Third Molar Agenesis Is Associated with Facial Size
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample
- -
- Age between 9 years and 50 years at the time of pre-treatment records. For patients younger than 12.5 years old, panoramic radiographs at older ages, higher than 12.5 years old, were checked to avoid misdiagnosis of late forming third molars
- -
- Agenesis of one or more third molars
- -
- European (White) ancestry
- -
- Lateral cephalometric radiographs in maximal intercuspation, of diagnostic quality, with a reference ruler at the mid-sagittal plane for magnification adjustment
- -
- Panoramic radiographs of diagnostic quality for the identification of the structures of interest
- -
- Presence of systemic diseases, syndromes, or any other conditions that affect craniofacial development, as reported in the subjects’ medical record
- -
- Agenesis of additional teeth, other than third molars
- -
- Presence of severe dental anomaly affecting tooth number, size, or form in any tooth except for third molars
- -
- History of previous intervention known to influence craniofacial morphology, such as orthodontic treatment, prior to cephalometric image acquisition
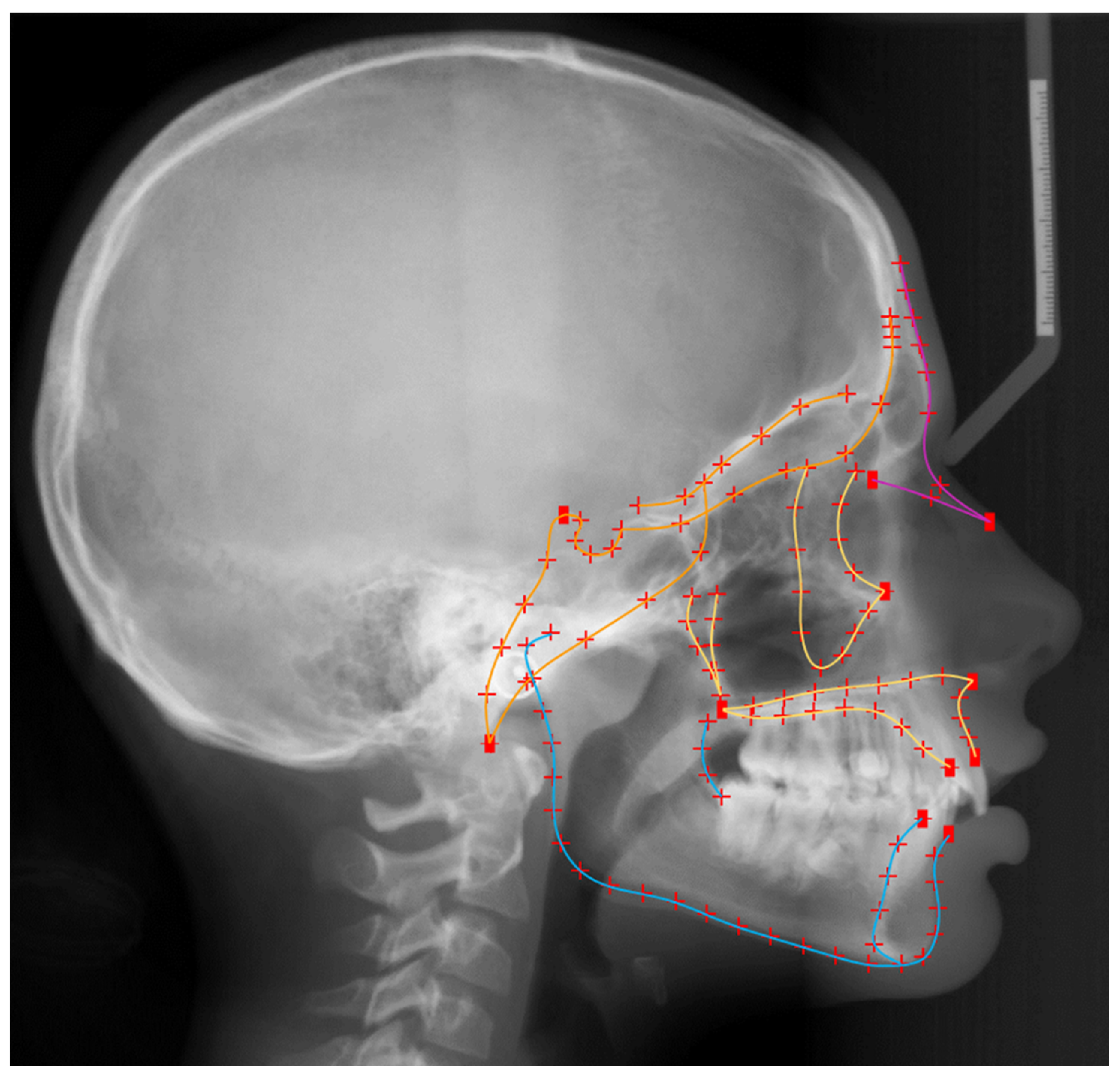
2.3. Data Collection
2.4. Size Assessment
2.5. Statistical Analyses
2.5.1. Method Error
2.5.2. Main Hypotheses Testing
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polder, B.J.; Hof, M.A.V.; der Linden, F.P.G.M.V.; Kuijpers-Jagtman, A.M. A Meta-Analysis of the Prevalence of Dental Agenesis of Permanent Teeth. Community Dent. Oral Epidemiol. 2004, 32, 217–226. [Google Scholar] [CrossRef]
- Khalaf, K.; Miskelly, J.; Voge, E.; Macfarlane, T.V. Prevalence of Hypodontia and Associated Factors: A Systematic Review and Meta-Analysis. J. Orthod. 2014, 41, 299–316. [Google Scholar] [CrossRef]
- Carter, K.; Worthington, S. Morphologic and Demographic Predictors of Third Molar Agenesis. J. Dent. Res. 2015, 94, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Scheiwiller, M.; Oeschger, E.S.; Gkantidis, N. Third Molar Agenesis in Modern Humans with and without Agenesis of Other Teeth. PeerJ 2020, 8, e10367. [Google Scholar] [CrossRef] [PubMed]
- Garn, S.M.; Lewis, A.B.; Vicinus, J.H. Third Molar Polymorphism and Its Significance to Dental Genetics. J. Dent. Res. 1963, 42, 1344–1363. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Sanpei, S.; Ishida, R.; Sanpei, S.; Abe, R.; Endo, T. Association between Third Molar Agenesis Patterns and Agenesis of Other Teeth in a Japanese Orthodontic Population. Odontology 2015, 103, 89–96. [Google Scholar] [CrossRef]
- Nassif, A.; Senussi, I.; Meary, F.; Loiodice, S.; Hotton, D.; Robert, B.; Bensidhoum, M.; Berdal, A.; Babajko, S. Msx1 Role in Craniofacial Bone Morphogenesis. Bone 2014, 66, 96–104. [Google Scholar] [CrossRef]
