Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethnobotanical Uses and Chemical Composition of Verbascum Used in Spanish Folk Medicine
2.2. In Silico Modelling of Verbascum spp. Chemical Constituents’ Affinities by Human Molecular Targets
2.3. Comparative Review of Ethnobotanical Uses and Physiopatological Molecular Targets
3. Results
3.1. Ethnobotanical Uses and Chemical Composition
3.1.1. Circulatory System Diseases
3.1.2. Digestive Apparatus
3.1.3. Respiratory Diseases
3.1.4. Musculature and Skeleton
3.1.5. Skin and Sense Organs
3.1.6. Other Uses
3.1.7. Chemical Composition
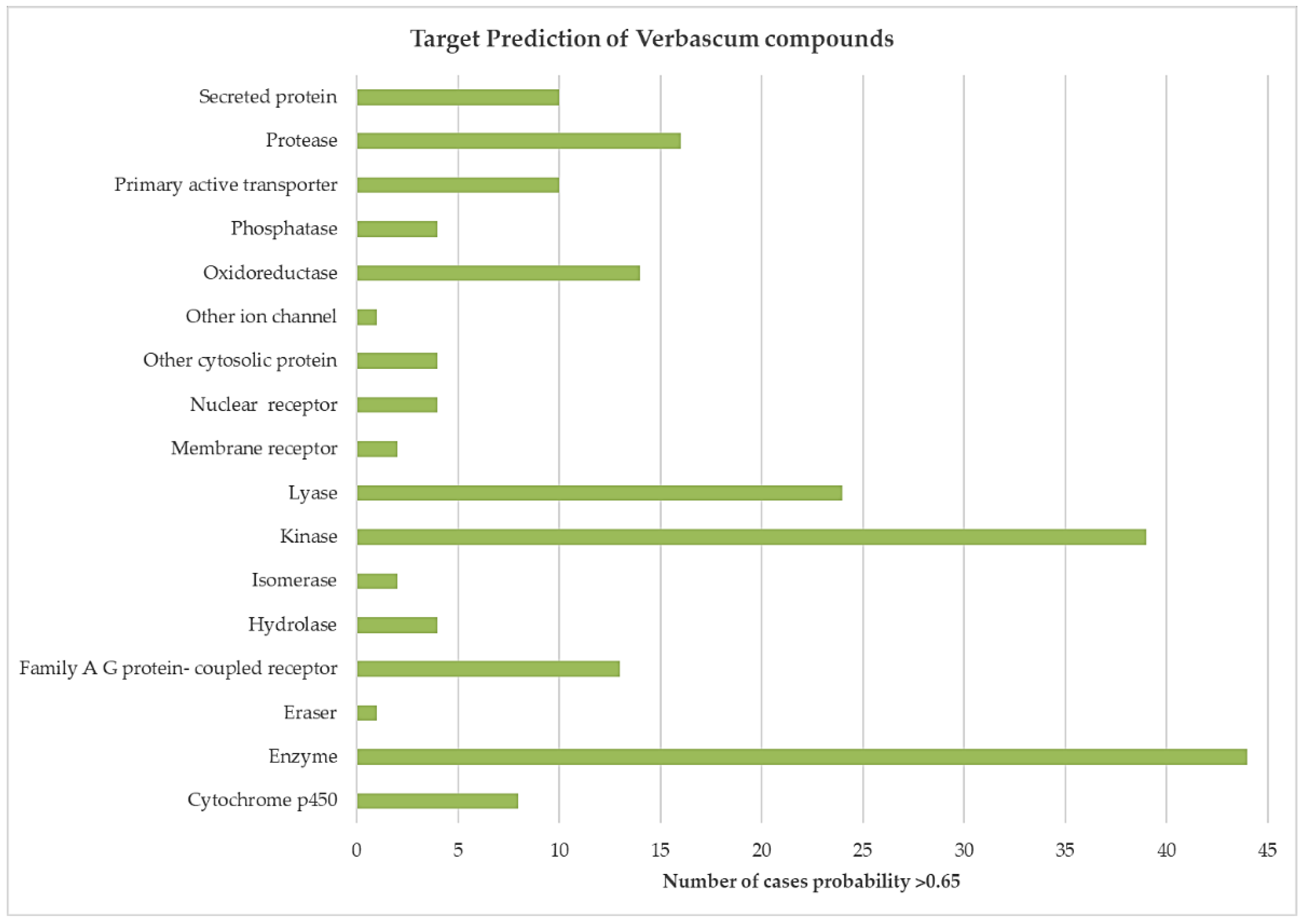
3.2. In Silico Modelling of Verbascum spp. Chemical Constituents’ Affinities by Human Molecular Targets
4. Discussion
4.1. Anti-Inflammatory Action of Verbascum
4.2. Circulatory System Diseases
4.3. Digestive Apparatus
4.4. Respiratory Diseases
4.5. Musculature and Skeleton
4.6. Skin and Sense Organs
4.7. Other Uses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Vp | Verbascum pulverulentum |
| Vs | V. sinuatum |
| Vt | V. tapsus |
| Vb | V. boerhavii |
| Vc | V. creticum |
| Vd | V. dentifolium |
| Vg | V. giganteum |
| Vl | V. lychnitis |
| Vr | V. rotundifolium |
| Vv | V. virgatum |
| SMILES | Simplified Molecular Input Line Entry Specification |
| IASP | International Association for the Study of Pain |
Appendix A
| Metabolite | Species | Reference | SMILES Code | |
|---|---|---|---|---|
| Monoterpene Iridioids | ||||
| 1 | Aucubin | Vs, Vt, Vl, Vv | [6,9,17] | C1=COC(C2C1C(C=C2CO)O)OC3C(C(C(C(O3)CO)O)O)O |
| 2 | 6-O-β-d-glucopyranosyl aucubin | Vs | [9] | [H][C@@]23C=CO[C@@H](OC1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O)[C@]2([H])C(CO)=C[C@H]3O[C@H]4[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]4O |
| 3 | Sinuatol | Vs | [9,11] | [H][C@@]34C=COC(O[C@H]2O[C@](CO)(O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)[C@@H](O)[C@H](O)[C@H]2O)[C@]3([H])C(CO)=CC4 |
| 4 | 6-O-β-d-xylopyranosyl aucubin | Vs, Vt | [6,9,10,17] | [H][C@@]23C=CO[C@@H](O[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O)[C@]2([H])C(CO)=C[C@H]3O[C@H]4[C@@H](O)O[C@H](CO)[C@@H]4O |
| 5 | 6-O-α-l-sinuatosyl aucubin | Vs | [9,12] | [H][C@@]23C=CO[C@@H](O[C@H]1O[C@@H](CO)[C@H](O)[C@@H](O)[C@@H]1O)[C@]2([H])C(CO)=C[C@H]3OC5O[C@@H](CO)[C@H](O)[C@@H](OC4OCC(O)C(O)C4O)[C@@H]5O |
| 6 | Sinuatoside | Vs | [9,13] | OCC4=C[C@@H](OC2OC(CO)C(O)C(OC1OCC(O)C(O)C1O)C2O)C5C=CO[C@@H](OC3OC(CO)C(O)C(O)C3O)C45 |
| 7 | Aucuboside | Vs, Vl | [9,13] | C1=COC(C2C1C(C=C2CO)O)OC3C(C(C(C(O3)CO)O)O)O |
| 8 | Catalpol | Vs, Vt, Vl | [6,9,17] | C1=COC(C2C1C(C3C2(O3)CO)O)OC4C(C(C(C(O4)CO)O)O)O |
| 9 | Isocatalpol | Vt, Vl | [6,9,17] | [H][C@@]24C=CO[C@@H](OC1OC(CO)C(O)[C@H](O)C1O)[C@]2([H])[C@@]3(CO)O[C@H]3[C@H]4O |
| 10 | Methylcatalpol | Vt, Vl | [6,9,17] | CO[C@@H]1[C@@H]2O[C@]2(CO)[C@H]2[C@H](O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)OC=C[C@@H]12 |
| 11 | 6-O-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | CC6O[C@H](O[C@H]3C2C=CO[C@@H](OC1OC(CO)C(O)[C@@H](O)[C@H]1O)C2[C@]4(O)O[C@H]34)C(COC(=O)C=Cc5ccccc5)C(O)[C@@H]6O |
| 12 | Saccatoside | Vt, Vv | [6,9,17] | C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@H]2[C@@H]3C=CO[C@H]([C@@H]3[C@@]4([C@H]2O4)CO)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O)OC(=O)/C=C/C6=CC=C(C=C6)O)O)O |
| 13 | 6-O-(3″-O-p-coumaroyl)-α-Lrhamnopyranosylcatalpol | Vs, Vt, Vv | [6,9,17] | C[C@@H]6O[C@@H](O[C@@H]3C2C=CO[C@H](O[C@@H]1OC(CO)[C@H](O)C(O)[C@@H]1O)C2[C@@]4(CO)O[C@@H]34)C(O)C(OC(=O)C=Cc5ccc(O)cc5)[C@@H]6O |
| 14 | 6-O-(4″-O-p-coumaroyl)-α-Lrhamnopyranosylcatalpol | Vt | [6,9,17] | [H][C@@]24C=CO[C@@H](O[C@@H]1OC(CO)[C@H](O)[C@H](O)C1O)[C@]2([H])[C@@]3(CO)O[C@H]3[C@H]4O[C@H]5O[C@@H](C)C(O)C(O)C5OC(=O)C=Cc6ccc(O)cc6 |
| 15 | 6-O-(2″-O-(p-methoxy-trans-cinnamoyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)OC1C(O)[C@H](O)[C@H](C)O[C@@H]1O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)cc6 |
| 16 | 6-O-(3″-O-(p-methoxy-trans-cinnamoyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)OC5[C@H](O)[C@H](C)O[C@H](O[C@H]3C2C=CO[C@@H](O[C@H]1OC(CO)[C@@H](O)C(O)[C@H]1O)C2[C@]4(CO)O[C@H]34)C5O)cc6 |
| 17 | Verbascoside A | Vt | [6,9,17] | C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1)O[C@H]2[C@@H]3C=CO[C@H]([C@@H]3[C@@]4([C@H]2O4)CO)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O)O)O)OC(=O)/C=C/C6=CC=C(C=C6)OC |
| 18 | 6-O-[2″-O-(3,4-dihydroxy-trans-cinnamoyl)]-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | C=C(C=Cc1ccc(O)c(O)c1)OC2C(O)[C@H](O)[C@H](C)O[C@@H]2O[C@H]5C4C=CO[C@@H](O[C@H]3OC(CO)[C@@H](O)C(O)[C@H]3O)C4[C@]6(CO)O[C@H]56 |
| 19 | 6-O-[4″-O-(3,4-dihydroxy-trans-cinnamoyl)]-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | C=C(C=Cc1ccc(O)c(O)c1)O[C@@H]6[C@H](C)O[C@H](O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)C(O)C6O |
| 20 | 6-O-[3″-O-(3,4-dimethoxy-trans-cinnamoyl)]-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | C=C(C=Cc1ccc(OC)c(OC)c1)OC6[C@H](O)[C@H](C)O[C@H](O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)C6O |
| 21 | 6-O-(2″-O-feruloyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6cc(C=CC(=O)OC1C(O)[C@H](O)[C@H](C)O[C@@H]1O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)ccc6O |
| 22 | 6-O-(4″-O-feruloyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6cc(C=CC(=O)O[C@@H]5[C@H](C)O[C@H](O[C@H]3C2C=CO[C@@H](O[C@H]1OC(CO)[C@@H](O)C(O)[C@H]1O)C2[C@]4(CO)O[C@H]34)C(O)C5O)ccc6O |
| 23 | 6-O-(2″-O-isoferuloyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)OC1C(O)[C@H](O)[C@H](C)O[C@@H]1O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)cc6O |
| 24 | 6-O-(3″-O-isoferuloyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)OC5[C@H](O)[C@H](C)O[C@H](O[C@H]3C2C=CO[C@@H](O[C@H]1OC(CO)[C@@H](O)C(O)[C@H]1O)C2[C@]4(CO)O[C@H]34)C5O)cc6O |
| 25 | 6-O-(4″-O-isoferuloyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)O[C@@H]5[C@H](C)O[C@H](O[C@H]3C2C=CO[C@@H](O[C@H]1OC(CO)[C@@H](O)C(O)[C@H]1O)C2[C@]4(CO)O[C@H]34)C(O)C5O)cc6O |
| 26 | Pulverulentoside I | Vp, Vs, Vt | [6,9,17] | C=C(C=Cc1ccc(OC)cc1)OC6[C@@H](O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)O[C@@H](C)[C@@H](O)C6OOC(C)=O |
| 27 | 6-O-(2″-O-p-methoxy-trans-cinnamoyl-4″-O-asetyl)-α-l-rhamnopyranosylcatalpol | Vt | [6,9,17] | COc6ccc(C=CC(=O)OC1C(O)[C@H](OC(C)=O)[C@H](C)O[C@@H]1O[C@H]4C3C=CO[C@@H](O[C@H]2OC(CO)[C@@H](O)C(O)[C@H]2O)C3[C@]5(CO)O[C@H]45)cc6 |
