --- | 0.16 ± 0.13 | 0.27 ± 0.05 | --- |
| 11 | 3-MeC23 | 9.93 ± 1.63 | 14.2 ± 6.14 | 12.78 ± 1.92 | 8.37 ± 1.98 | 8.06 ± 1.04 |
| 12 | X-C24:1 | 0.18 ± 0.09 | 0.03 ± 0.03 | 0.16 ± 0.12 | 0.27 ± 0.11 | 0.04 ± 0.02 |
| 13 | n-C24 | 0.83 ± 0.16 | 1.07 ± 0.53 | 1 ± 0.16 | 1.52 ± 0.41 | 1.83 ± 0.53 |
| 14 | 3,7 di-MeC23 | 0.29 ± 0.11 | 0.07 ± 0.04 | 0.23 ± 0.12 | 0.47 ± 0.09 | 0.04 ± 0.03 |
| 15 | X,Y-C25:2 | 0.41 ± 0.15 | 0.05 ± 0.02 | 0.66 ± 0.17 | 0.39 ± 0.08 | 0.02 ± 0.01 |
| 16 | 4-MeC24 | 0.3 ± 0.06 | 0.06 ± 0.04 | 0.31 ± 0.17 | 0.44 ± 0.08 | 0.04 ± 0.02 |
| 17 | X’,Y’-C25:2 | 11.66 ± 9.84 | 9.99 ± 4.83 | 6.13 ± 1.6 | 3.84 ± 3.05 | 4.09 ± 1.62 |
| 18 | X-C25:1 | 30.97 ± 8.84 | 42.77 ± 10.37 | 37.45 ± 5.28 | 21.66 ± 4.89 | 26.16 ± 3.7 |
| 19 | X’-C25:1 | 2.12 ± 0.54 | 2 ± 1.15 | 2.64 ± 0.62 | 1.82 ± 0.69 | 1.32 ± 0.4 |
| 20 | n-C25 | 4.8 ± 1.54 | 5.19 ± 3.99 | 7.18 ± 1.06 | 5.69 ± 1.06 | 17.59 ± 4.68 |
| 21 | 9-, 11-, 13-MeC25 | 0.56 ± 0.26 | --- | --- | 1.19 ± 0.53 | --- |
| 22 | 5-MeC25 | 1.17 ± 0.27 | 0.32 ± 0.19 | 1.34 ± 0.31 | 1.26 ± 0.41 | 0.25 ± 0.15 |
| 23 | 3-MeC25 | 2.1 ± 0.56 | 1.28 ± 0.75 | 1.9 ± 0.68 | 2.31 ± 0.67 | |
| 24 | 5,x-diMeC25 | 0.29 ± 0.1 | 0.07 ± 0.06 | 0.53 ± 0.36 | 0.36 ± 0.1 | 0.04 ± 0.02 |
| 25 | 5,17-diMeC25 | 0.56 ± 0.27 | --- | 0.52 ± 0.17 | 0.41 ± 0.16 | --- |
| 26 | n-C26 | 0.37 ± 0.13 | 0.1 ± 0.08 | 0.34 ± 0.23 | 0.63 ± 0.16 | 0.13 ± 0.09 |
| 27 | 3,9-diMeC25 | 0.32 ± 0.18 | 0.01 ± 0.01 | 0.86 ± 0.58 | 0.57 ± 0.22 | 0.02 ± 0.01 |
| 28 | X,Y-C27:2 | 1.13 ± 0.59 | 0.16 ± 0.1 | 1.16 ± 0.3 | 0.94 ± 0.62 | 0.15 ± 0.19 |
| 29 | X-C27:1 | 1.1 ± 0.39 | 0.54 ± 0.51 | 1.13 ± 0.16 | 1.18 ± 0.54 | 0.26 ± 0.23 |
| 30 | n-C27 | 0.87 ± 0.22 | 0.31 ± 0.25 | 1.54 ± 0.51 | 1.24 ± 0.46 | 0.8 ± 0.42 |
| 31 | 9-, 11-, 13-MeC27 | 0.57 ± 0.21 | 0.02 ± 0.01 | 0.83 ± 0.42 | 1.06 ± 0.56 | --- |
| 32 | 5-MeC27 | 0.43 ± 0.11 | --- | 0.78 ± 0.36 | 1.15 ± 0.57 | --- |
| 33 | 4-MeC27 | 0.42 ± 0.08 | --- | 0.53 ± 0.28 | 0.93 ± 0.26 | --- |
| 34 | 3-MeC27 | 0.5 ± 0.18 | --- | 0.96 ± 0.55 | 0.93 ± 0.31 | --- |
| 35 | 5,15- + 5,17-diMeC27 | 0.74 ± 0.22 | --- | 0.97 ± 0.24 | --- | |
| 36 | n-C28 + unknown | 0.32 ± 0.12 | --- | 0.77 ± 0.38 | 0.73 ± 0.22 | 0.02 ± 0.01 |
| 37 | X,Y-C29:2 | 1.27 ± 0.7 | --- | 0.99 ± 0.54 | 1.1 ± 0.42 | 0.31 ± 0.12 |
| 38 | X-C29:1 | 1.17 ± 0.36 | 0.12 ± 0.09 | --- | 1.01 ± 0.32 | 0.02 ± 0.01 |
| 39 | n-C29 | 1.09 ± 0.24 | 0.15 ± 0.11 | 1.59 ± 0.56 | 1.89 ± 0.73 | 0.53 ± 0.21 |
| 40 | 13-, 11-MeC29 | 1.35 ± 0.23 | 0.05 ± 0.04 | 1.57 ± 0.51 | 1.72 ± 0.61 | 0.08 ± 0.06 |
| 41 | 5,17-diMeC29 | 1.44 ± 0.34 | 0.04 ± 0.04 | 1.22 ± 0.36 | 1.77 ± 0.53 | 0.25 ± 0.19 |
| 42 | n-C30 + unknown | 0.54 ± 0.15 | 0.02 ± 0.02 | 0.27 ± 0.23 | 0.92 ± 0.34 | 0.03 ± 0.01 |
| 43 | X-C31:1 | 1.7 ± 1.15 | 0.11 ± 0.12 | --- | 1.76 ± 0.67 | 2.54 ± 1.89 |
| 44 | X’-C31:1 | 0.66 ± 0.15 | 0.04 ± 0.04 | --- | 1.1 ± 0.47 | 0.25 ± 0.08 |
| 45 | n-C31 | 1.08 ± 0.43 | 0.03 ± 0.04 | --- | 1.77 ± 0.51 | 0.02 ± 0.01 |
References
- Michalakis, Y. Parasitism and the evolution of life-history traits. In Ecology and Evolution of Parasitism; Thomas, F., Guégan, J.-F., Renaud, F., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 19–30. [Google Scholar]
