Postoperative Stroke after Spinal Anesthesia and Responses of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients
2.3. Animals
2.4. Subarachnoid Catheterization
2.5. Intrathecal Administration
2.6. Myelogram
2.7. General Preparation for Physiological Experiments
2.8. Measurement of Blood Pressure, Heart Rate and Spontaneous Baroreflexes
2.9. Measurement of Carotid Blood Flow
2.10. Measurement of Microvascular Perfusion, Tissue Oxygen Level and Temperature in the Cerebral Cortex
2.11. Sample Size Calculation
2.12. Statistical Analyses
3. Results
3.1. Part 1. Patients
3.2. Part 2. Animals
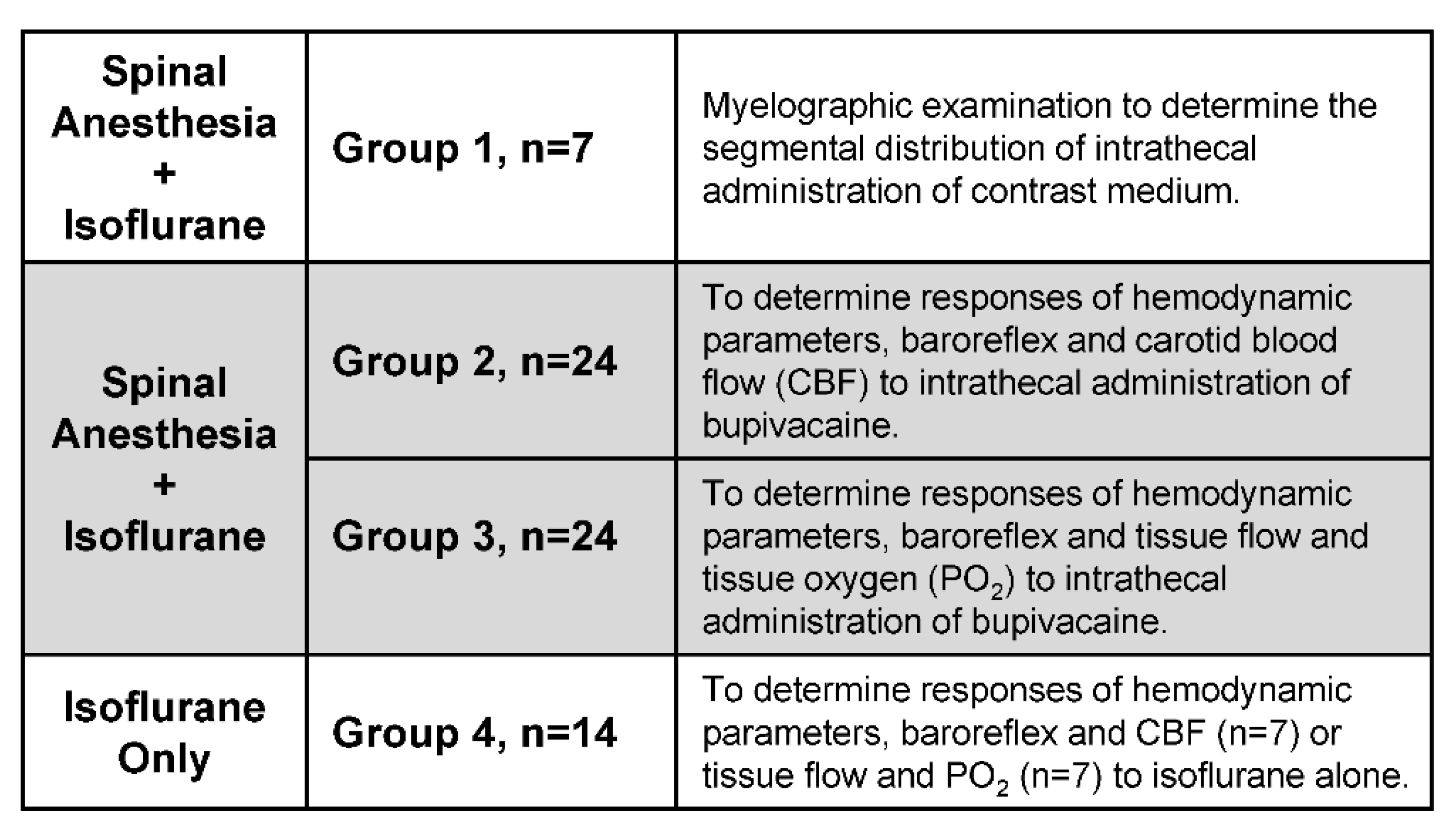
3.2.1. Experimental Setup
3.2.2. Distribution of Contrast Medium in Spinal Subarachnoid Space after Intrathecal Administration
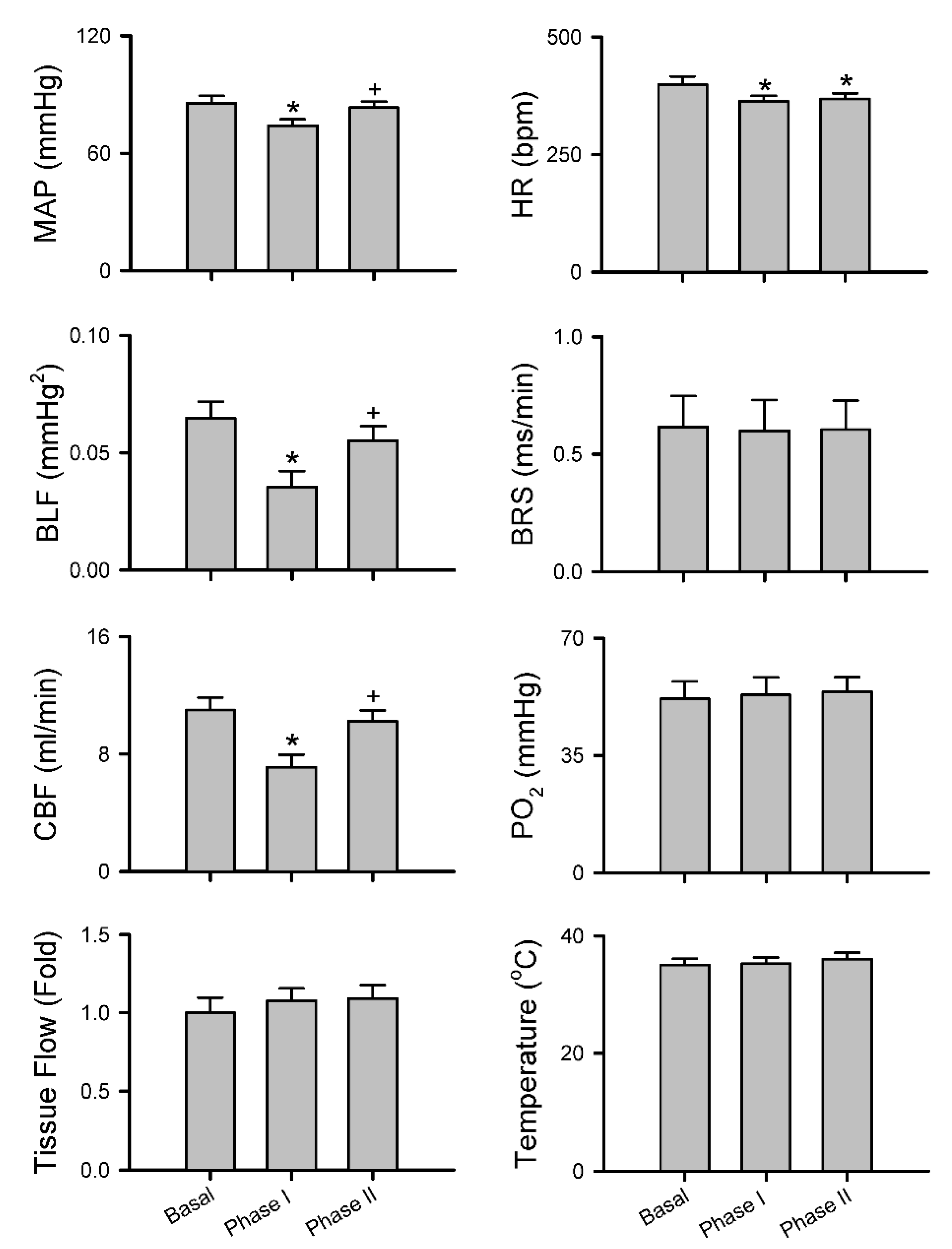
3.2.3. Common Response Pattern of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine
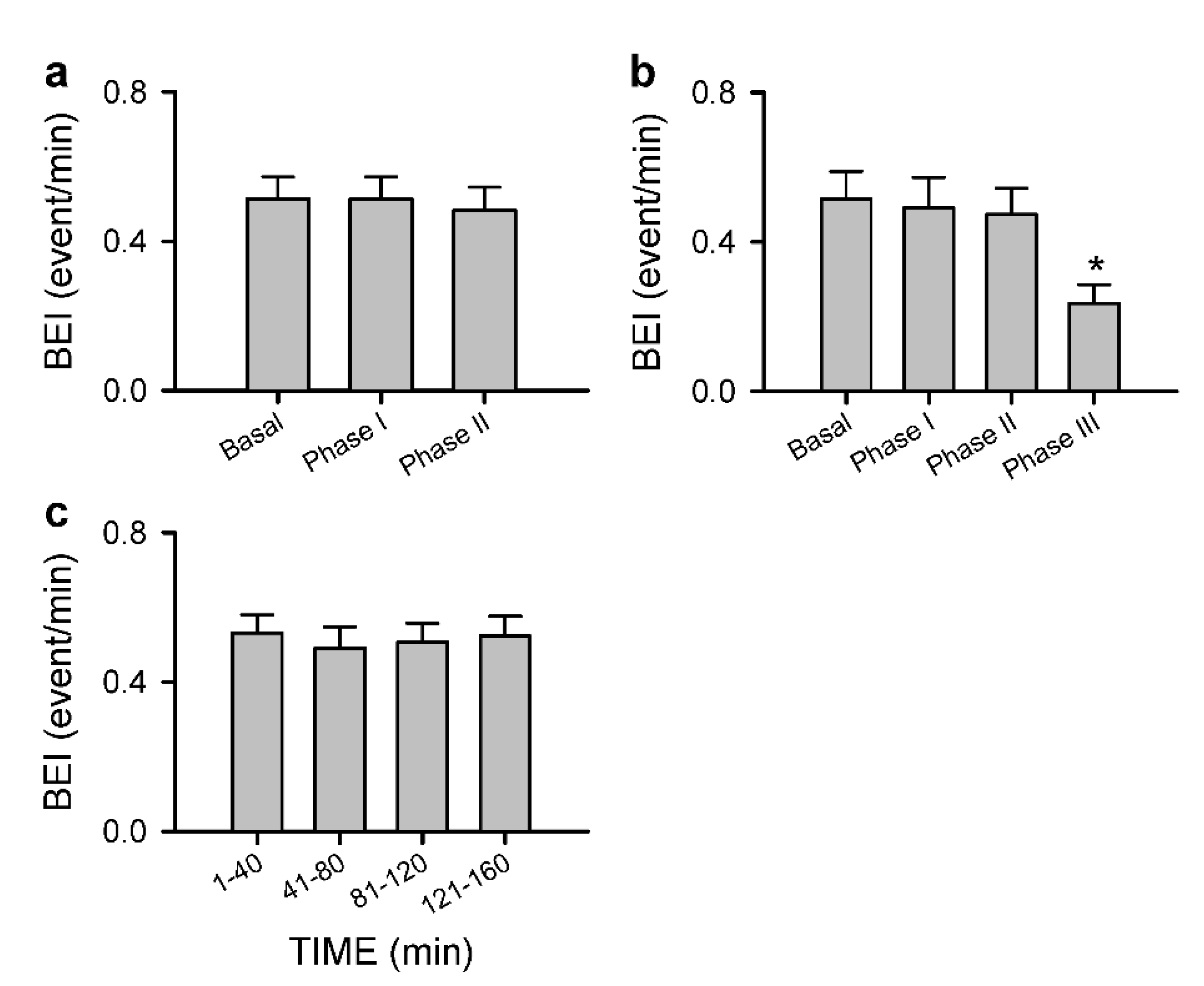
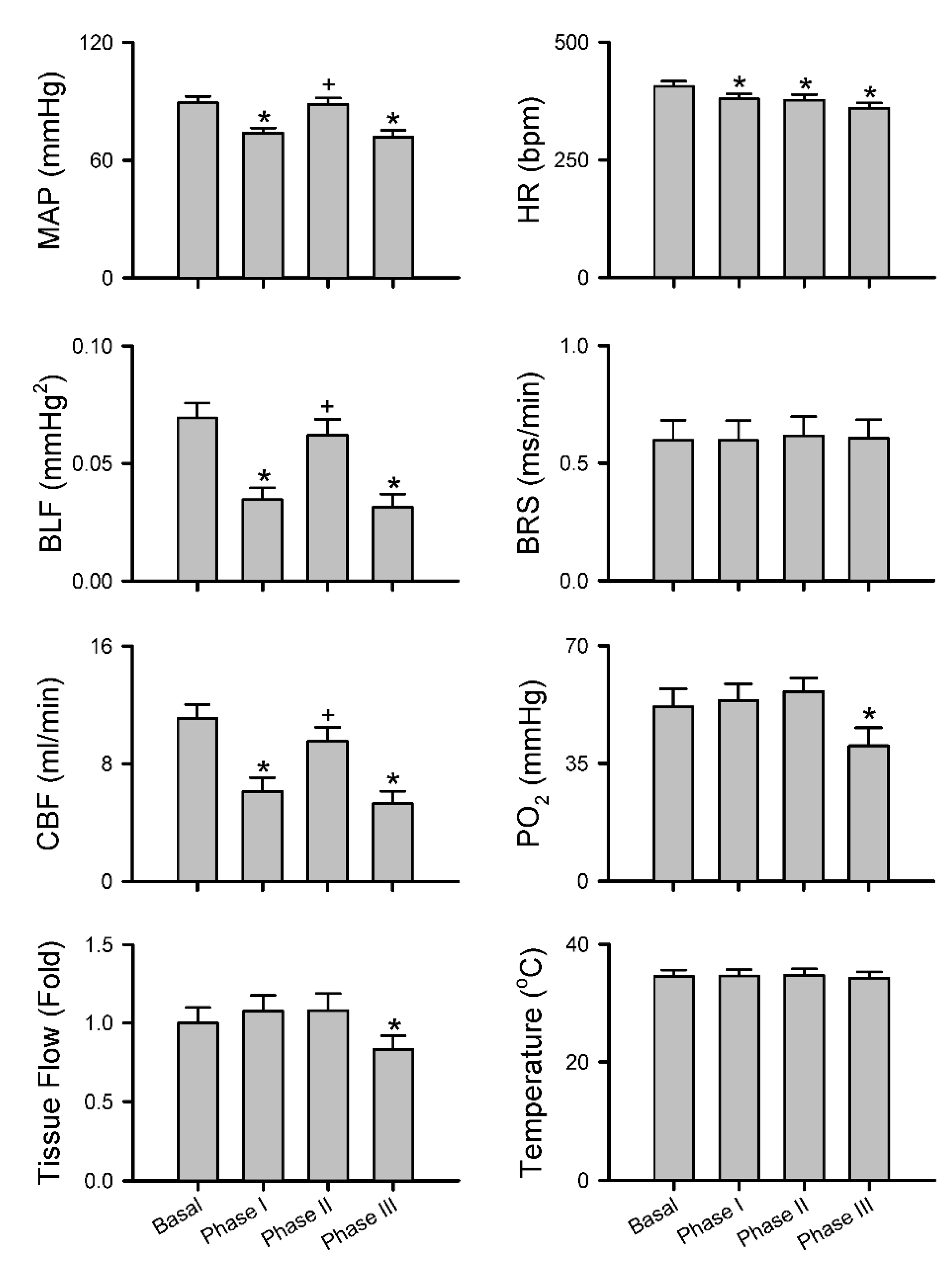
3.2.4. Anomalous Response Pattern of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine
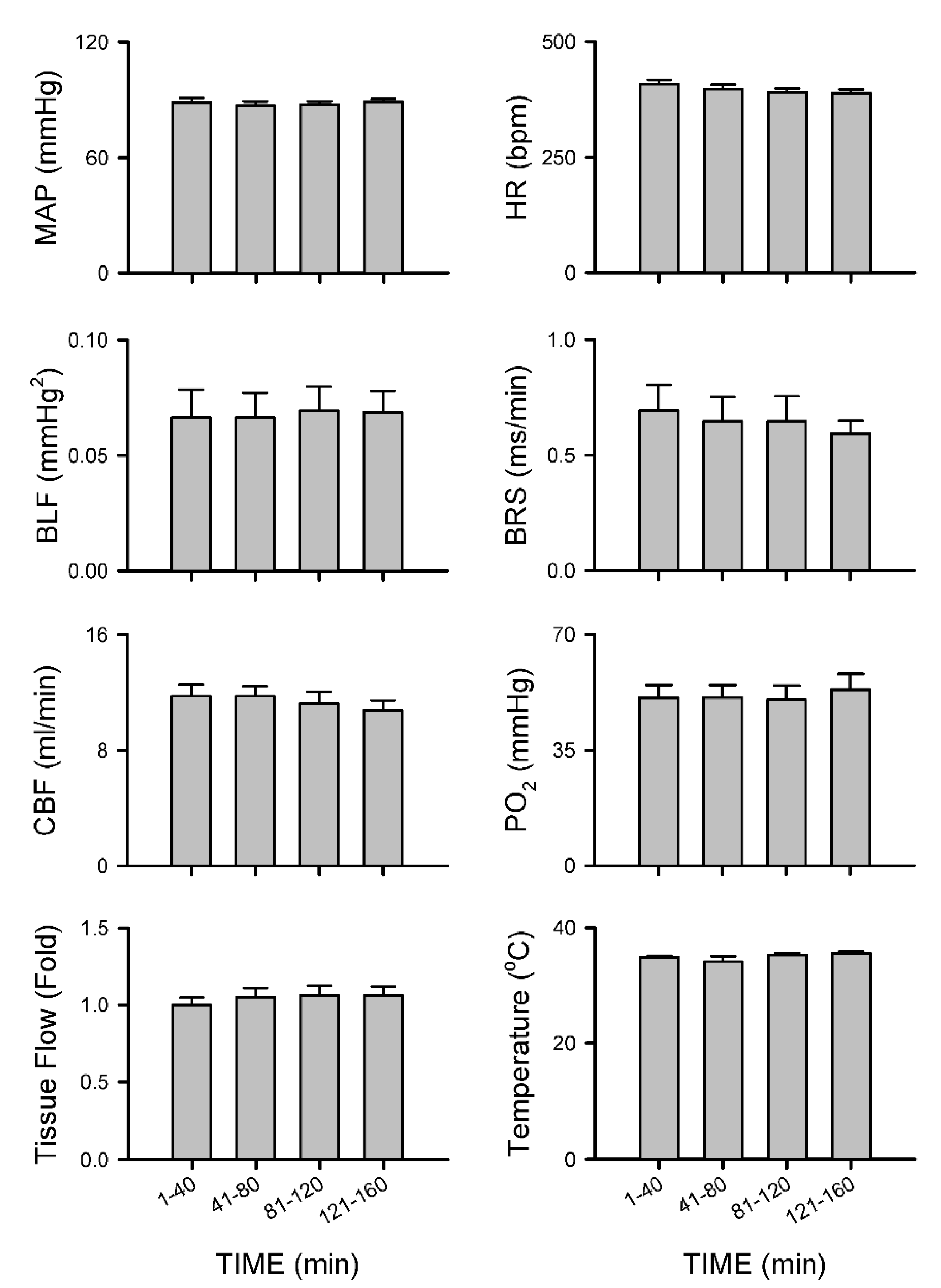
3.2.5. Insignificant Changes in Carotid or Cerebral Blood Flow and Baroreflex Functionality under Isoflurane Anesthesia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wulf, H.F.W. The Centennial of Spinal Anesthesia. Anesthesiology 1998, 89, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Konrad, C.; Schupfer, G.; Wietlisbach, M.; Gerber, H. Learning manual skills in anesthesiology: Is there a recommended number of cases for anesthetic procedures? Anesth. Analg. 