The Role of Food Supplementation in Microcirculation—A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Microcirculation in Physiological Conditions
1.2. The Role of Microcirculation in Pathological Conditions
1.3. Food Supplementation
2. The Importance of Food Safety and Food Quality in the Food Supplement Industry
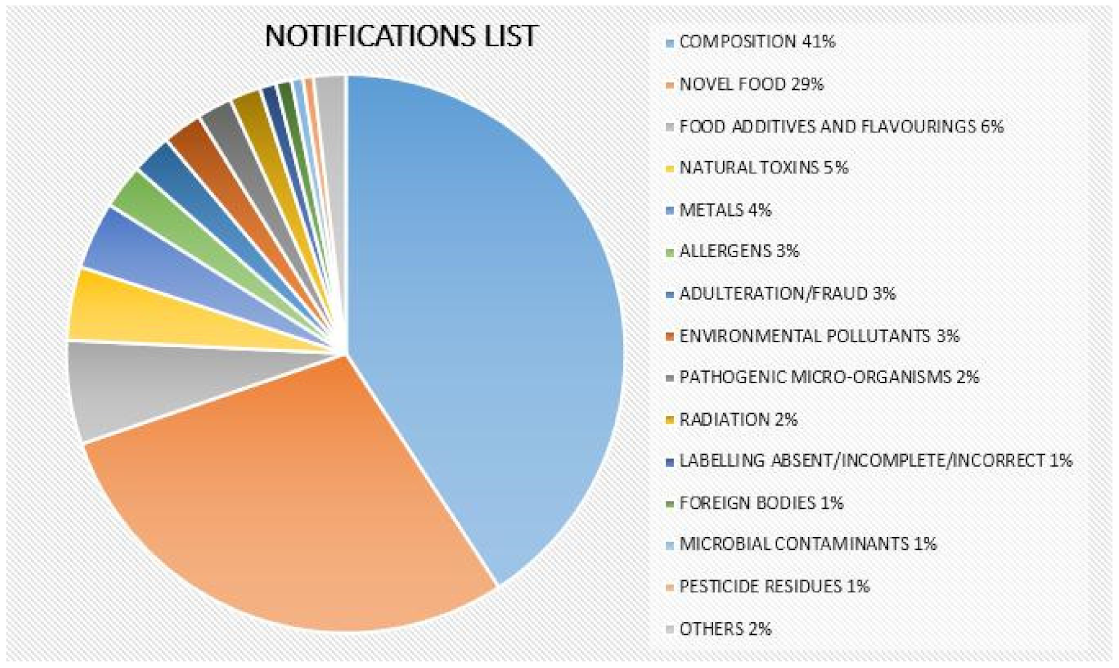
- A food supplement for sale on the market that has not been previously noted or included in the corresponding food supplements list or does not coincide with what has been noted and commercialized;
- The food supplement labeling does not comply with the provisions of the general and specific regulations for labeling food supplements;
- The labeling declares a nonharmonized substance and does not justify prior marketing in another Member State;
- The labeling of the food supplement includes unauthorized harmonized substances or substances in larger quantities than those authorized in those cases for which a maximum limit has been established.
- The presence of toxic compounds;
- The presence of pharmacologically active substances;
- The presence of addictive or psychotropic substances;
- Adverse reactions to, and drug interactions with, otherwise nontoxic substances;
- Genetic variants among plant species;
- Differences in processing and manufacturing conditions. Some other problems are addressed in this section;
- Misidentification of the initial plant source;
- Adulteration by other plants;
- Environmental contamination (e.g., with heavy metals and pesticide or herbicide residues);
- Biological contamination (mycotoxins, microorganisms);
- Addition of illegal substances.