- Williams, M.A.; Letra, A. The Changing Landscape in the Genetic Etiology of Human Tooth Agenesis. Genes 2018, 9, 255. [Google Scholar] [CrossRef]
- González-Forero, M.; Gardner, A. Inference of Ecological and Social Drivers of Human Brain-Size Evolution. Nature 2018, 557, 554–557. [Google Scholar] [CrossRef]
- DeCasien, A.R.; Williams, S.A.; Higham, J.P. Primate Brain Size Is Predicted by Diet but Not Sociality. Nat. Ecol. Evol. 2017, 1, 0112. [Google Scholar] [CrossRef]
- Evans, A.R.; Daly, E.S.; Catlett, K.K.; Paul, K.S.; King, S.J.; Skinner, M.M.; Nesse, H.P.; Hublin, J.-J.; Townsend, G.C.; Schwartz, G.T.; et al. A Simple Rule Governs the Evolution and Development of Hominin Tooth Size. Nature 2016, 530, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Bastir, M.; Rosas, A.; Stringer, C.; Cuétara, J.M.; Kruszynski, R.; Weber, G.W.; Ross, C.F.; Ravosa, M.J. Effects of Brain and Facial Size on Basicranial Form in Human and Primate Evolution. J. Hum. Evol. 2010, 58, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.D.; Evans, A.R.; Jernvall, J. Predicting Evolutionary Patterns of Mammalian Teeth from Development. Nature 2007, 449, 427–432. [Google Scholar] [CrossRef]
- Lavelle, C.L.B.; Moore, W.J. The Incidence of Agenesis and Polygenesis in the Primate Dentition. Am. J. Phys. Anthropol. 1973, 38, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lacy, S.A. Evidence of Dental Agenesis in Late Pleistocene Homo. Int. J. Paleopathol. 2021, 32, 103–110. [Google Scholar] [CrossRef]
- Oeschger, E.S.; Kanavakis, G.; Halazonetis, D.J.; Gkantidis, N. Number of Teeth Is Associated with Facial Size in Humans. Sci. Rep. UK 2020, 10, 1820. [Google Scholar] [CrossRef]
- Trinkaus, E. Neandertal Faces Were Not Long; Modern Human Faces Are Short. Proc. Natl. Acad. Sci. USA 2003, 100, 8142–8145. [Google Scholar] [CrossRef]
- Castro, J.M.B.D. Third Molar Agenesis in Human Prehistoric Populations of the Canary Islands. Am. J. Phys. Anthropol. 1989, 79, 207–215. [Google Scholar] [CrossRef]
- Brook, A.H. A Unifying Aetiological Explanation for Anomalies of Human Tooth Number and Size. Arch. Oral Biol. 1984, 29, 373–378. [Google Scholar] [CrossRef]
- Carter, K.E.; Worthington, S. The Evolution of Anthropoid Molar Proportions. BMC Evol. Biol. 2016, 16, 110. [Google Scholar] [CrossRef]
- Katz, D.C.; Grote, M.N.; Weaver, T.D. Changes in Human Skull Morphology across the Agricultural Transition Are Consistent with Softer Diets in Preindustrial Farming Groups. Proc. Natl. Acad. Sci. USA 2017, 114, 9050–9055. [Google Scholar] [CrossRef]
- Cocos, A.; Halazonetis, D.J. Craniofacial Shape Differs in Patients with Tooth Agenesis: Geometric Morphometric Analysis. Eur. J. Orthod. 2016, 39, 345–351. [Google Scholar] [CrossRef][Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Wijk, A.J.V.; Tan, S.P.K. A Numeric Code for Identifying Patterns of Human Tooth Agenesis: A New Approach. Eur. J. Oral Sci. 2006, 114, 97–101. [Google Scholar] [CrossRef]