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, Princeton, NJ, USA, 1998; p. 392. [Google Scholar]
- Buschinger, A. Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 219–235. [Google Scholar]
- Grüter, C.; Jongepier, E.; Foitzik, S. Insect societies fight back: The evolution of defensive traits against social parasites. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170200. [Google Scholar] [CrossRef]
- Forel, A. La parabiose chez les fourmis. Bull. Soc. Vaud. Sc. Nat. 1898, 34, 380–384. [Google Scholar]
- Emery, C. Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biol. Zentralbl. 1909, 29, 352–362. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Springer: Berlin/Heidelberg, Germany, 1990; p. 732. [Google Scholar]
- Lowe, R.M.; Ward, S.A.; Crozier, R.H. The evolution of parasites from their hosts: Intra–and interspecific parasitism and Emery’s rule. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1301–1305. [Google Scholar] [CrossRef]
- Brandt, M.; Foitzik, S.; Fischer-Blass, B.; Heinze, J. The coevolutionary dynamics of obligate ant social parasite systems—between prudence and antagonism. Biol. Rev. 2005, 80, 251–267. [Google Scholar] [CrossRef]
- Cini, A.; Sumner, S.; Cervo, R. Inquiline social parasites as tools to unlock the secrets of insect sociality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180193. [Google Scholar] [CrossRef]
- Lenoir, A.; d’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef]
- d’Ettorre, P.; Moore, A.J. Chemical communication and the coordination of social interactions in insects. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 81–96. [Google Scholar]
- Nash, D.R.; Boomsma, J.J. Communication between hosts and social parasites. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 55–79. [Google Scholar]
- van Zweden, J.; d’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 222–243. [Google Scholar]
- Barbero, F. Cuticular Lipids as a Cross-Talk among Ants, Plants and Butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Bonelli, S.; Thomas, J.A.; Balletto, E.; Schonrogge, K. Acoustical mimicry in a predatory social parasite of ants. J. Exp. Biol. 2009, 212, 4084–4090. [Google Scholar] [CrossRef]
- Barbero, F.; Thomas, J.A.; Bonelli, S.; Balletto, E.; Schonrogge, K. Queen Ants Make Distinctive Sounds That Are Mimicked by a Butterfly Social Parasite. Science 2009, 323, 782–785. [Google Scholar] [CrossRef]
- Thomas, J.A.; Schonrogge, K.; Bonelli, S.; Barbero, F.; Balletto, E. Corruption of ant acoustical signals by mimetic social parasites: Maculinea butterflies achieve elevated status in host societies by mimicking the acoustics of queen ants. Commun. Integr. Biol. 2010, 3, 169–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Settele, J.; Barbero, F.; Musche, M.; Thomas, J.A.; Schönrogge, K. Singing the blues: From experimental biology to conservation application. J. Exp. Biol. 2011, 214, 1407–1410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbero, F.; Patricelli, D.; Witek, M.; Balletto, E.; Casacci, L.P.; Sala, M.; Bonelli, S. Myrmica ants and their butterfly parasites with special focus on the acoustic communication. Psyche 2012, 2012, 725237. [Google Scholar] [CrossRef]