1998, 86, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, D.J.; Neal, J.M.; Pollock, J.E. The regional anesthesia “learning curve”. What is the minimum number of epidural and spinal blocks to reach consistency? Reg. Anesth. 1996, 21, 182–190. [Google Scholar]
- Memtsoudis, S.G.; Sun, X.; Chiu, Y.-L.; Stundner, O.; Liu, S.S.; Banerjee, S.; Mazumdar, M.; Sharrock, N.E. Perioperative Comparative Effectiveness of Anesthetic Technique in Orthopedic Patients. Anesthesiology 2013, 118, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Pugely, A.; Martin, C.T.; Gao, Y.; Mendoza-Lattes, S.; Callaghan, J.J. Differences in Short-Term Complications Between Spinal and General Anesthesia for Primary Total Knee Arthroplasty. J. Bone Jt. Surg. Am. Vol. 2013, 95, 193–199. [Google Scholar] [CrossRef]
- Van Waesberghe, J.; Stevanovic, A.; Rossaint, R.; Coburn, M. General vs. neuraxial anaesthesia in hip fracture patients: A systematic review and meta-analysis. BMC Anesthesiol. 2017, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.O.; Bomer, W.; Krauth, J.; Marquardt, B. Incidence and time course of cardiovascular side effects during spinal anesthesia after prophylactic administration of intravenous fluids or vasoconstrictors. Anesth. Analg. 1998, 87, 347–354. [Google Scholar]