- Gkantidis, N.; Katib, H.; Oeschger, E.; Karamolegkou, M.; Topouzelis, N.; Kanavakis, G. Patterns of Non-Syndromic Permanent Tooth Agenesis in a Large Orthodontic Population. Arch. Oral Biol. 2017, 79, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mitteroecker, P.; Gunz, P. Advances in Geometric Morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Gunz, P.; Mitteroecker, P. Semilandmarks: A Method for Quantifying Curves and Surfaces. Hystrix 2013, 24, 103–109. [Google Scholar] [CrossRef]
- Bookstein, F.L. Landmark Methods for Forms without Landmarks: Morphometrics of Group Differences in Outline Shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes Method for the Optimal Superimposition of Landmarks. Syst. Zool. 1990, 39, 40. [Google Scholar] [CrossRef]
- Slice, D.E. Geometric Morphometrics. Annu. Rev. Anthropol. 2007, 36, 261–281. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar]
- Kruger, E.; Thomson, W.M.; Konthasinghe, P. Third Molar Outcomes from Age 18 to 26: Findings from a Population-Based New Zealand Longitudinal Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 150–155. [Google Scholar] [CrossRef]
- Ricketts, R.M. A Principle of Arcial Growth of the Mandible. Angle Orthod. 1972, 42, 368–386. [Google Scholar]
- Turner, C.G. Late Pleistocene and Holocene Population History of East Asia Based on Dental Variation. Am. J. Phys. Anthropol. 1987, 73, 305–321. [Google Scholar] [CrossRef]
- Kajii, T.S.; Sato, Y.; Kajii, S.; Sugawara, Y.; Iida, J. Agenesis of Third Molar Germs Depends on Sagittal Maxillary Jaw Dimensions in Orthodontic Patients in Japan. Angle Orthod. 2004, 74, 337–342. [Google Scholar]
- Swee, J.; Silvestri, A.R.; Finkelman, M.D.; Rich, A.P.; Alexander, S.A.; Loo, C.Y. Inferior Alveolar Nerve Block and Third-Molar Agenesis A Retrospective Clinical Study. J. Am. Dent. Assoc. 2013, 144, 389–395. [Google Scholar] [CrossRef]
- Shimizu, T.; Morita, W.; Maeda, T. Genetic Mapping of Agenesis of the Third Molars in Mice. Biochem. Genet. 2013, 51, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Vastardis, H. The Genetics of Human Tooth Agenesis: New Discoveries for Understanding Dental Anomalies. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 0650–0656. [Google Scholar] [CrossRef]
- Roseman, C.C. Detecting Interregionally Diversifying Natural Selection on Modern Human Cranial Form by Using Matched Molecular and Morphometric Data. Proc. Natl. Acad. Sci. USA 2004, 101, 12824–12829. [Google Scholar] [CrossRef]
- Gkantidis, N.; Halazonetis, D.J. Morphological Integration between the Cranial Base and the Face in Children and Adults. J. Anat. 2011, 218, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.B.R.B.; Figueiroa, J.N.; Dantas, R.M.X.; Kurita, L.M.; dos Anjos Pontual, M.L.; de Moraes Ramos-Perez, F.M.; da Cruz Perez, D.E.; dos Anjos Pontual, M.L. Evaluation of Third Molar Development in the Estimation of Chronological Age. Forensic Sci. Int. 2015, 254, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Atchley, W.R.; Plummer, A.A.; Riska, B. Genetics of mandible form in the mouse. Genetics 1985, 111, 555–577. [Google Scholar] [CrossRef]