- Sala, M.; Casacci, L.P.; Balletto, E.; Bonelli, S.; Barbero, F. Variation in Butterfly Larval Acoustics as a Strategy to Infiltrate and Exploit Host Ant Colony Resources. PLoS ONE 2014, 9, e094341. [Google Scholar] [CrossRef]
- Barbero, F.; Casacci, L.P. Butterflies that trick ants with sound. Phys. Today 2015, 68, 64–65. [Google Scholar] [CrossRef]
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454. [Google Scholar] [CrossRef]
- Radchenko, A.; Elmes, G.W. A taxonomic revision of the socially parasitic Myrmica ants (Hymenoptera: Formicidae) of the Palaearctic region. Ann. Zool. 2003, 217–243. [Google Scholar]
- Jansen, G.; Savolainen, R.; Vepsäläinen, K. Phylogeny, divergence-time estimation, biogeography and social parasite–host relationships of the Holarctic ant genus Myrmica (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 2010, 56, 294–304. [Google Scholar] [CrossRef]
- Witek, M.; Barbero, F.; Marko, B. Myrmica ants host highly diverse parasitic communities: From social parasites to microbes. Insectes Soc. 2014, 61, 307–323. [Google Scholar] [CrossRef]
- Radchenko, A.; Elmes, G. Myrmica Ants (Hymenoptera: Formicidae) of the Old World; Natura Optima Dux Foundation: Warsaw, Poland, 2010; p. 789. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsalainen, K. Ants of Poland—With Reference to the Myrmecofauna of Europe; Natura Optima Dux Foundation: Warsaw, Poland, 2012; p. 496. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags - und Vertriebsgesellschaft: Tauer, Germany, 2018; p. 408. [Google Scholar]
- Witek, M.; Babik, H.; Czechowski, W.; Czechowska, W. Myrmica karavajevi (Arn.) (Hymenoptera: Formicidae) in Poland: A species not as rare as it is thought to be. Fragm. Faunist. 2013, 56, 17–24. [Google Scholar] [CrossRef][Green Version]
- Wardlaw, J.; Elmes, G.; Thomas, J. Techniques for studying Maculinea butterflies: I. Rearing Maculinea caterpillars with Myrmica ants in the laboratory. J. Insect Conserv. 1998, 2, 79–84. [Google Scholar] [CrossRef]
- Csata, E.; Timuş, N.; Witek, M.; Casacci, L.P.; Lucas, C.; Bagnères, A.-G.; Sztencel-Jabłonka, A.; Barbero, F.; Bonelli, S.; Rákosy, L.; et al. Lock-picks: Fungal infection facilitates the intrusion of strangers into ant colonies. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- von Beeren, C.; Schulz, S.; Hashim, R.; Witte, V. Acquisition of chemical recognition cues facilitates integration into ant societies. BMC Ecol. 2011, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Barbero, F.; Bonelli, S.; Balletto, E.; Casacci, L.P. The acoustic repertoire of lycaenid butterfly larvae. Bioacoustics 2017, 26, 77–90. [Google Scholar] [CrossRef]
- Audacity Team. Audacity®: Free Audio Editor and Recorder [Computer application]. 2021. Available online: https://www.audacityteam.org/ (accessed on 20 May 2018).
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer. Available online: https://www.fon.hum.uva.nl/praat/ (accessed on 1 April 2019).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 5 May 2021).