- Carpenter, R.L.; Caplan, R.A.; Brown, D.L.; Stephenson, C.; Wu, R. Incidence and Risk Factors for Side Effects of Spinal Anesthesia. Anesthesiology 1992, 76, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Gentili, M.; Huu, P.C.; Enel, D.; Hollande, J.; Bonnet, F. Sedation depends on the level of sensory block induced by spinal anaesthesia. Br. J. Anaesth. 1998, 81, 970–971. [Google Scholar] [CrossRef][Green Version]
- Inagaki, Y.; Mashimo, T.; Kuzukawa, A.; Tsuda, Y.; Yoshiya, I. Epidural lidocaine delays arousal from isoflurane anes-thesia. Anesth. Analg. 1994, 79, 368–372. [Google Scholar] [CrossRef]
- Greene, N.M. Preganglionic Sympathetic Blockade in Man: A Study of Spinal Anesthesia. Acta Anaesthesiol. Scand. 1981, 25, 463–469. [Google Scholar] [CrossRef]
- Sancetta, S.M.; Lynn, R.B.; Simeone, F.A.; Scott, R.W.; Heckman, G.; Janouskovec, H. Studies of Hemodynamic Changes in Humans Following Induction of Low and High Spinal Anesthesia. Circulation 1952, 6, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.F.; Butterworth, J.F., 4th; Kitzman, D.W.; Berman, J.M.; Kashtan, H.I.; McKinley, A.C. Treatment of hypo-tension after hyperbaric tetracaine spinal anesthesia. A randomized, double-blind, cross-over comparison of phe-nylephrine and epinephrine. Anesthesiology 1997, 86, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, J. Physiology of spinal anesthesia: What are the implications for management? Reg. Anesth. Pain Med. 1998, 23, 370–373. [Google Scholar] [CrossRef]
- Critchley, L.A.; Conway, F. Hypotension during subarachnoid anaesthesia: Haemodynamic effects of colloid and me-taraminol. Br. J. Anaesth. 1996, 76, 734–736. [Google Scholar] [CrossRef]