| 28 | Pulverulentoside II | Vp | [9] | COc6ccc(C=CC(=O)OC5C(O)C(C)OC(OC3C2C=COC(OC1OC(CO)C(O)C(O)C1O)C2C4(CO)OC34)C5OC(C)=O)cc6O |
| 29 | Catalposide | Vl | [5,9] | C1=COC(C2C1C(C3C2(O3)CO)OC(=O)C4=CC=C(C=C4)O)OC5C(C(C(C(O5)CO)O)O)O |
| 30 | Specioside | Vl | [4,83] | C1C(C(=CC(=O)OC2=C1C=CC(=C2)OC3C(C(C(C(O3)CO)O)O)O)C4=CC=C(C=C4)O)O.C1C(C(=CC(=O)OC2=C1C=CC(=C2)O)C3=CC=C(C=C3)O)OC4C(C(C(C(O4)CO)O)O)O |
| 31 | Ajugol | Vt, Vv | [6,9,17] | CC1(CC(C2C1C(OC=C2)OC3C(C(C(C(O3)CO)O)O)O)O)O |
| 32 | 6-O-benzoyl ajugol | Vt | [6,9,17] | [H][C@@]23C=CO[C@@H](O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)[C@]2([H])[C@@](C)(O)C[C@H]3OC(=O)c4ccccc4 |
| 33 | 6-O-syringoyl ajugol | Vt | [6,9,17] | CC1(CC(C2C1C(OC=C2)OC3C(C(C(C(O3)CO)O)O)O)OC(=O)C4=CC(=C(C(=C4)OC)O)OC)O |
| 34 | 6-O-vanilloyl ajugol | Vt | [6,9,17] | CC1(CC(C2C1C(OC=C2)OC3C(C(C(C(O3)CO)O)O)O)OC(=O)C4=CC(=C(C=C4)O)OC)O |
| 35 | Harpagide | Vs, Vt | [6,9,17] | CC1(CC(C2(C1C(OC=C2)OC3C(C(C(C(O3)CO)O)O)O)O)O)O |
| 36 | Harpagoside | Vp, Vs, Vt | [6,9,17] | CC1(CC(C2(C1C(OC=C2)OC3C(C(C(C(O3)CO)O)O)O)O)O)OC(=O)C=CC4=CC=CC=C4 |
| 37 | Lychnitoside | Vl | [9] | OCC2=CO[C@@H](O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C3C=CCC23 |
| 38 | Lateroside | Vt | [8] | [H][C@@]24C=CO[C@@H](O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)[C@]2([H])[C@@](C)(OC(=O)C=Cc3ccccc3)C[C@H]4O |
| 39 | 5-O-α-l-rhamnopyranosy (1α-3)-[α-d-glucuronopyranosyl (1α-6)]-α-d-glucopyranoside | Vt | [8] | CC8OC(OC1C(O)C(O)C(O)OC1OC2C(O)C(O)C(C(=O)O)OC2OC7CC[C@]6(C)C5CC=C4C3CC(C)(C)CC(O)[C@]3(C)CC[C@@]4(C)[C@]5(C)CCC6C7(C)CO)C(O)C(O)C8O |
| 40 | Ningpogenin | Vt | [8] | [H][C@]12C=C(CO)[C@@H](CO)[C@@]1([H])CC(=C)O2 |
| 41 | 10-deoxyeucommiol | Vt | [8] | CC1=C(CO)C(CCO)[C@@H](O)C1 |
| 42 | Jioglutolide | Vt | [8] | C[C@@]1(C[C@H]([C@H]2[C@@H]1COC(=O)C2)O)O |
| 43 | 6-β-hydroxy-2-oxabicyclo [4.3.0]Δ8-9-nonen-1-one | Vt | [8] | [H][C@@]12CCOC(=O)C1=C(C)C[C@H]2O |
| 44 | 8-cinnamoylmyoporoside | Vt | [8] | C[C@@]1(C[C@H](C2[C@@H]1[C@@H](OC=C2)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O)O)OC(=O)/C=C/C4=CC=CC=C4 |
| 45 | Verbthasin A | Vt | [8] | [H][C@@]12COC(=C)[C@]1([H])C[C@@H](O)C2=CCO |
| SESQUITERPENES | ||||
| 46 | Buddlindeterpene A | Vt | [8] | CC2=CCC[C@]1(C)O[C@@H]1CC(C)(C)C=CC2=O |
| 47 | Buddlindeterpene B | Vt | [8] | CC1=CCC(C)(C)C=CC(=O)C(C)=CCC1 |
| 48 | Buddlindeterpene C | Vt | [8] | C=C[C@@]1(C)CCC2C(O)(C1=O)[C@H]4CC3[C@@](C)(C)CCC[C@]23CO4 |
| Triterpene Saponines | ||||
| 49 | Thapsuine B | Vt, Vl | [3,4,9] | [H][C@@]49C=C[C@]23OC[C@@]1(CCC(C)(C)C[C@]12[H])CC[C@@]3(C)[C@]4(C)CC[C@]%10([H])[C@](C)(CO)C(O[C@H]8OC(COC6OC(C)C(OC5OC(CO)C(O)C(O)C5O)C(O)C6O)[C@@H](O)C(OC7CC(C)C(O)C(O)C7O)[C@H]8O)CC[C@]9%10C |
| 50 | Hydroxythapsuine B | Vt | [3,4,9] | [H][C@@]49C=C[C@]23OC[C@@]1(CCC(C)(O)C[C@]12[H])CC[C@@]3(C)[C@]4(C)CC[C@]%10([H])[C@](C)(CO)C(O[C@H]8OC(COC6OC(C)C(OC5OC(CO)C(O)C(O)C5O)C(O)C6O)[C@@H](O)C(OC7CC(C)C(O)C(O)C7O)[C@H]8O)CC[C@]9%10C |
| 51 | Saikogenin A | Vt | [3,4,9] | CC1(CCC2(C(CC3(C(=C2C1)C=CC4C3(CCC5C4(CCC(C5(C)CO)O)C)C)C)O)CO)C |
| 52 | Thapsuine A | Vt, Vl | [3,4,9] | CC1C(C(C(C(O1)OC2C(C(OC(C2O)OC3CCC4(C(C3(C)CO)CCC5(C4C=CC67C5(CCC8(C6CC(CC8)(C)C)CO7)C)C)C)COC9C(C(C(C(O9)C)OC1C(C(C(C(O1)CO)O)O)O)O)O)O)O)O)O |
| 53 | Hydroxythapsuine A | Vt | [3,4,9] | CC%10OC(OC9C(O)C(COC2OC(C)C(OC1OC(CO)C(O)C(O)C1O)C(O)C2O)OC(OC8CC[C@]7(C)[C@H]6C=C[C@]45OC[C@@]3(CCC(C)(O)C[C@H]34)CC[C@@]5(C)[C@]6(C)CC[C@H]7[C@]8(C)CO)C9O)C(O)C(O)C%10O |
| 54 | Ursolic acid | Vt, Vl | [8] | CC1CCC2(CCC3(C(=CCC4C3(CCC5C4(CCC(C5(C)C)O)C)C)C2C1C)C)C(=O)O |
| 55 | Veratric acid | Vt | [8] | COC1=C(C=C(C=C1)C(=O)O)OC |
| 56 | β-spinasterol | Vt | [8] | [H]C(=C([H])C(CC)C(C)C)[C@@H](C)[C@@]4([H])CC[C@@]3([H])C2=CC[C@@]1([H])C[C@@H](O)CC[C@]1(C)[C@@]2([H])CC[C@@]34C |
| 57 | Hydroxythapsuine | Vt | [9] | [H][C@@]49C=C[C@]23OC[C@@]1(CCC(C)(O)C[C@]12[H])CC[C@@]3(C)[C@]4(C)CC[C@]%10([H])[C@](C)(CO)C(O[C@H]8OC(COC6OC(C)C(OC5OC(CO)C(O)C(O)C5O)C(O)C6O)[C@@H](O)C(OC7CC(C)C(O)C(O)C7O)[C@H]8O)CC[C@]9%10C |
| 58 | 3-O-fucopyranosyl saikogenin F | Vt | [9] | C[C@@H]7O[C@H](OC6CC[C@]5(C)C4C=C[C@]23OC[C@@]1(CC[C@](C)(C)CC12)[C@@H](O)C[C@@]3(C)[C@]4(C)CCC5[C@]6(C)O)[C@@H](O)[C@H](O)[C@@H]7O |
| Phenilpropanoid Glycosides | ||||
| 59 | Verbascoside (=acetoside) | Vs, Vl | [9,16] | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)O)CO)OCCC4=CC(=C(C=C4)O)O)O)O)O)O |
| 60 | Poliumoside | Vs, Vt, Vb | [9] | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OCCC3=CC(=C(C=C3)O)O)O)OC4C(C(C(C(O4)C)O)O)O)OC(=O)C=CC5=CC(=C(C=C5)O)O)O)O)O |
| 61 | Forsythoside B | Vt, Vl | [9] | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)O)COC4C(C(CO4)(CO)O)O)OCCC5=CC(=C(C=C5)O)O)O)O)O)O |
| 62 | Arenarioside | Vt | [9] | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)O)COC4C(C(C(CO4)O)O)O)OCCC5=CC(=C(C=C5)O)O)O)O)O)O |
| 63 | Alyssonoside | Vt | [9] | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)/C=C/C3=CC(=C(C=C3)O)OC)COC4C(C(CO4)(CO)O)O)OCCC5=CC(=C(C=C5)O)O)O)O)O)O |
| 64 | Leucosceptoside B | Vt | [9] | [H][C@@]5(O[C@@H]3C[C@H](OCCc1ccc(OC)c(O)c1)O[C@H](CO[C@@H]2OC[C@](O)(CO)[C@H]2O)[C@H]3OC(=C)C=Cc4ccc(O)c(OC)c4)O[C@@H](C)[C@H](O)[C@@H](O)[C@H]5O |
| 65 | Cistanoside B | Vt | [9] | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)/C=C/C3=CC(=C(C=C3)O)OC)COC4C(C(C(C(O4)CO)O)O)O)OCCC5=CC(=C(C=C5)O)OC)O)O)O)O |
| Flavones | ||||
| 66 | Apigenin | Vl | [8] | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O |
| 67 | Apigenin-7-glucuronide | Vl | [8] | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O |
| 68 | Luteolin | Vt, Vl | [9,15] | C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O)O |
| 69 | Luteolin-5-glucoside | Vl | [8] | C1=CC(=C(C=C1C2=CC(=O)C3=C(O2)C=C(C=C3OC4C(C(C(C(O4)CO)O)O)O)O)O)O |
| 70 | Luteolin-7-glucuronide | Vl | [8] | C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O)O |
| 71 | 7-methoxy-luteolin | Vl | [8] | COc3cc(O)c2c(=O)cc(c1ccc(O)c(O)c1)oc2c3 |
| 72 | Cynaroside | Vt | [9] | C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)O)O)O |
| 73 | Apigetrin | Vt | [9] | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)O)O |
| 74 | 4′,7-dihydroxyflavone-4′-rhamnoside | Vt | [9] | C[C@H]4O[C@H](Oc3ccc(c2cc(=O)c1ccc(O)cc1o2)cc3)[C@@H](O)[C@@H](O)[C@@H]4O |
| 75 | 6-hydroxyluteolin-7-glucoside | Vt | [9] | C1=CC(=C(C=C1C2=CC(=O)C3=C(C(=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)O)O)O)O |
| Flavonols | ||||
| 76 | Quercetin | Vt, Vl | [9] | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O |