3. European and American Legal Framework of Food Supplements
4. Food Supplements with Beneficial Effects on Microcirculation
4.1. Ruscus aculeatus
4.2. Hawthorn
4.3. Centella asiatica
4.4. Ginseng
4.5. Aesculus hippocastanum L.
4.6. Hamamelis virginiana L.
4.7. Vitis vinifera L.
4.8. Ginkgo biloba L.
4.9. Salvia miltiorrhiza Bunge
4.10. Mangifera indica L.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.S. Regulation of blood flow in the microcirculation. Microcirculation 2005, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, D.D.; Chabowski, D.S.; Kadlec, A.O.; Durand, M.J.; Freed, J.K.; Aissa, K.A.; Beyer, A.M. The Human Microcirculation—Regulation of flow and Beyond. Circ. Res. 2016, 118, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Bagher, P.; Segal, S.S. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol. 2011, 202, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, Z.; Khazaei, M. A review on angiogenesis and its assays. Iran. J. Basic Med. Sci. 2012, 15, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- McCarron, J.G.; Lee, M.D.; Wilson, C. The Endothelium Solves Problems That Endothelial Cells Do Not Know Exist. Trends Pharmacol. Sci. 2017, 38, 322–338. [Google Scholar] [CrossRef]
- Abularrage, C.J.; Sidawy, A.N.; Aidinian, G.; Singh, N.; Weiswasser, J.M.; Arora, S. Evaluation of the microcirculation in vascular disease. J. Vasc. Surg. 2005, 42, 574–581. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Noel, C.; Merz, B. Clinical update Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef]
- Stehouwer, C.D.A. Microvascular Dysfunction and Hyperglycemia: A Vicious Cycle with Widespread Consequences. Diabetes 2018, 67, 1729–1741. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial Dysfunction. A Marker of Atherosclerotic Risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Backer, D.D.; Donadello, K.; Taccone, F.S.; Ospina-Tascon, G.; Salgado, D.; Vincent, J. Microcirculatory alterations: Potential mechanisms and implications for therapy. Ann. Intensive Care 2011, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Buerk, D.G.; Barbee, K.A.; Jaron, D. Nitric Oxide Signaling in the Microcirculation. Crit. Rev. Biomed. Eng. 2011, 39, 397–433. [Google Scholar] [CrossRef]
- Böhm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Bronzato, S.; Durante, A. Dietary Supplements and Cardiovascular Diseases. Int. J. Prev. Med. 2018, 9, 80. [Google Scholar]
- Deaton, C.; Froelicher, E.S.; Wu, L.H.; Ho, C.; Shishani, K.; Jaarsma, T. The Global Burden of Cardiovascular Disease. Eur. J. Cardiovasc. Nurs. 2011, 10, S5–S13. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third Who Report on Neglected Tropical Diseases 2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Vasquez, E.C.; Pereira, T.M.C.; Peotta, V.A.; Baldo, M.P.; Campos-Toimil, M. Review Article Probiotics as Beneficial Dietary Supplements to Prevent and Treat Cardiovascular Diseases: Uncovering Their Impact on Oxidative Stress. Oxid. Med. Cell. Longev. 2019, 2019, 3086270. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L. Annals of Internal Medicine Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Tomaino, L.; Dernini, S.; Berry, E.M.; Lairon, D.; de la Cruz, J.N.; Bach-Faig, A.; Donini, L.M.; Medina, F.X.; Belahsen, R.; et al. Updating the mediterranean diet pyramid towards sustainability: Focus on environmental concerns. Int. J. Environ. Res. Public Health 2020, 17, 8758. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian diet: An overview through the perspective of quality of life domains. Int. J. Environ. Res. Public Health 2021, 18, 4067. [Google Scholar] [CrossRef] [PubMed]
- New Trends in the Food Supplement Industry. Available online: https://natacgroup.com/news/new-trends-in-the-food-supplement-industry/ (accessed on 25 February 2021).
- Pey, J. ¿A qué nos referimos cuando hablamos de OTC y de EFP? Available online: https://www.clubdelafarmacia.com/para-estar-al-dia/el-blog-del-club/a-que-nos-referimos-cuando-hablamos-de-otc-y-de-efp/ (accessed on 25 February 2021).
- Petroczi, A.; Taylor, G.; Naughton, D.P. Mission impossible? Regulatory and enforcement issues to ensure safety of dietary supplements. Food Chem. Toxicol. 2011, 49, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (Cranberry)-based dietary supplements: Variation in mass uniformity, proanthocyanidin dosage and anthocyanin profile demonstrates quality control standard needed. Nutrients 2020, 12, 992. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Comm. 2002, L31, 1–24. [Google Scholar]
- European Commission. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the member states relating to food supplements. Off. J. Eur. Union 2000, L183, 51–57. [Google Scholar]
- Cerezo, A.B.; Leal, Á.; Álvarez-Fernández, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; García-Parrilla, M.C. Quality control and determination of melaonin in food supplements. J. Food Compos. Anal. 2016, 45, 80–86. [Google Scholar] [CrossRef]
- Agencia Española de Consumo y Seguridad Alimentaria y Nutrición (AECOSAN). Ministerio de Sanidad y Consumo de España. Programa 10. Control de Complementos Alimenticios: Notificación, Etiquetado y Composición. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/pncoca/P10_Complementos.pdf (accessed on 28 April 2021).
- Fibigr, J.; Šatínský, D.; Solich, P. Current trends in the analysis and quality control of food supplements based on plant extracts. Anal. Chim. Acta 2018, 1036, 1–15. [Google Scholar] [CrossRef]
- French Food Safety Agency. Food Supplements, the Need for Informed Consumption. 2021. Available online: https://www.anses.fr/en/content/food-supplements-need-informed-consumption (accessed on 28 April 2021).