- Cheverud, J.; Hartman, S.; Richtsmeier, J.; Atchley, W. A Quantitative Genetic Analysis of Localized Morphology in Mandibles of Inbred Mice Using Finite Element Scaling Analysis. J. Craniofacial Genet. Dev. Biol. 1991, 11, 122–137. [Google Scholar]
- Zelditch, M.L.; Wood, A.R.; Bonett, R.M.; Swiderski, D.L. Modularity of the Rodent Mandible: Integrating Bones, Muscles, and Teeth. Evol. Dev. 2008, 10, 756–768. [Google Scholar] [CrossRef]
- Noback, M.L.; Harvati, K. The Contribution of Subsistence to Global Human Cranial Variation. J. Hum. Evol. 2015, 80, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Organ, C.; Nunn, C.L.; Machanda, Z.; Wrangham, R.W. Phylogenetic Rate Shifts in Feeding Time during the Evolution of Homo. Proc. Natl. Acad. Sci. USA 2011, 108, 14555–14559. [Google Scholar] [CrossRef]
- Beecher, R.M.; Corruccini, R.S. Effects of Dietary Consistency on Maxillary Arch Breadth in Macaques. J. Dent. Res. 1981, 60, 68. [Google Scholar] [CrossRef] [PubMed]
| Number of Missing Third Molars | Frequency | ||
|---|---|---|---|
| Females | Males | Total | |
| 0 | 186 | 124 | 310 |
| 1 | 23 | 20 | 43 |
| 2 | 27 | 23 | 50 |
| 3 | 9 | 8 | 17 |
| 4 | 31 | 19 | 50 |
| Total | 276 | 194 | 470 |
| Size Configurations * | Parameter | β-Coefficient | 95% CI | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | p-Value | |||
| Cranial Base | Intercept | 4.955 | 4.943 | 4.966 | <0.001 |
| Age | 0.001 | 0.001 | 0.002 | <0.001 | |
| Number of missing third molars | −0.002 | −0.004 | 0.001 | 0.276 | |
| Female (male: reference) | −0.026 | −0.033 | −0.018 | <0.001 | |
| Maxilla | Intercept | 4.984 | 4.969 | 4.999 | <0.001 |
| Age | 0.004 | 0.004 | 0.005 | <0.001 | |
| Number of missing third molars | −0.013 | −0.016 | −0.009 | <0.001 | |
| Female (male: reference) | −0.025 | −0.034 | −0.015 | <0.001 | |
| Mandible | Intercept | 5.276 | 5.259 | 5.293 | <0.001 |
| Age | 0.006 | 0.005 | 0.007 | <0.001 | |
| Number of missing third molars | −0.012 | −0.016 | −0.008 | <0.001 | |
| Female (male: reference) | −0.037 | −0.048 | −0.026 | <0.001 | |
| Entire facial configuration | Intercept | 6.196 | 6.184 | 6.208 | <0.001 |
| Age | 0.004 | 0.004 | 0.005 | <0.001 | |
| Number of missing third molars | −0.007 | −0.010 | −0.004 | <0.001 | |
| Female (male: reference) | −0.033 | −0.041 | −0.025 | <0.001 | |
| Size Configurations * | Control | Missing Third Molars | Mean Difference | |||
|---|---|---|---|---|---|---|
| ln (Cs) | mm | ln (Cs) | mm | |||
| Cranial base | Females | 4.95 | 140.76 | 4.95 | 141.35 | NS |
| Males | 4.97 | 144.76 | 4.98 | 145.13 | NS | |
| Maxilla | Females | 5.02 | 151.88 | 4.99 | 147.45 | −3.00% |