| 77 | Quercetin-7-glucuronide | Vl | [8] | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O)O)O |
| 78 | 3′-methylquercetin | Vt | [9] | COC1=C(C=CC(=C1)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O |
| 79 | Kaempferol | Vt | [9] | C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O |
| 80 | Rutin | Vt | [9] | CC1C(C(C(C(O1)OCC2C(C(C(C(O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC(=C(C=C5)O)O)O)O)O)O)O)O |
| O-Metilated Flavones | ||||
| 81 | Acacetin | Vl | [9] | COC1=CC=C(C=C1)C2=CC(=O)C3=C(C=C(C=C3O2)O)O |
| 82 | Acacetin-7-O-α-d-glucoside | Vt | [8] | O=c3cc(c1ccc(O)c(O)c1)oc4cc(OC2OC(CO)C(O)C(O)C2O)cc(O)c34 |
| 83 | Patuletin | Vl | [8,9] | COC1=C(C2=C(C=C1O)OC(=C(C2=O)O)C3=CC(=C(C=C3)O)O)O |
Appendix B
| Target Class | Target | Uniprot ID | Metabolite | Probability | Chemical Group |
|---|---|---|---|---|---|
| Cytochrome P450 | Cytochrome P450 19A1 | P11511 | Apigenin | 1.00 | Flavone |
| Quercetin | 1.00 | Flavonol | |||
| Cytochrome P450 1B1 | Q16678 | 3′-methylquercetin | 1.00 | Flavonol | |
| Kaempferol | 1.00 | ||||
| Quercetin | 1.00 | ||||
| Acacetin | 1.00 | O-metilated Flavone | |||
| Apigenin | 1.00 | Flavone | |||
| Luteolin | 1.00 | ||||
| Enzyme | 11-beta- hydroxysteroid dehydrogenase 1 | P28845 | Ursolic acid | 0.66 | Triterpene |
| Aldehyde reductase (by homology) | P14550 | Quercetin | 0.66 | Flavonol | |
| Aldo-keto reductase family 1 member B10 | O60218 | Apigenin | 0.68 | Flavone | |
| Luteolin | 0.68 | ||||
| Ursolic acid | 0.66 | Triterpene | |||
| Aldo-keto reductase family 1 member C1 (by homology) | Q04828 | Quercetin | 0.70 | Flavonol | |
| Aldo-keto reductase family 1 member C2 (by homology) | P52895 | Quercetin | 0.73 | Flavonol | |
| Aldo-keto reductase family 1 member C4 (by homology) | P17516 | Quercetin | 0.80 | Flavonol | |
| Aldo-keto-reductase family 1 member C3 (by homology) | P42330 | Quercetin | 0.80 | Flavonol | |
| Aldose reductase | P15121 | Luteolin | 1.00 | Flavone | |
| Apigenin | 1.00 | ||||
| Apigenin-7-glucuronide | 1.00 | ||||
| 7-methoxy-luteolin | 1.00 | ||||
| Luteolin-7-glucuronide | 1.00 | ||||
| Quercetin | 1.00 | Flavonol | |||
| Kaempferol | 1.00 | ||||
| Arachidonate 12-lipoxygenase | P18054 | Quercetin | 1.00 | Flavonol | |
| Arachidonate 15-lipoxygenase | P16050 | Quercetin | 1.00 | Flavonol | |
| Arginase-1 (by homology) | P05089 | Luteolin | 1.00 | Flavone | |
| DNA polymerase beta | P06746 | Ursolic acid | 1.00 | Triterpene | |
| DNA-(apurinic or apyrimidinic site) lyase | P27695 | Quercetin | 1.00 | Flavonol | |
| Estradiol 17-beta-dehydrogenase 1 | P14061 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Kaempferol | 1.00 | Flavonol | |||
| Estradiol 17-beta-dehydrogenase 2 | P37059 | Kaempferol | 1.00 | Flavonol | |
| P37059 | Quercetin | 1.00 | Flavonol | ||
| Glyoxalase I | Q04760 | Luteolin | 1.00 | Flavone | |
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Liver glycogen phosphorylase | P06737 | Quercetin | 1.00 | Flavonol | |
| Lymphocyte differentiation antigen CD38 | P28907 | Luteolin | 1.00 | Flavone | |
| Myeloperoxidase | P05164 | Quercetin | 1.00 | Flavonol | |
| NADPH oxidase 4 | Q9NPH5 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Phospholipase A2 group B | P04054 | Quercetin | 1.00 | Flavonol | |
| PI3-kinase p110-gamma subunit | P48736 | Quercetin | 1.00 | Flavonol | |
| PI3-kinase p85-alpha subunit | P27986 | Quercetin | 1.00 | Flavonol | |
| Poly [ADP-ribose] polymerase-1 | P09874 | Luteolin | 1.00 | Flavone | |
| Tankyrase-1 | O95271 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Tankyrase-2 | Q9H2K2 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Eraser | Lysine-specific demethylase 4D-like | B2RXH2 | Quercetin | 0.68 | Flavonol |
| Family A G protein- coupled receptor | Adrenergic receptor alpha-2 | P18825 | Rutin | 1.00 | Flavonol |
| Alpha-2a adrenergic receptor | P08913 | Rutin | 1.00 | Flavonol | |
| Neuromedin-U receptor 2 | Q9GZQ4 | Rutin | 1.00 | Flavonol | |
| Adenosine A1 receptor (by homology) | P30542 | Luteolin | 1.00 | Flavone | |
| Apigenin | 1.00 | ||||
| Kaempferol | 0.80 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Adenosine A2a receptor (by homology) | P29274 | Apigenin | 1.00 | Flavone | |
| Quercetin | 1.00 | Flavonol | |||
| Dopamine D4 receptor | P21917 | Quercetin | 1.00 | Flavonol | |
| G-protein coupled receptor 35 | Q9HC97 | Quercetin | 1.00 | Flavonol | |
| Interleukin-8 receptor A | P25024 | Quercetin | 1.00 | Flavonol | |
| Vasopressin V2 receptor | P30518 | Quercetin | 1.00 | Flavonol | |
| Hydrolase | Acetylcholinesterase | P22303 | Apigenin | 1.00 | Flavone |
| Kaempferol | 0.77 | Flavonol | |||
| Quercetin | 0.68 | ||||
| Rutin | 1.00 | ||||
| Isomerase | DNA topoisomerase I (by homology) | P11387 | Luteolin | 1.00 | Flavone |
| DNA topoisomerase II alpha | P11388 | Quercetin | 0.68 | Flavonol | |
| Kinase | ALK tyrosine kinase receptor | Q9UM73 | Quercetin | 1.00 | Flavonol |
| CaM kinase II beta | Q13554 | Quercetin | 1.00 | Flavonol | |
| Casein kinase II alpha | P68400 | Apigenin | 1.00 | Flavone | |
| Quercetin | 1.00 | Flavonol | |||
| Cyclin-dependent kinase 1 | P06493 | Quercetin | 1.00 | Flavonol | |
| Cyclin-dependent kinase 5/CDK5 activator 1 | Q15078 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | Flavone | |||
| Cyclin-dependent kinase 6 | Q00534 | Apigenin | 1.00 | Flavone | |
| Death-associated protein kinase 1 | P53355 | Quercetin | 1.00 | Flavonol | |
| Epidermal growth factor receptor erbB1 | P00533 | Quercetin | 1.00 | Flavonol | |
| Focal adhesion kinase 1 | Q05397 | Quercetin | 1.00 | Flavonol | |
| Glycogen synthase kinase-3 beta | P49841 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Kaempferol | 0.66 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Hepatocyte growth factor receptor | P08581 | Quercetin | 1.00 | Flavonol | |
| Insulin receptor | P06213 | Quercetin | 0.68 | Flavonol | |
| Insulin-like growth factor I receptor | P08069 | Quercetin | 1.00 | Flavonol | |
| Myosin light chain kinase, smooth muscle | Q15746 | Quercetin | 0.68 | Flavonol | |
| NUAK family SNF1-like kinase 1 | O60285 | Quercetin | 1.00 | Flavonol | |
| Protein kinase N1 | Q16512 | Quercetin | 1.00 | Flavonol | |
| Protein kinase C alpha | P17252 | Verbascoside | 0.78 | Phenilpropanoid | |
| Serine/threonine-protein kinase AKT | P31749 | Quercetin | 1.00 | Flavonol | |
| Serine/threonine-protein kinase Aurora-B | Q96GD4 | Quercetin | 1.00 | Flavonol | |