- Rooke, G.A.; Freund, P.R.; Jacobson, A.F. Hemodynamic response and change in organ blood volume during spinal anesthesia in elderly men with cardiac disease. Anesth. Analg. 1997, 85, 99–105. [Google Scholar] [PubMed]
- Critchley, L.A.; Chan, S.; Tam, Y.H. Spectral analysis of sudden bradycardia during intrathecal meperidine anesthesia. Reg. Anesth. Pain Med. 1998, 23, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Crystal, G.J.; Salem, M.R. The Bainbridge and the "reverse" Bainbridge reflexes: History, physiology, and clinical rele-vance. Anesth. Analg. 2012, 114, 520–532. [Google Scholar] [CrossRef]
- Mather, L.E.; Chang, D.H. Cardiotoxicity with modern local anaesthetics: Is there a safer choice? Drugs 2001, 61, 333–342. [Google Scholar] [CrossRef]
- Cowley, A.W., Jr.; Liard, J.F.; Guyton, A.C. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ. Res. 1973, 32, 564–576. [Google Scholar] [CrossRef]
- Thrasher, T.N.; Burattini, R.; Borgdorff, P.; Westerhof, N. Baroreceptors and the long-term control of blood pressure. Exp. Physiol. 2004, 89, 331–335. [Google Scholar] [CrossRef]
- McHenry, L.C., Jr.; West, J.W.; Cooper, E.S.; Goldberg, H.I.; Jaffe, M.E. Cerebral autoregulation in man. Stroke 1974, 5, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Gratadour, P.; Viale, J.P.; Parlow, J.; Sagnard, P.; Counioux, H.; Bagou, G.; Annat, G.; Hughson, R.; Quintin, L. Sym-pathovagal effects of spinal anesthesia assessed by the spontaneous cardiac baroreflex. Anesthesiology 1997, 87, 1359–1367. [Google Scholar] [CrossRef]