- Food Standards Agency (UK). Food Supplements. What Food Supplements Are and What You Need to Do as a Business to Sell Them. 2018. Available online: https://www.food.gov.uk/business-guidance/food-supplements (accessed on 28 April 2021).
- BFR. Frequently Asked Questions on Food Supplements. 2018. Available online: https://www.bfr.bund.de/en/frequently_asked_questions_on_food_supplements-70347.html (accessed on 28 April 2021).
- Rocha, T.; Amaral, J.S.; Oliveira, M.B.P.P. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 43–62. [Google Scholar] [CrossRef]
- Hachem, R.; Assemat, G.; Martins, N.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J. Pharm. Biomed. Anal. 2016, 124, 34–47. [Google Scholar] [CrossRef]
- Sanzini, E.; Badea, M.; Dos Santos, A.; Restani, P.; Sievers, H. Quality control of plant food supplements. Food Funct. 2011, 2, 740–746. [Google Scholar] [CrossRef] [PubMed]
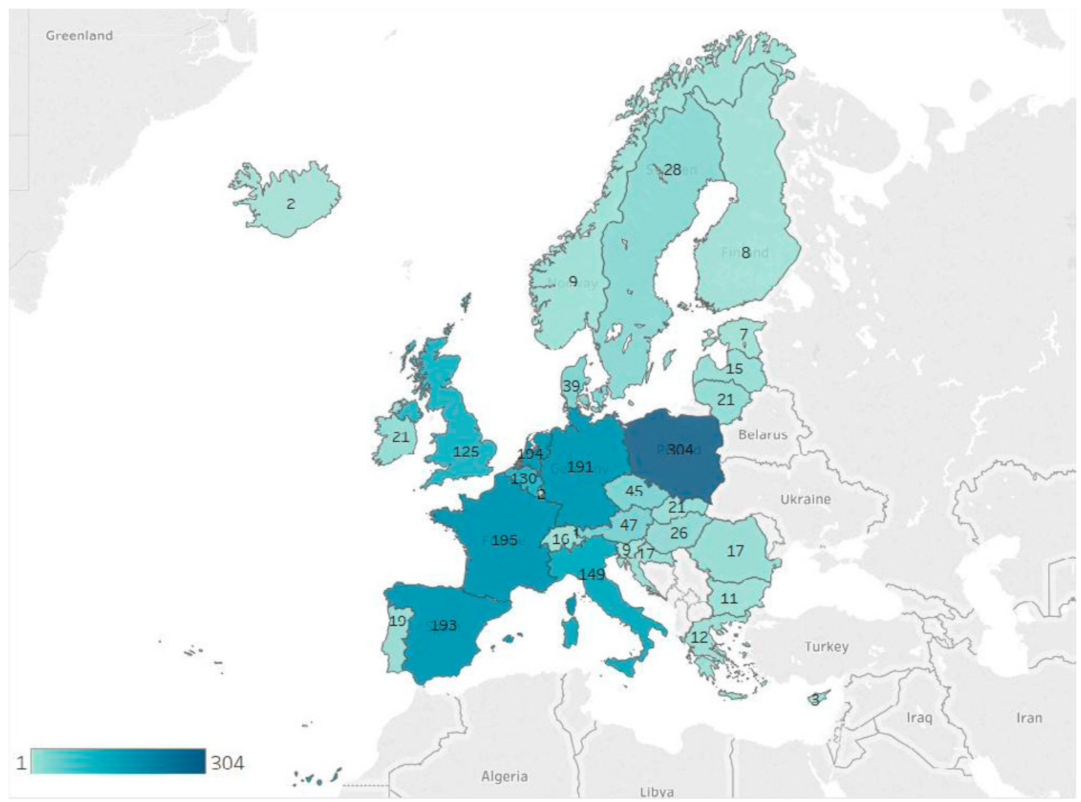
- European Commission. RASFF—Food and Feed Safety Alerts. 2021. Available online: https://ec.europa.eu/food/safety/rasff_en (accessed on 28 April 2021).
- European Commission. RASFF—The Rapid Alert System for Food and Feed—Annual Report 2019. 2020. Available online: https://op.europa.eu/en/publication-detail/-/publication/2c5c7729-0c31-11eb-bc07-01aa75ed71a1/language-en/format-PDF/source-174742448 (accessed on 28 April 2021).