| Serine/threonine-protein kinase NEK2 | P51955 | Quercetin | 1.00 | Flavonol | |
| Serine/threonine-protein kinase NEK6 | Q9HC98 | Quercetin | 1.00 | Flavonol | |
| Serine/threonine-protein kinase PIM1 | P11309 | Quercetin | 1.00 | Flavonol | |
| Serine/threonine-protein kinase PLK1 | P53350 | Quercetin | 1.00 | Flavonol | |
| Tyrosine-protein kinase receptor FLT3 | P36888 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Tyrosine-protein kinase receptor UFO | P30530 | Quercetin | 1.00 | Flavonol | |
| Tyrosine-protein kinase SRC | P12931 | Quercetin | 1.00 | Flavonol | |
| Tyrosine-protein kinase SYK | P43405 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Kaempferol | 0.66 | Flavonol | |||
| Quercetin | 0.70 | ||||
| Vascular endothelial growth factor receptor2 | P35968 | Quercetin | 1.00 | Flavonol | |
| Lyase | Carbonic anhydrase I | P00915 | Quercetin | 1.00 | Flavonol |
| Carbonic anhydrase II | P00918 | Luteolin | 1.00 | Flavone | |
| 1.00 | |||||
| 3′-methylquercetin | 1.00 | Flavonol | |||
| Kaempferol | 1.00 | ||||
| Quercetin | 1.00 | ||||
| Carbonic anhydrase III | P07451 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydrase IV | P22748 | Luteolin | 1.00 | Flavone | |
| 3′-methylquercetin | 1.00 | Flavonol | |||
| Kaempferol | 1.00 | ||||
| Quercetin | 1.00 | ||||
| Carbonic anhydrase IX | Q16790 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydrase VA | P35218 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydrase VI | P23280 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydrase VII | P43166 | Luteolin | 1.00 | Flavone | |
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Carbonic anhydrase XII | O43570 | Luteolin | 1.00 | Flavone | |
| 3′-methylquercetin | 1.00 | Flavonol | |||
| Kaempferol | 1.00 | ||||
| Quercetin | 1.00 | ||||
| Carbonic anhydrase XIII (by homology) | Q8N1Q1 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydrase XIV | Q9ULX7 | Quercetin | 1.00 | Flavonol | |
| Carbonic anhydraseVII | P43166 | 3′-methylquercetin | 1.00 | Flavonol | |
| Membrane receptor | Beta amyloid A4 protein | P05067 | Luteolin | 1.00 | Flavone |
| Receptor-type tyrosine-protein phosphatase F (LAR) | P10586 | Ursolic acid | 0.70 | Triterpene | |
| Nuclear receptor | Estrogen receptor alpha | P03372 | Apigenin | 1.00 | Flavone |
| Estrogen receptor beta | Q92731 | Apigenin | 1.00 | Flavone | |
| Estrogen-related receptor alpha | P11474 | Kaempferol | 1.00 | Flavonol | |
| Nuclear receptor ROR-gamma | P51449 | Ursolic acid | 0.70 | Triterpene | |
| Other cytosolic protein | Cyclin-dependent kinase 1/cyclin B | Q8WWL | Apigenin | 1.00 | Flavone |
| Cyclin-dependent kinase 1/cyclin B | Q8WWL7 | Luteolin | 1.00 | Flavone | |
| Heat shock protein HSP 90-alpha | P07900 | Catalposide | 0.83 | Iridoid | |
| Specioside | 1.00 | ||||
| Other ion channel | Cystic fibrosis transmembrane conductance regulator | P13569 | Apigenin | 1.00 | Flavone |
| Oxidoreductase | Arachidonate 5-lipoxygenase | P21397 | Apigenin | 1.00 | Flavone |
| P35354 | Apigenin | 1.00 | |||
| P47989 | Apigenin | 1.00 | |||
| P09917 | Luteolin | 1.00 | |||
| P21397 | Luteolin | 1.00 | |||
| P47989 | Luteolin | 1.00 | |||
| P47989 | 3′-methylquercetin | 1.00 | Flavonol | ||
| P09917 | Kaempferol | 1.00 | |||
| P14679 | Kaempferol | 1.00 | |||
| P21397 | Kaempferol | 0.66 | |||
| P47989 | Kaempferol | 1.00 | |||
| P09917 | Quercetin | 1.00 | |||
| P21397 | Quercetin | 1.00 | |||
| P47989 | Quercetin | 1.00 | |||
| Phosphatase | Low molecular weight phosphotyrosine protein phosphatase | P24666 | Ursolic acid | 0.7 | Triterpene |
| Protein-tyrosine phosphatase 1B | P18031 | Ursolic acid | 0.95 | Triterpene | |
| Receptor-type tyrosine-protein phosphatase S | Q13332 | Luteolin | 0.90 | Flavone | |
| T-cell protein-tyrosine phosphatase | P17706 | Ursolic acid | 0.74 | Triterpene | |
| Primary active transporter | ATP-binding cassette sub-family G member 2 | Q9UNQ0 | Luteolin | 1.00 | Flavone |
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Apigenin | 1.00 | Flavone | |||
| Multidrug resistance-associated protein 1 | P33527 | Apigenin | 1.00 | Flavone | |
| Luteolin | 0.66 | ||||
| Kaempferol | 1.00 | Flavonol | |||
| Quercetin | 1.00 | ||||
| P-glycoprotein 1 | P08183 | Kaempferol | 1.00 | Flavonol | |
| Quercetin | 1.00 | ||||
| Protease | Beta-secretase 1 | P56817 | Quercetin | 1.00 | Flavonol |
| Matrix metalloproteinase 12 | P39900 | Luteolin | 1.00 | Flavone | |
| Poliumoside | 0.68 | Phenilpropanoid | |||
| Verbascoside | 0.74 | ||||
| Matrix metalloproteinase 13 | P45452 | Quercetin | 1.00 | Flavonol | |
| Matrix metalloproteinase 2 | P08253 | Luteolin | 1.00 | Flavone | |
| Kaempferol | 0.66 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Poliumoside | 0.68 | Phenilpropanoid | |||
| Verbascoside | 0.74 | ||||
| Matrix metalloproteinase 3 | P08254 | Quercetin | 1.00 | Flavonol | |
| Matrix metalloproteinase 9 | P14780 | Luteolin | 1.00 | Flavone | |
| Kaempferol | 0.66 | Flavonol | |||
| Quercetin | 1.00 | ||||
| Plasminogen | P00747 | 7-methoxy-luteolin | 1.00 | Flavone | |
| Thrombin | P00734 | Quercetin | 1.00 | Flavonol | |
| Secreted protein | Interleukin-2 | P60568 | 6-hydroxyluteolin-7-glucoside | 0.70 | Flavone |
| Apigetrin | 0.66 | ||||
| Cynaroside | 1.00 | ||||
| Acacetin-7-O-α-D-glucoside | 1.00 | O-metilated Flavone | |||
| TNF-alpha | P01375 | 6-hydroxyluteolin-7-glucoside | 0.70 | Flavone | |
| Apigetrin | 1.00 | ||||
| Cynaroside | 1.00 | ||||
| Acacetin-7-O-α-D-glucoside | 1.00 | O-metilated Flavone | |||
| Transthyretin | P02766 | Apigenin | 1.00 | Flavone | |
| Luteolin | 1.00 | ||||
| Transcription factor | Aryl hydrocarbon receptor | P35869 | Kaempferol | 1.00 | Flavonol |
| Zinc finger protein GLI1 | P08151 | Buddlindeterpene B | 0.74 | Sesquiterpene | |
| Zinc finger protein GLI2 | P10070 | Buddlindeterpene B | 0.74 | Sesquiterpene | |
| Unclassified protein | Microtubule-assicuated protein tau | P10636 | Quercetin | 0.68 | Flavonol |
References
- Benedí, C. Verbascum. Flora Iberica. In Flora Iberica; Benedí Gonzalez, C., Rico Hernández, E., Güemes Heras, J., Herrero Nieto, A., Eds.; CSIC: Madrid, Spain, 2009; Volume 13, pp. 49–97. [Google Scholar]
- Pardo de Santayana, M.; Morales, R.; Aceituno-Mata, L.; Molina, M. (Eds.) Inventario Español de Conocimientos Tradicionales Relativos a la Biodiversidad. Fase II (2); Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2018; ISBN 978-84-491-1472-4.