- Minville, V.; Asehnoune, K.; Salau, S.; Bourdet, B.; Tissot, B.; Lubrano, V.; Fourcade, O. The Effects of Spinal Anesthesia on Cerebral Blood Flow in the Very Elderly. Anesth. Analg. 2009, 108, 1291–1294. [Google Scholar] [CrossRef]
- Bonnet, M.-P.; Larousse, E.; Asehnoune, K.; Benhamou, D. Spinal Anesthesia with Bupivacaine Decreases Cerebral Blood Flow in Former Preterm Infants. Anesth. Analg. 2004, 98, 1280–1283. [Google Scholar] [CrossRef]
- Kam, P.C.A.; Calcroft, R.M. Peri-operative stroke in general surgical patients. Anaesthesia 1997, 52, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Cohen, J.R. Perioperative stroke after general surgical procedures. N. Y. State J. Med. 1993, 93, 162–165. [Google Scholar] [PubMed]
- Selim, M. Perioperative stroke. N. Engl. J. Med. 2007, 356, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Szeder, V.; Torbey, M.T. Prevention and Treatment of Perioperative Stroke. Neurologist 2008, 14, 30–36. [Google Scholar] [CrossRef]
- Knapp, R.B.; Topkins, M.J.; Artusio, J.F. The Cerebrovascular Accident and Coronary Occlusion in Anesthesia. JAMA 1962, 182, 332–334. [Google Scholar] [CrossRef]