- González, I.; Lê Cao, K.; Déjean, S. MixOmics: Omics Data Integration Project. Available online: http://www.math.univ-toulouse.fr/~biostat/mixOmics (accessed on 4 May 2020).
- Doyle, N.; Swain, D.; Roberts, J.; Cozzolino, D. The use of qualitative analysis in food research and technology: Considerations and reflections from an applied point of view. Food Anal. Methods 2017, 10, 964–969. [Google Scholar] [CrossRef]
- Roberts, J.; Cozzolino, D. An overview on the application of chemometrics in food science and technology—An approach to quantitative data analysis. Food Anal. Methods 2016, 9, 3258–3267. [Google Scholar] [CrossRef]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Bookstein, F. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evol. Biol. 2011, 38, 100–114. [Google Scholar] [CrossRef]
- Indahl, U.G.; Liland, K.H.; Næs, T. Canonical partial least squares—A unified PLS approach to classification and regression problems. J. Chemom. 2009, 23, 495–504. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, M.; Wagner, H. Package ‘Vegan’. Community Ecology Package, Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 4 May 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.V. Least-squares means: The R Package lsmeans. J. Stat. Softw. 2016, 69. [Google Scholar] [CrossRef]
- Casacci, L.P.; Thomas, J.A.; Sala, M.; Treanor, D.; Bonelli, S.; Balletto, E.; Schönrogge, K. Ant pupae employ acoustics to communicate social status in their colony’s hierarchy. Curr. Biol. 2013, 23, 323–327. [Google Scholar] [CrossRef]
- Castro, S.; Àlvarez, M.; Munguira, M. Morphology of the stridulatory organs of Iberian myrmicine ants (Hymenoptera: Formicidae). Ital. J. Zool. (Modena) 2015, 82, 387–397. [Google Scholar] [CrossRef]
- Schultner, E.; Pulliainen, U. Brood recognition and discrimination in ants. Insectes Soc. 2020, 67, 11–34. [Google Scholar] [CrossRef]
- Thomas, J.A.; Elmes, G.W. Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol. Entomol. 1998, 23, 457–464. [Google Scholar] [CrossRef]
- Cervo, R.; Macinai, V.; Dechigi, F.; Turillazzi, S. Fast growth of immature brood in a social parasite wasp: A convergent evolution between avian and insect cuckoos. Am. Nat. 2004, 164, 814–820. [Google Scholar] [CrossRef]
- Thomas, J.; Wardlaw, J. The capacity of a Myrmica ant nest to support a predacious species of Maculinea butterfly. Oecologia 1992, 91, 101–109. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: New York, NY, USA, 2010; p. 492. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Akino, T.; Knapp, J.J.; Thomas, J.A.; Elmes, G.W. Chemical mimicry and host specificity in the butterfly Maculinea rebeli, a social parasite of Myrmica ant colonies. Proc. R. Soc. Lond. B 1999, 266, 1419–1426. [Google Scholar] [CrossRef]
- Akino, T. Chemical strategies to deal with ants: A review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News. 2008, 11, 173–181. [Google Scholar]
- Johnson, C.A. Slave-maker ant competition for a shared host and the effect on coevolutionary dynamics. Ecol. Monogr. 2008, 78, 445–460. [Google Scholar] [CrossRef]
- Tsuneoka, Y.; Akino, T. Chemical camouflage of the slave-making ant Polyergus samurai queen in the process of the host colony usurpation (Hymenoptera: Formicidae). Chemoecology 2012, 22, 89–99. [Google Scholar] [CrossRef]
- Hojo, M.K.; Wada-Katsumata, A.; Akino, T.; Yamaguchi, S.; Ozaki, M.; Yamaoka, R. Chemical disguise asparticular caste of host ants in the ant inquiline parasite Niphanda fusca (Lepidoptera: Lycaenidae). Proc. R. Soc. B Biol. Sci. 2009, 276, 551–558. [Google Scholar] [CrossRef]
- Franks, N.; Blum, M.; Smith, R.K.; Allies, A.B. Behavior and chemical disguise of cuckoo ant Leptothorax kutteri in relation to its host Leptothorax acervorum. J. Chem. Ecol. 1990, 16, 1431–1444. [Google Scholar] [CrossRef]
- Lambardi, D.; Dani, F.R.; Turillazzi, S.; Boomsma, J.J. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 2007, 61, 843–851. [Google Scholar] [CrossRef]
- Nehring, V.; Evison, S.E.F.; Santorelli, L.A.; D’Ettorre, P.; Hughes, W.O.H. 2011: Kin-informative recognition cues in ants. Proc. R. Soc. B. 2011, 278, 1942–1948. [Google Scholar] [CrossRef]