- De Pascual, T.J.; Diaz, F.; Grande, M. Components of Verbascum thapsus L. I. Triterpenes. An. Quim. 1978, 74, 311–314. [Google Scholar]
- De Pascual, T.J.; Diaz, F.; Grande, M. Components of Verbascum thapsus L. III. Contribution to the study of saponins. An. Quim. Ser. C 1980, 76, 107–110. [Google Scholar]
- Klimek, B. 6-O-p-coumaroylcatapol from Verbascum lychnitis. Planta Med. 1991, 57, 298. [Google Scholar] [CrossRef] [PubMed]
- Warashina, T.; Miyase, T.; Ueno, A. Phenylethanoid and lignan glycosides from Verbascum thapsus. Phytochemistry 1992, 31, 961–965. [Google Scholar] [CrossRef]
- Klimek, B. Flavonoid glucuronides from Verbascum lychnitis and V. nigrum. Acta Pol. Pharm. 1995, 52, 53–56. [Google Scholar]
- Tatli, I.I.; Akdemir, Z.Ş. Chemical constituents of Verbascum L. species. Fabad J. Pharm. Sci. 2004, 29, 93–107. [Google Scholar]
- Riaz, M.; Zia-Ul-Haq, M.; Jaafar, H.Z.E. Common mullein, pharmacological and chemical aspects. Braz. J. Pharmacogn. 2013, 23, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Guiso, M.; Iavarone, C.; Passacantilli, P.; Trogolo, C. 6-O-ß-D-Xylopyranosylaucubin from Ver bascum sinuatum. Phytochemistry 1980, 19, 571–573. [Google Scholar] [CrossRef]
- Bianco, A.; Guiso, M.; Iavarone, C.; Passacantilli, P.; Trogolo, C. Sinuatol (6-O-a-L-rhamnopyranosyl-aucu bin) from Verbascum sinuatum. Planta Med. 1981, 41, 75–79. [Google Scholar] [CrossRef]
- Bianco, A.; Guiso, M.; Iavarone, C.; Passacantilli, P.; Trogolo, C. 6-O-a-Sinuatosyl aucubin from Verbascum sinuatum. Phytochemistry 1981, 20, 465–468. [Google Scholar] [CrossRef]
- Falsone, G.; Laryea, M.; Crea, A.; Finner, E. Iridoids from Verbascum sinuatum. J. Med. Plant Res. 1982, 44, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, J. Determinacion de la estructura de los glicosidos de Verbascum lychnitis por espectrometria de masas. Quim. Ind. 1985, 31, 503–507. [Google Scholar]
- Souleles, C.; Geronikaki, A. Flavonoids from Verbascum thapsus. Sci. Pharm. 1989, 57, 59–61. [Google Scholar]
- Mehrotra, R.; Ahmed, B.; Vishwakarma, R.; Thakur, R. Verbacoside: A new luteolin glycoside from Verbascum thapsus. J. Nat. Prod. 1989, 52, 640–643. [Google Scholar] [CrossRef]
- Warashina, T.; Miyase, T.; Ueno, A. Iridoid glycosides from Verbascum thapsus L. Chem. Pharm. Bull. 1991, 39, 3261–3264. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Sharma, R.; Kumar, A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Pahwa, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V.; et al. Chemo- and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 5 May 2021).
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucl. Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Pardo de Santayana, M.; Morales, R.; Aceituno-Mata, L.; Molina, M. (Eds.) Inventario Español de los Conocimientos Tradicionales Relativos a la Biodiversidad. Fase II (1); Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2018; ISBN 978-84-491-1472-4.
- Akerreta, S.; Calvo, M.; Cavero, R. Sabiduría Popular y Plantas Curativas (Recopilación Extraída de un Estudio Etnobotánico en Navarra); Ediciones I: Madrid, Spain, 2013. [Google Scholar]
- Agelet, A. Estudis d’Etnobotànica Farmacèutica al Pallars. Ph.D. Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 1999. [Google Scholar]
- Villar, L.; Palacín, J.; Calvo, C.; Gómez, D.; Montserrat, G. Lantas Medicinales del Pirineo Aragonés y Demás Tierras Oscenses; CSIC: Madrid, Spain; Diputación de Huesca: Huesca, Spain, 1987.
- Conca, A.; Oltra, J. Plantes Medicinales y Comestibles; Caixa d’Estalvis I Monte de Pietat d’Ontinyent: Valencia, Spain, 2005. [Google Scholar]
- Pellicer, J. Costumari Botànic. Recerques Etnobotàniques a les Comarques Centrals Valencianes; Edicions del Bullent: Picanya, Spain, 2000; Volume 1. [Google Scholar]
- Bonet, M. Estudis Etnobotànics a la Vall del Tenes (Vallès Oriental). Master’s Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 1991. [Google Scholar]
- Pascual Gil, J.; Ingeniería Técnica Agrícola. Etnobotánica de La Pernía, Polentinos y Valle de Castillería (Palencia); Proyecto Fin de Carrera; Universidad de Valladolid: Palencia, Spain, 2013. [Google Scholar]
- Bonet, M. Estudi Etnobotànic del Montseny. Ph.D. Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 2001. [Google Scholar]
- Verde, A.; Rivera, D.; Obón, C. Etnobotánica en las Sierras de Segura y Alcaraz: Las Plantas y el Hombre; Instituto de Estudios Albacetenses: Albacete, Spain, 1998. [Google Scholar]
- Velasco, J.; Criado, J.; Blanco, E. Usos Tradicionales de las Plantas en la Provincia de Salamanca; Diputación de Salamanca: Salamanca, Spain, 2010. [Google Scholar]
- Granzow de la Cerda, I. Etnobotánica. El Mundo Vegetal en la Tradición; Centro de Cultura Tradicional, Diputación de Salamanca: Salamanca, Spain, 1993. [Google Scholar]
- Carrió, E. Contribució al Coneixement Etnobotànic de Mallorca. La Biodiversitat Vegetal i la Seva Gestió en una Illa Mediterrània. Ph.D. Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 2013. [Google Scholar]
- Verde, A. Estudio Etnofarmacológico de Tres Áreas de Montaña de Castilla-La Mancha. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2002. [Google Scholar]
- Calvo, M.; Cavero, R. Medicinal plants used for cardiovascular diseases in Navarra and their validation from official sources. J. Ethnopharmacol. 2014, 157, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Verde, A.; Rivera, D.; Fajardo, J.; Obón, C.; Cebrián, F. Guía de las Plantas Medicinales de Castilla-La Mancha (y Otros Recursos de Uso Tradicional); Altabán: Albacete, Spain, 2008. [Google Scholar]
- Belda, A.; Bellod, F.; Ríos Ruiz, S. Avance sobre la flora medicinal en la Sierra de Mariola (Valencia-Alicante). Flora Montiber. 2004, 28, 29–48. [Google Scholar]
- Panero, J. Sayago: Costumbres, Creencias y Tradiciones; Aderisa: Zamora, Spain, 2005. [Google Scholar]
- Tejerina, A. Usos y Saberes Sobre las Plantas de Monfragüe. Etnobotánica de la Comarca Natural; Itomonfragüe: Cáceres, Spain, 2010. [Google Scholar]
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, western Spain. J. Ethnopharmacol. 2010, 131, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M. Aproximación a la Etnobotánica de la Provincia de Jaén. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1997. [Google Scholar]
- Obón, C.; Rivera, D. Las Plantas Medicinales de Nuestra Región; Consejería de Cultura y Educación, Editora Regional de Murcia: Murcia, Spain, 1991. [Google Scholar]
- Fajardo, J.; Verde, A.; Rivera, D.; Obón, C. Etnobotánica en La Serranía de Cuenca. Las plantas y el Hombre; Diputación de Cuenca: Cuenca, Spain, 2007. [Google Scholar]
- Rigat, M. Estudi Etnobotànic de la Vall de Camprodon (Alta Vall del Ter, Pirineus). Master’s Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 2005. [Google Scholar]
- Rivera, D.; Alcaraz, F.; Verde, A.; Fajardo, J.; Obón, C. Las Plantas en la Cultura Popular. Enciclopedia Divulgativa de la Historia Natural de Jumilla-Yecla 9; Caja de Ahorros del Mediterráneo: Alicante, Spain; Sociedad Mediterránea de Historia Natural: Murcia, Spain, 2008. [Google Scholar]
- Parada, M. Estudi etnobotànic de L’Alt Empordà. Ph.D. Thesis, Cultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 2008. [Google Scholar]
- Moll, M. Les Plantes a Menorca: Noms i Usos; Institut Menorquí d’Estudis: Mahón, Menorca, Spain, 2005. [Google Scholar]
- Pérez Ramírez, I. Conocimiento Local y Uso de las Plantas Aromáticas y Medicinales en Tres Localidades del sur de Extremadura. Master’s Thesis, Universidad Internacional de Andalucía, Jaén, Spain, 2013. [Google Scholar]
- Segarra, E. Etnobotanica Farmacéutica de Gàtova: Serra Calderona; Facultad de Farmacia, Universidad de Valencia: Valencia, Spain, 2008. [Google Scholar]
- Anllo, J. Estudio Etnobotánico de la Comarca de Terra Chá. Ph.D. Thesis, Universidad de Santiago de Compostela, Santiago de Compostela, Spain, 2011. [Google Scholar]
- Barandiaran, J.; Manterola, A. Medicina Popular en Vasconia. Atlas Etnográfico de Vasconia; Euskalerria, E., Jaurlaritza, E., Eds.; Gobierno de Navarra: Bilbao, Spain, 2004; Volume 5.