- Larsen, S.F.; Zaric, D.; Boysen, G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol. Scand. 1988, 32, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Thoennissen, J.; Herkner, H.; Lang, W.; Domanovits, H.; Laggner, A.N.; Müllner, M. Does bed rest after cervical or lumbar puncture prevent headache? A systematic review and meta-analysis. Can. Med. Assoc. J. 2001, 165, 1311–1316. [Google Scholar]
- Poon, Y.Y.; Chang, A.Y.W.; Ko, S.F.; Chan, S.H.H. An Improved Procedure for Catheterization of the Thoracic Spinal Subarachnoid Space in the Rat. Anesth. Analg. 2005, 101, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Poon, Y.-Y.; Tsai, C.-Y.; Cheng, C.-D.; Chang, A.Y.W.; Chan, S.H.H. Endogenous nitric oxide derived from NOS I or II in thoracic spinal cord exerts opposing tonic modulation on sympathetic vasomotor tone via disparate mechanisms in anesthetized rats. Am. J. Physiol. Circ. Physiol. 2016, 311, H555–H562. [Google Scholar] [CrossRef]
- Li, P.-L.; Chao, Y.-M.; Chan, S.H.H.; Chan, J.Y.H. Potentiation of Baroreceptor Reflex Response by Heat Shock Protein 70 in Nucleus Tractus Solitarii Confers Cardiovascular Protection During Heatstroke. Circulation 2001, 103, 2114–2119. [Google Scholar] [CrossRef]
- Laude, D.; Baudrie, V.; Elghozi, J.-L. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R142–R150. [Google Scholar] [CrossRef]
- Mukda, S.; Tsai, C.-Y.; Leu, S.; Yang, J.-L.; Chan, S.H.H. Pinin protects astrocytes from cell death after acute ischemic stroke via maintenance of mitochondrial anti-apoptotic and bioenergetics functions. J. Biomed. Sci. 2019, 26, 1–14. [Google Scholar] [CrossRef]