- Thomas, J.A.; Elmes, G.W.; Sielezniew, M.; Stankiewicz-Fiedurek, A.; Simcox, D.J.; Settele, J.; Schonrogge, K. Mimetic host shifts in an endangered social parasite of ants. Proc. R. Soc. Lond. B 2013, 280. [Google Scholar] [CrossRef]
- Scarparo, G.; d’Ettorre, P.; Di Giulio, A. Chemical deception and structural adaptation in Microdon (Diptera, Syrphidae, Microdontinae), a genus of hoverflies parasitic on social insects. J. Chem. Ecol. 2019, 45, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Schönrogge, K.; Wardlaw, J.C.; Peters, A.J.; Everett, S.; Thomas, J.A.; Elmes, G.W. Changes in chemical signature and host specificity from larval retrieval to full social integration in the myrmecophilous butterfly Maculinea rebeli. J. Chem. Ecol. 2004, 30, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Bagnères, A.-G.; Lorenzi, M.C. Chemical deception/mimicry using cuticular hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology, Blomquist, G.J., Bagneres, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 282–323. [Google Scholar]
- Uboni, A.; Bagnères, A.-G.; Christidès, J.-P.; Lorenzi, M.C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef]
- Bonavita-Cougourdan, A.; Theraulaz, G.; Bagnères, A.-G.; Roux, M.; Pratte, M.; Provost, E.; Clément, J.-L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. 1991, 100B, 667–680. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef]
- Cini, A.; Gioli, L.; Cervo, R. A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 2009, 5, 459–461. [Google Scholar] [CrossRef]
- Masoni, A.; Frizzi, F.; Nieri, R.; Casacci, L.; Mazzoni, V.; Turillazzi, S.; Santini, G. Ants modulate stridulatory signals depending on the behavioural context. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schonrogge, K.; Barbero, F.; Casacci, L.P.; Settele, J.; Thomas, J.A. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 2017, 134, 249–256. [Google Scholar] [CrossRef]
- Di Giulio, A.; Maurizi, E.; Barbero, F.; Sala, M.; Fattorini, S.; Balletto, E.; Bonelli, S. The Pied Piper: A Parasitic Beetle’s Melodies Modulate Ant Behaviours. PLoS ONE 2015, 10, e0130541. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, R.; Vepsäläinen, K. Sympatric speciation through intraspecific social parasitism. Proc. Natl. Acad. Sci. USA 2003, 100, 7169–7174. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, K.; Ebsen, J.; Savolainen, R.; Boomsma, J. Genetic differentiation between the ant Myrmica rubra and its microgynous social parasite. Insectes Soc. 2009, 56, 425–437. [Google Scholar] [CrossRef]
- Huang, M.H.; Dornhaus, A. A meta-analysis of ant social parasitism: Host characteristics of different parasitism types and a test of Emery’s rule. Ecol. Entomol. 2008, 33, 589–596. [Google Scholar] [CrossRef]
- Guillem, R.M.; Drijfhout, F.P.; Martin, S.J. Using chemo-taxonomy of host ants to help conserve the large blue butterfly. Biol. Conserv. 2012, 148, 39–43. [Google Scholar] [CrossRef]
- Tinbergen, N. The Study of Instinct; Pygmalion Press, an imprint of Plunkett Lake Press: Lexington, KY, USA, 2020; p. 19441. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casacci, L.P.; Barbero, F.; Ślipiński, P.; Witek, M. The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies. Biology 2021, 10, 654. https://doi.org/10.3390/biology10070654
Casacci LP, Barbero F, Ślipiński P, Witek M. The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies. Biology. 2021; 10(7):654. https://doi.org/10.3390/biology10070654
Chicago/Turabian StyleCasacci, Luca Pietro, Francesca Barbero, Piotr Ślipiński, and Magdalena Witek. 2021. "The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies" Biology 10, no. 7: 654. https://doi.org/10.3390/biology10070654
APA StyleCasacci, L. P., Barbero, F., Ślipiński, P., & Witek, M. (2021). The Inquiline Ant Myrmica karavajevi Uses Both Chemical and Vibroacoustic Deception Mechanisms to Integrate into Its Host Colonies. Biology, 10(7), 654. https://doi.org/10.3390/biology10070654