- Pardo de Santayana, M. Estudios Etnobotánicos en Campoo (Cantabria): Conocimiento y Uso Tradicional de Plantas; CSIC: Madrid, Spain, 2008.
- Rigat, M.; Vallès, J.; Iglésias, J.; Garnatje, T. Traditional and alternative natural therapeutic products used in the treatment of respiratory tract infectious diseases in the eastern Catalan Pyrenees (Iberian Peninsula). J. Ethnopharmacol. 2013, 148, 411–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verde, A.; Fajardo, J.; Rivera, D.; Obón, C. Etnobotánica en el Entorno del Parque Nacional de Cabañeros; Ministerio de Medio Ambiente, Parques Nacionales: Madrid, Spain, 2000. [Google Scholar]
- Pardo de Santayana, M. Guía de las Plantas Medicinales de Cantabria; Estvdio: Santander, Spain, 2004. [Google Scholar]
- Cavero, R.Y.; Akerreta, S.; Calvo, M.I. Pharmaceutical ethnobotany in the Middle Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 137, 844–855. [Google Scholar] [CrossRef] [PubMed]
- González-Tejero, M. Investigaciones Etnobotánicas en la Provincia de Granada. Ph.D. Thesis, Facultad de Farmacia, Universidad de Granada, Granada, Spain, 1989. [Google Scholar]
- Martínez Lirola, M.; González-Tejero, M.; Molero Mesa, J. Investigaciones Etnobotánicas en el Parque Natural de Cabo de Gata-Níjar (Almería); Sociedad Almeriense de Historia Natural: Almería, Spain, 1997. [Google Scholar]
- Carrió, E.; Vallès, J. Ethnobotany of medicinal plants used in Eastern Mallorca (Balearic Islands, Mediterranean Sea). J. Ethnopharmacol. 2012, 141, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Mulet, L. Estudio Etnobotánico de la Provincia de Castellón; Diputación de Castellón: Castellón de la Plana, Spain, 1991. [Google Scholar]
- Benítez Cruz, G. Etnobotánica y Etnobiología del Poniente Granadino. Ph.D. Thesis, Facultad de Farmacia, Universidad de Granada, Granada, Spain, 2009. [Google Scholar]
- Benítez Cruz, G.; González-Tejero, M.; Molero Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (Southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Pardo-de-Santayana, M.; Aceituno, L.; Acosta, R.; Álvarez, A.; Barroso, E.; Blanco Salas, J.; Bonet, M.; Carrió, E.; Cavero, R.; Delgado, L.; et al. Medicinal and veterinary plants in the Spanish inventory of traditional knowledge related to biodiversity. In Proceedings of the Joint Society Conference Society for Economic Botany and Indigenous Plant Use Forum, Clanwilliam, South Africa, 29 June–2 July 2015. [Google Scholar]
- Cavero, R.Y.; Calvo, M.I. Medicinal plants used for respiratory affections in Navarra and their pharmacological validation. J. Ethnopharmacol. 2014, 158, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J. Estudio Etnobotánico de la Provincia de La Coruña. Ph.D. Thesis, Facultad de Farmacia, Universidad de Valencia, Valencia, Spain, 2008. [Google Scholar]
- Selga, A. Estudis Etnobotànics a les Guilleries. Ph.D. Thesis, Facultat de Farmàcia, Universitat de Barcelona, Barcelona, Spain, 1998. [Google Scholar]
- Turker, A.U.; Camper, N.D. Biological activity of common mullein, a medicinal plant. J. Ethnopharmacol. 2002, 82, 117–125. [Google Scholar] [CrossRef]
- Fernández Ocaña, A. Estudio Etnobotánico en el Parque Natural de las Sierras de Cazorla, Segura y Las Villas. Investigación Química de un Grupo de Especies Interesantes. Ph.D. Thesis, Facultad de Ciencias Experimentales, Universidad de Jaén, Jaén, Spain, 2000. [Google Scholar]
- Blanco, E. El Caurel, las Plantas y Sus Habitantes (Lugo); Fundación Caixa Galicia: La Coruña, Spain, 1996. [Google Scholar]
- Blanco, E. Diccionario de Etnobotánica Segoviana; Ayuntamiento de Segovia: Segovia, Spain, 1998.
- Aceituno-Mata, L. Estudio Etnobotánico y Agroecológico de la Sierra Norte de Madrid. Ph.D. Thesis, Facultad de Ciencias, Universidad Autónoma de Madrid, Madrid, Spain, 2010. [Google Scholar]
- Martínez-Lirola, M.; González-Tejero, M.; Molero-Mesa, J. Ethnobotanical resources in the province of Almeria, Spain: Campos de Níjar. Econ. Bot. 1996, 50, 40–56. [Google Scholar] [CrossRef]
- Espinosa, J.; Fernández, C.; Díaz, M.; Ramírez, M. Plantas útiles en Castillo de Locubín (Jaén, sur de la Península Ibérica). II. Blancoana 2002, 19, 3–16. [Google Scholar]
- Gil Palomo, C.; Juárez Castillo, J. Sobre las Plantas Silvestres de Cástaras. Usos y Costumbres Tradicionales en un Lugar de la Alpujarra; Ediciones RaRo: Jaén, Spain, 2005. [Google Scholar]
- Bonet, M.; Parada, M.; Selga, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the regions of L’Alt Empordà and Les Guilleries (Catalonia, Iberian Peninsula). J. Ethnopharmacol. 1999, 68, 145–168. [Google Scholar] [CrossRef]
- Mesa, S. Tudio Etnobotánico y Agroecológico de la Comarca de la Sierra de Mágina (Jaén). Ph.D. Thesis, Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain, 1996. [Google Scholar]
- Carazo, M.; Camacho, A.; Fernández Ocaña, A.; Fernández, C.; Calero, J.; Montiel, M. Utilización de plantas vasculares en Carchelejo (Sierra Mágina, Jaén). I. Blancoana 1998, 15, 42–55. [Google Scholar]
- Gallego, E.; Gallego, Á. Usos, Tradiciones y Conocimiento de las Plantas por las Gentes de Sayago; Náyade Editorial: Medina del Campo, Spain, 2008. [Google Scholar]
- Molina, N.; ETSI Agrónomos y Montes. Estudio de la Flora de Interés Etnobotánico en el Municipio de Carcabuey (Córdoba); Proyecto Fin de Carrera; Universidad de Córdoba: Córdoba, Spain, 2001. [Google Scholar]
- Sánchez Romero, M.; ETSI Agrónomos y Montes. Estudio de la Flora de Interés Etnobotánico en el Municipio de Rute (Córdoba); Proyecto Fin de Carrera; Universidad de Córdoba: Córdoba, Spain, 2003. [Google Scholar]
- Barber, A.; Redero, S.; Corbi, M.; Alba, B.; Molina, J.; Barber, J. Aproximació al Coneixement Etnobiològic i Etnoecològic d’Ibi (Foia de Castalla, l’Alcoià, Alacant). Una Anàlisi sobre la Relació dels Éssers Humans i l’Entorn a Ibi; Identia Institute: Barcelona, Spain, 2005. [Google Scholar]
- O’Mahony, J.M.; McCarthy, E. What is in a name? Can mullein weed beat TB where modern drugs are failing? Evid. Based Complement. Altern. Med. 2011, 2011, 239237. [Google Scholar]