- Poon, Y.-Y.; Tsai, C.-Y.; Huang, Y.; Wu, J.C.C.; Chan, S.H.H.; Chan, J.Y.H. Disproportional cardiovascular depressive effects of isoflurane: Serendipitous findings from a comprehensive re-visit in mice. Lab Anim. 2021, 50, 26–31. [Google Scholar] [CrossRef]
- Gelderd, J.B.; Chopin, S.F. The vertebral level of origin of spinal nerves in the rat. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1977, 188, 45–47. [Google Scholar] [CrossRef]
- Steel, W.A. Blood Pressure Maintenance in Spinal Anesthesia. J. Am. Med. Assoc. 1925, 84, 79. [Google Scholar] [CrossRef]
- Bijker, J.B.; Persoon, S.; Peelen, L.M.; Moons, K.G.; Kalkman, C.J.; Kappelle, L.J.; van Klei, W.A. Intraoperative hypo-tension and perioperative ischemic stroke after general surgery: A nested case-control study. Anesthesiology 2012, 116, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Abkur, T.M.; Mohamed, M.B.; Peters, C. Multiple territory watershed infarcts following spinal anaesthesia. BMJ Case Rep. 2014, 2014, 2014204995. [Google Scholar] [CrossRef] [PubMed]
- White, R.P.; Markus, H.S. Impaired dynamic cerebral autoregulation in carotid artery stenosis. Stroke 1997, 28, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L.; Bochorishvili, G.; Depuy, S.D.; Burke, P.G.; Abbott, S.B. C1 neurons: The body’s EMTs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R187–R204. [Google Scholar] [CrossRef] [PubMed]
- Strack, A.; Sawyer, W.; Marubio, L.; Loewy, A. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988, 455, 187–191. [Google Scholar] [CrossRef]