| Males | 5.05 | 156.41 | 5.02 | 151.36 | −3.34% | |
| Mandible | Females | 5.32 | 204.77 | 5.29 | 198.20 | −3.31% |
| Males | 5.36 | 213.59 | 5.32 | 206.15 | −3.60% | |
| Entire facial configuration | Females | 6.22 | 504.96 | 6.21 | 497.14 | −1.57% |
| Males | 6.26 | 524.20 | 6.24 | 513.99 | −1.99% | |
| Size Configurations * | Parameter | β-Coefficient | 95% CI | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | p-Value | |||
| Individuals with third molar agenesis only in the maxilla (N = 37) and no agenesis (N = 310) | |||||
| Maxilla | Intercept | 4.957 | 4.940 | 4.973 | <0.001 |
| Age | 0.005 | 0.004 | 0.006 | <0.001 | |
| Sex | 0.026 | 0.015 | 0.036 | <0.001 | |
| Number of missing third molars | −0.010 | −0.020 | 0 | 0.048 | |
| Mandible | Intercept | 5.231 | 5.213 | 5.249 | <0.001 |
| Age | 0.006 | 0.005 | 0.007 | <0.001 | |
| Sex | 0.039 | 0.027 | 0.051 | <0.001 | |
| Number of missing third molars | −0.007 | −0.018 | 0.004 | 0.219 | |
| Individuals with third molar agenesis only in the mandible (N = 49) and no agenesis (N = 310) | |||||
| Maxilla | Intercept | 4.959 | 4.943 | 4.974 | <0.001 |
| Age | 0.004 | 0.003 | 0.005 | <0.001 | |
| Sex | 0.026 | 0.015 | 0.036 | <0.001 | |
| Number of missing third molars | −0.018 | −0.026 | −0.011 | <0.001 | |
| Mandible | Intercept | 5.236 | 5.219 | 5.253 | <0.001 |
| Age | 0.006 | 0.005 | 0.007 | <0.001 | |
| Sex | 0.038 | 0.026 | 0.050 | <0.001 | |
| Number of missing third molars | −0.021 | −0.030 | −0.013 | <0.001 | |
| Size Configurations | No Third Molar Agenesis (N = 310) | Missing Third Molars Only in the Maxilla (N = 37) | Missing Third Molars Only in the Mandible (N = 49) | |||||
|---|---|---|---|---|---|---|---|---|
| ln (Cs) | mm | ln (Cs) | mm | Size Difference to Controls | ln (Cs) | mm | Size Difference to Controls | |
| Maxillary size | 5.03 | 152.93 | 5.01 | 149.90 | −2% | 4.99 | 146.94 | −4% * |
| Mandibular size | 5.34 | 208.51 | 5.31 | 202.35 | −3% | 5.29 | 198.34 | −5% * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkantidis, N.; Tacchi, M.; Oeschger, E.S.; Halazonetis, D.; Kanavakis, G. Third Molar Agenesis Is Associated with Facial Size. Biology 2021, 10, 650. https://doi.org/10.3390/biology10070650
Gkantidis N, Tacchi M, Oeschger ES, Halazonetis D, Kanavakis G. Third Molar Agenesis Is Associated with Facial Size. Biology. 2021; 10(7):650. https://doi.org/10.3390/biology10070650
Chicago/Turabian StyleGkantidis, Nikolaos, Manuel Tacchi, Elias S. Oeschger, Demetrios Halazonetis, and Georgios Kanavakis. 2021. "Third Molar Agenesis Is Associated with Facial Size" Biology 10, no. 7: 650. https://doi.org/10.3390/biology10070650
APA StyleGkantidis, N., Tacchi, M., Oeschger, E. S., Halazonetis, D., & Kanavakis, G. (2021). Third Molar Agenesis Is Associated with Facial Size. Biology, 10(7), 650. https://doi.org/10.3390/biology10070650