- Rho, H.S.; Ghimeray, A.K.; Yoo, D.S.; Ahn, S.M.; Kwon, S.S.; Lee, K.H.; Cho, D.H.; Cho, J.Y. Kaempferol and kaempferol rhamnosides with depigmenting and anti-inflammatory properties. Molecules 2011, 16, 3338–3344. [Google Scholar] [CrossRef] [Green Version]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Zampolli, A. From Asthma to Atherosclerosis—5-Lipoxygenase, Leukotrienes, and Inflammation. N. Engl. J. Med. 2004, 350, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisastra, R.; Dekker, F.J. Inflammation, cancer and oxidative lipoxygenase activity are intimately linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.L.; Murphy, R.C. The Eicosanoids: Cyclooxygenase, Lipoxygenase and Epoxygenase Pathways. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 259–296. ISBN 9780444634382. [Google Scholar]
- Calder, P.C. Eicosanoids. Essays Biochem. 2020, 64, 423–441. [Google Scholar] [PubMed]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wang, C.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.; Sun, H. Luteolin protects HUVECs from TNF-α-induced oxidative stress and inflammation via its effects on the Nox4/ROS-NF-κB and MAPK pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Ma, W.; Li, N.; Wang, K.J. Antioxidant and anti-inflammatory flavonoids from the flowers of chuju, a medical cultivar of chrysanthemum morifolim ramat. J. Mex. Chem. Soc. 2017, 61, 282–289. [Google Scholar] [CrossRef]
- Zhao, N.; Dong, Q.; Fu, X.X.; Du, L.L.; Cheng, X.; Du, Y.M.; Liao, Y.H. Acacetin blocks Kv1.3 channels and inhibits human T cell activation. Cell. Physiol. Biochem. 2014, 34, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, M.; Xu, L.J. Apigetrin treatment attenuates LPS-induced acute otitis media though suppressing inflammation and oxidative stress. Biomed. Pharmacother. 2019, 109, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Park, B.R.; Moon, S.M.; Shin, S.H.; Kim, J.S.; Kim, D.K.; Kim, C.S. Cynaroside protects human periodontal ligament cells from lipopolysaccharide-induced damage and inflammation through suppression of NF-κB activation. Arch. Oral Biol. 2020, 120, 104944. [Google Scholar] [CrossRef]
- Lin, H.; Song, P.; Zhao, Y.; Xue, L.J.; Liu, Y.; Chu, C.Q. Targeting Th17 cells with small molecules and small interference RNA. Mediat. Inflamm. 2015, 2015, 290657. [Google Scholar] [CrossRef]
- Cium, L.; Milaciu, M.V.; Runcan, O.; Vesa, C.; Negrean, V.; Pern, M.; Donca, V.I. The Effects of Flavonoids in Cardiovascular Diseases. Molecules 2020, 25, 4320. [Google Scholar]
- Gallego, E. Estudio Etnobotánico del Occidente Alistano; CSIC, Diputación de Zamora; Instituto de Estudios Zamoranos “Florián de Ocampo”: Zamora, Spain, 2009. [Google Scholar]
- Li, W.; Du, L.; Li, M. Alkaloids and flavonoids as α(1)-adrenergic receptor antagonists. Curr. Med. Chem. 2011, 18, 4923–4932. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Drug Eval. 2013, 22, 1063–1079. [Google Scholar]
- Lorenz, P.; Conrad, J.; Stintzing, F.C. Metabolic fate of depsides and alkaloid constituents in aqueous extracts from Mercurialis perennis L. during fermentation. Chem. Biodivers. 2013, 10, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ren, D.; Luo, Y.; Yang, X. Protective effects of ursolic acid against hepatotoxicity and endothelial dysfunction in mice with chronic high choline diet consumption. Chem. Biol. Interact. 2016, 258, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, D.; Bauman, D.R.; Heredia, V.V.; Penning, T.M. The aldo-keto reductase superfamily homepage. Chem. Biol. Interact. 2003, 143, 621–631. [Google Scholar] [CrossRef]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Babamoradi, N.; Yousefi, S.; Ziarati, P. Optimization of ultrasound-assisted extraction of functional polysaccharides from common mullein (Verbascum thapsus L.) flowers. J. Food Process Eng. 2018, 41, e12851. [Google Scholar] [CrossRef]
- Bylka, W.; Witkowska-Banaszczak, E.; Studzińska-Sroka, E.; Matławska, I. Phytotherapy of respiratory tract diseases. Wiad. Lek. 2012, 65, 124–131. [Google Scholar]
- Kumar, A.; Choudhir, G.; Shukla, S.K.; Sharma, M.; Tyagi, P.; Bhushan, A.; Rathore, M. Identification of phytochemical inhibitors against main protease of {COVID}-19 using molecular modeling approaches. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Mitra, D.; Verma, D.; Mahakur, B.; Kamboj, A.; Srivastava, R.; Gupta, S.; Pandey, A.; Arora, B.; Pant, K.; Panneerselvam, P.; et al. Molecular docking and simulation studies of natural compounds of Vitex negundo L. against papain-like protease (PLpro) of SARS CoV-2 (coronavirus) to conquer the pandemic situation in the world. J. Biomol. Struct. Dyn. 2021, 18, 1–22. [Google Scholar]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.Y.; Kim, D.; Naguyen, T.T.H.; Park, S.J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Rana, K.V.; Parama, D.; Banik, K.; Girisa, S.; Sahu, H.; Thakur, K.K.; Dutta, U.; Garodia, P.; Gupta, S.C.; et al. COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci. 2020, 119201. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Ludwig, S.; Zarbock, A. Coronaviruses and SARS-CoV-2: A Brief Overview. Anesth. Analg. 2020, 131, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.E.; Sandeep, B.V.; Rao, B.G.; Kalpana, V.L. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front. Pharmacol. 2021, 11, 2305. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saakre, M.; Mathew, D.; Ravisankar, V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni Suef Univ. J. Basic Appl. Sci. 2021, 10, 21. [Google Scholar] [CrossRef]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto-Junior, A.; Lima-Tolouei, S.E.; dos Reis Lívero, F.A.; Gasparotto, F.; Boeing, T.; de Souza, P. Natural Agents Modulating ACE-2: A Review of Compounds with Potential against SARS-CoV-2 Infections. Curr. Pharm. Des. 2021, 27, 1588–1596. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, C.; He, F.; Xie, Y.; Zhou, H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19). Pharmacol. Res. 2020, 158. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G.; Kerimi, A. Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction. Biochem. Pharmacol. 2020, 178, 114123. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Khan, A.; Bertuccioli, A.; Maffioli, P.; Derosa, G.; Khan, S.; Khan, B.A.; Nigar, R.; Ujjan, I.; Devraian, B.R. Quercetin Phytosome® as a potential candidate for managing COVID-19. Minerva Gastroenterol. 2021, 67, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.A. Revised definition of pain by ‘International Association for the Study of Pain’: Concepts, challenges and compromises. Anaesth. Pain Intensive Care 2020, 24, 481–483. [Google Scholar] [CrossRef]