- Domoto, T.; Teramoto, M.; Tanigawa, K.; Tamura, K.; Yasui, Y. Origins of nerve fibers containing nitric oxide synthase in the rat celiac-superior mesenteric ganglion. Cell Tissue Res. 1995, 281, 215–221. [Google Scholar] [CrossRef]
- Fink, G.D.; Osborn, J.W. The Splanchnic Circulation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 211–213. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poon, Y.-Y.; Liu, Y.-W.; Huang, Y.-H.; Chan, S.H.H.; Tsai, C.-Y. Postoperative Stroke after Spinal Anesthesia and Responses of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine in Rats. Biology 2021, 10, 617. https://doi.org/10.3390/biology10070617
Poon Y-Y, Liu Y-W, Huang Y-H, Chan SHH, Tsai C-Y. Postoperative Stroke after Spinal Anesthesia and Responses of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine in Rats. Biology. 2021; 10(7):617. https://doi.org/10.3390/biology10070617
Chicago/Turabian StylePoon, Yan-Yuen, Yueh-Wei Liu, Ya-Hui Huang, Samuel H. H. Chan, and Ching-Yi Tsai. 2021. "Postoperative Stroke after Spinal Anesthesia and Responses of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine in Rats" Biology 10, no. 7: 617. https://doi.org/10.3390/biology10070617
APA StylePoon, Y.-Y., Liu, Y.-W., Huang, Y.-H., Chan, S. H. H., & Tsai, C.-Y. (2021). Postoperative Stroke after Spinal Anesthesia and Responses of Carotid or Cerebral Blood Flow and Baroreflex Functionality to Spinal Bupivacaine in Rats. Biology, 10(7), 617. https://doi.org/10.3390/biology10070617