- Gerdle, B.; Ghafouri, B. Proteomic studies of common chronic pain conditions—A systematic review and associated network analyses. Exp. Rev. Proteom. 2020, 17, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Holmquist, G.L. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009, 10, S20–S29. [Google Scholar] [CrossRef] [Green Version]
- Stoyanov, S.; Fleming, T.; Konrade, I.; Haag, G.; Humpert, P.; Rabbani, N.; Thornalley, P.; Brownlee, M.; Nawroth, P.; Bierhaus, A. The Glyoxalase I (GLO-1) system as modulator of pain in early diabetic neuropathy. Diabetol. Stoffwechs. 2008, 3, A36. [Google Scholar] [CrossRef]
- Persson, L. Prenatal nutrition, socioenvironmental conditions, and child development. Lancet Glob. Health 2017, 5, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Indiana, M.; de Souza, F.H.V.; Eduardo, J.; Dantas Nascimento, P.G.B. Protein Kinases and Pain. In Protein Kinases; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Ma, H.; Qin, S.; Zhao, S. Osteoarthritis is Prevented in Rats by Verbascoside via Nuclear Factor kappa B (NF-kB) Pathway Downregulation. Med. Sci. Monit. 2020, 26, e921276. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Garduño-Siciliano, L.; Chávez-Rueda, A.K.; Siordia-Reyes, A.G.; Zamilpa, A.; Jiménez-Arellanes, M.A. In vivo anti-arthritic and antioxidant effects from the standardized ethanolic extract of Moussonia deppeana. Rev. Bras. Farmacogn. 2018, 28, 198–206. [Google Scholar] [CrossRef]
- Haleagrahara, N.; Miranda-Hernandez, S.; Alim, M.A.; Hayes, L.; Bird, G.; Ketheesan, N. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed. Pharmacother. 2017, 90, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Li, N.; Liu, Y.; Xu, Q.; Liu, Q.; You, Y.; Wei, Z.; Jiang, Y.; Liu, M.; Guo, T.; et al. Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int. Immunopharmacol. 2018, 55, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chen, X.; Chai, J.; Li, R.; Han, X.; Chen, X.; Liu, S.; Chen, M.; Xu, X. Antipyretic, anti-inflammatory and analgesic activities of Periplaneta americana extract and underlying mechanisms. Biomed. Pharmacother. 2020, 123, 109753. [Google Scholar] [CrossRef] [PubMed]
| Uses | Vp | Vs | Vt | Vb | Vc | Vd | Vg | Vl | Vr | Vv | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Circulatory | Anti-hemorrhoidal | B/T | B/T | S/B/T | B/T | T | B | T | |||
| Leg treatment | B | ||||||||||
| Anti-hypertensive | I/B | I | B | ||||||||
| Digestive | Teeth pain, gumboil | B/T | B | B/T | T | ||||||
| Digestive | I/B/T | B/T | |||||||||
| Gastric ulcer/inflammation | B/T | I/B/T | B | ||||||||
| Liver inflammation | I/B | T | I/B/T | I/B | |||||||
| Gallstone | I | I | I/B | I | |||||||
| Anti-diarrhoea | T | I | T | ||||||||
| Constipation | B | E | |||||||||
| Respiratory | Hoarse, tonsillitis | B/T | I/T | I/B/T | |||||||
| Cold | B | I | I/B | I | B | ||||||
| Cough, asthma, bronchitis, hemoptysis | B | I/B/M | I/B | I | I | B | |||||
| Musculature & Skeleton | Anti-inflammatory (swelling) | B/T | I/B/T | ||||||||
| Contusion, broken bones | I/T | T | I/B/T | ||||||||
| Arthrosis, rheumatism | B/T | B/T | I | T | |||||||
| Skin | Eczema, exanthema | B/T | B/T | T | |||||||
| Cysts and zits | T | T | I/B/T | T | T | ||||||
| Wounds, ulcers, burns | B/T | M/T | I/B/M/ | T | T | ||||||
| Horsefly bite | M/T | ||||||||||
| Chilblain | B/T | B | B/T | B/T | B/T | ||||||
| Nail conditions | B/T | ||||||||||
| Sense | Conjunctivitis | M | M | M | |||||||
| Otitis | B/M | M | B | ||||||||
| Infectious parasitic diseases | Diphtheria | T | |||||||||
| Helminthiasis | B | ||||||||||
| Tuberculosis | I | ||||||||||
| Typhus | T | ||||||||||
| Mange | T |
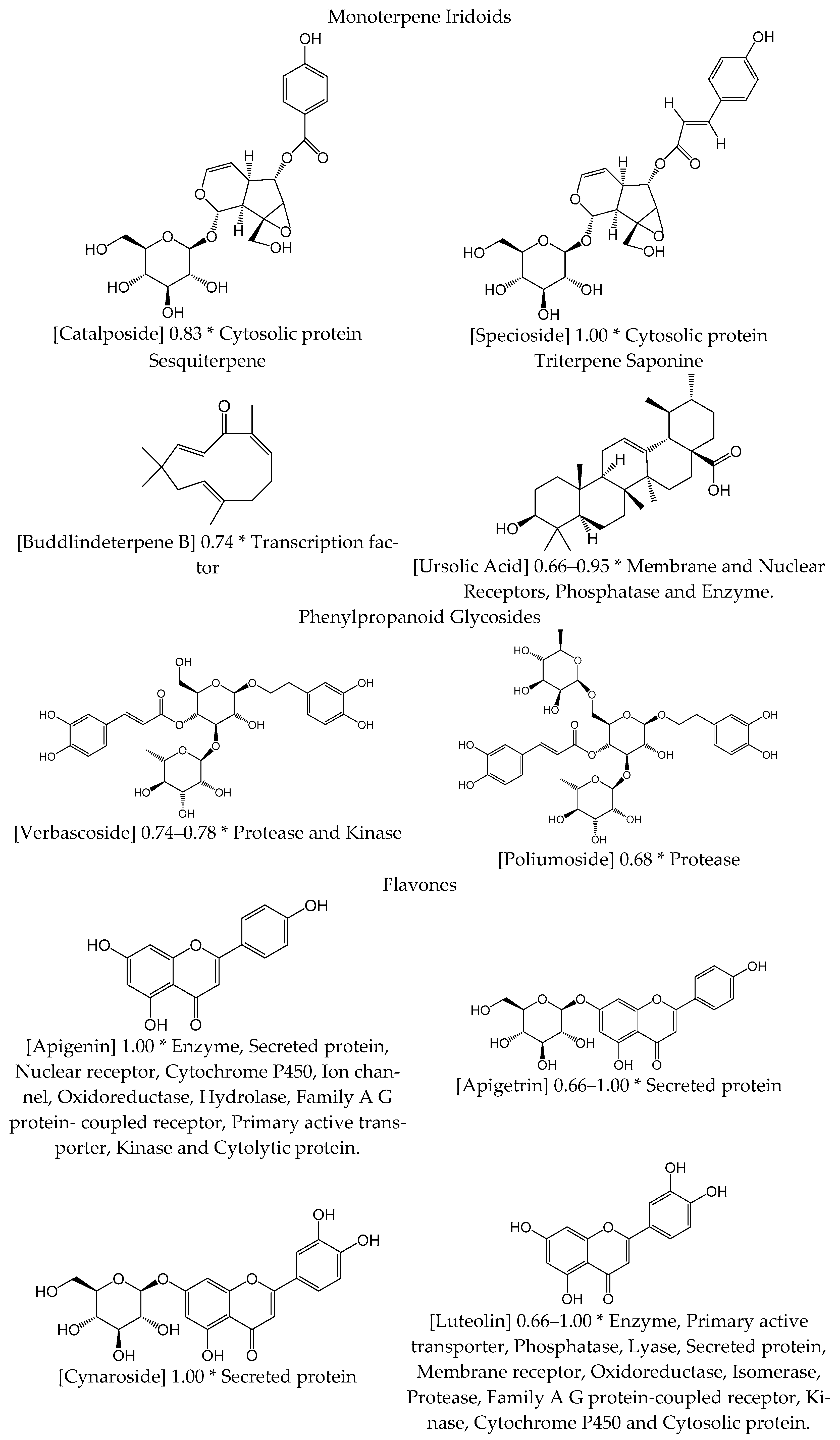
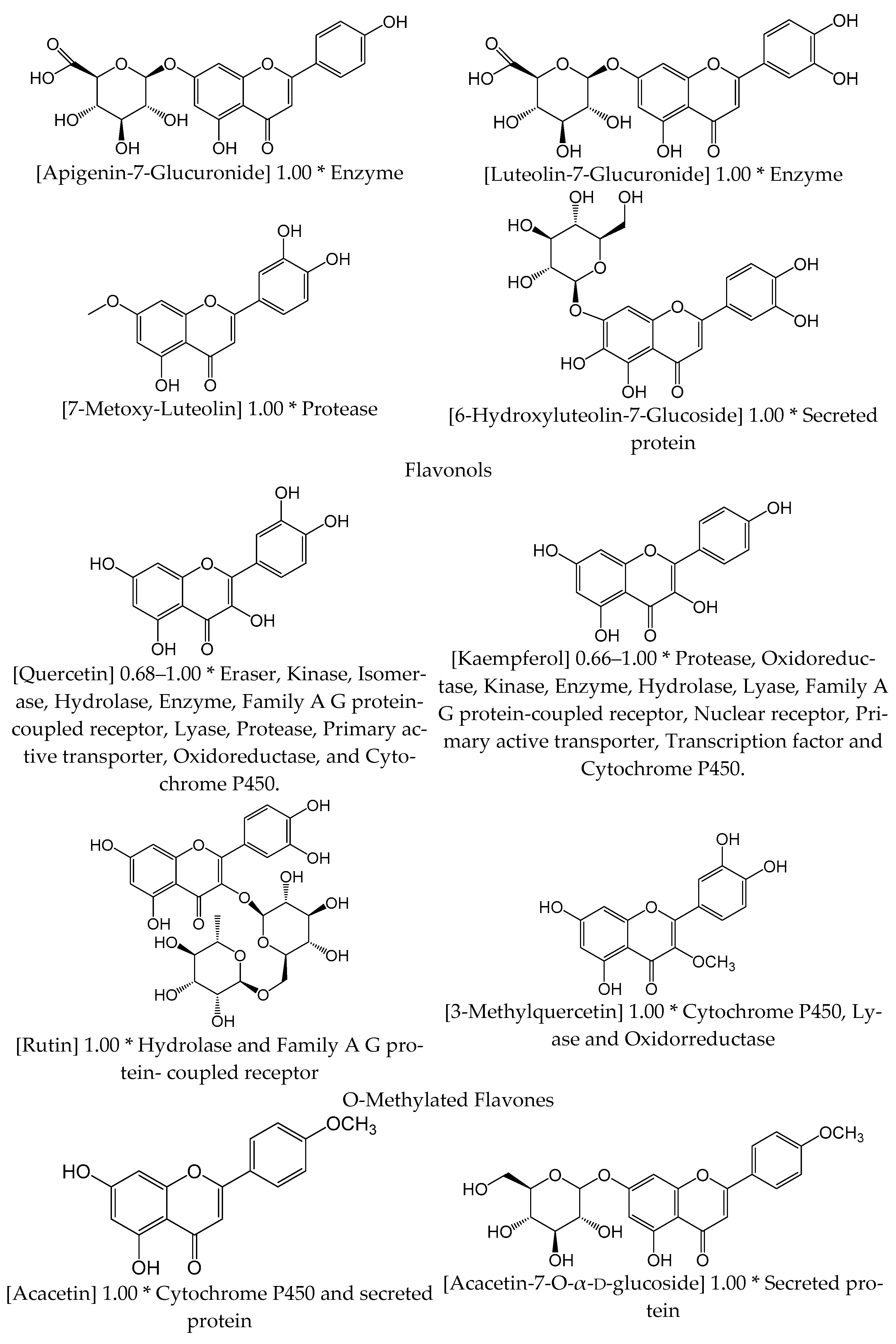
| Chemical Group | Component | Species |
|---|---|---|
| Monoterpene iridoid | Catalposide | Vl |
| Specioside | Vl | |
| Sesquiterpene | Buddlindeterpene B | Vt |
| Triterpene saponin | Ursolic acid | Vt, Vl |
| Phenypropanoid Glycosides | Verbascoside | Vs, Vl |
| Poliumoside | Vs, Vt, Vb | |
| Flavones | Apigenin | Vt |
| Apigenin-7-glucuronide | Vt | |
| Apigetrin | Vt, Vl | |
| Cynaroside | Vt, Vl | |
| Luteolin | Vt, Vl | |
| Luteolin-7-glucuronide | Vl | |
| 6-hydroxyluteolin-7-glucoside | Vt, Vl | |
| 7-methoxy-luteolin | Vl | |
| Flavonol | Quercetin | Vt, Vl |
| 3′-methylquercetin | Vt, Vl | |
| Kaempferol | Vt | |
| Rutin | Vt | |
| O-metilated Flavone | Acacetin | Vt |
| Acacetin-7-O-α-d-glucoside | Vt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Salas, J.; Hortigón-Vinagre, M.P.; Morales-Jadán, D.; Ruiz-Téllez, T. Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology 2021, 10, 618. https://doi.org/10.3390/biology10070618
Blanco-Salas J, Hortigón-Vinagre MP, Morales-Jadán D, Ruiz-Téllez T. Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology. 2021; 10(7):618. https://doi.org/10.3390/biology10070618
Chicago/Turabian StyleBlanco-Salas, José, María P. Hortigón-Vinagre, Diana Morales-Jadán, and Trinidad Ruiz-Téllez. 2021. "Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae)" Biology 10, no. 7: 618. https://doi.org/10.3390/biology10070618
APA StyleBlanco-Salas, J., Hortigón-Vinagre, M. P., Morales-Jadán, D., & Ruiz-Téllez, T. (2021). Searching for Scientific Explanations for the Uses of Spanish Folk Medicine: A Review on the Case of Mullein (Verbascum, Scrophulariaceae). Biology, 10(7), 618. https://doi.org/10.3390/biology10070618