- Gershwin, M.E.; Borchers, A.T.; Keen, C.L.; Hendler, S.; Hagie, F.; Greenwood, M.R.C. Public safety and dietary supplementation. Ann. N. Y. Acad. Sci. 2010, 1190, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Cassileth, B.R.; Heitzer, M.; Wesa, K. The public health impact of herbs and nutritional supplements. Pharm. Biol. 2009, 47, 761–767. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Thirumavalavan, N.; Srivatsav, A.; Yu, J.; Lipshultz, L.I.; Pastuszak, A.W. Testosterone imposters: An analysis of popular online testosterone boosting supplements. J. Sex. Med. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Rubio, C.; Dominik-Jakubiec, M.; Paz, S.; Gutiérrez, Á.J.; González-Weller, D.; Hardisson, A. Dietary exposure to trace elements (B, Ba, Li, Ni, Sr, and V) and toxic metals (Al, Cd, and Pb) from the consumption of commercial preparations of Spirulina platensis. Environ. Sci. Pollut. Res. 2021, 28, 22146–22155. [Google Scholar] [CrossRef]
- Starek, M.; Mierzwa, J.; Gumułka, P.; Dąbrowska, M. Vitamin D–current stage of knowledge about analysis and supplementation. Crit. Rev. Food Sci. Nutr. 2021, 62, 4607–4621. [Google Scholar] [CrossRef]
- Ichim, M.C.; de Boer, H.J. A Review of Authenticity and Authentication of Commercial Ginseng Herbal Medicines and Food Supplements. Front. Pharmacol. 2020, 11, 612071. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Zhang, C.; Zhang, B.; Wang, Z.; Zhu, L.; Mu, Y.; Qi, D. Effects of Dietary Cottonseed Oil and Cottonseed Meal Supplementation on Liver Lipid Content, Fatty Acid Profile and Hepatic Function in Laying Hens. Animals 2021, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: A systematic review and meta-analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef] [PubMed]
- Ucak, I.; Khalily, R.; Carrillo, C.; Tomasevic, I.; Barba, F.J. Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets. Foods 2020, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, L404, 9–25. [Google Scholar]
- EFSA (European Food Safety Authority). Outcome of a public consultation on the draft guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA Support. Publ. 2018, 15, 1364E. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, L136, 1–40. [Google Scholar]
- European Commission. Nutrition and Health Claims. 2021. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search (accessed on 25 January 2021).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to soy isoflavones and protection of DNA, proteins and lipids from oxidative damage (ID 1286, 4245), maintenance of normal blood LDL-cholesterol concentrations (ID 1135, 1704a, 3093a), reduction of vasomotor symptoms associated with menopause (ID 1654, 1704b, 2140, 3093b, 3154, 3590), maintenance of normal skin tonicity (ID 1704a), contribution to normal hair growth (ID 1704a, 4254), “cardiovascular health” (ID 3587), treatment of prostate cancer (ID 3588) and “upper respiratory tract” (ID 3589) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2264. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to niacin and energy-yielding metabolism (ID 43, 49, 54), function of the nervous system (ID 44, 53), maintenance of the skin and mucous membranes (ID 45, 48, 50, 52), maintenance of normal LDL-cholesterol, HDL cholesterol and triglyceride concentrations (ID 46), maintenance of bone (ID 50), maintenance of teeth (ID 50), maintenance of hair (ID 50, 2875) and maintenance of nails (ID 50, 2875) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1224. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to: Flavonoids and ascorbic acid in fruit juices, including berry juices (ID 1186); flavonoids from citrus (ID 1471); flavonoids from Citrus paradisi Macfad. (ID 3324, 3325); flavonoids (ID 1470, 1693, 1920); flavonoids in cranberry juice (ID 1804); carotenoids (ID 1496, 1621, 1622, 1796); polyphenols (ID 1636, 1637, 1640, 1641, 1642, 1643); rye bread (ID 1179); protein hydrolysate (ID 1646); carbohydrates with a low/reduced glycaemic load (ID 476, 477, 478, 479, 602) and carbohydrates which induce a low/reduced glycaemic response (ID 727, 1122, 1171); alfalfa (ID 1361, 2585, 2722, 2793); caffeinated carbohydrate-containing energy drinks (ID 1272); and soups (ID 1132, 1133) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2082. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin E and protection of DNA, proteins and lipids from oxidative damage (ID 160, 162, 1947), maintenance of the normal function of the immune system (ID 161, 163), maintenance of normal bone (ID 164), maintenance of normal teeth (ID 164), maintenance of normal hair (ID 164), maintenance of normal skin (ID 164), maintenance of normal nails (ID 164), maintenance of normal cardiac function (ID 166), maintenance of normal vision by protection of the lens of the eye (ID 167), contribution to normal cognitive function (ID 182, 183), regeneration of the reduced form of vitamin C (ID 203), maintenance of normal blood circulation (ID 216) and maintenance of normal a scalp (ID 2873) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1816. [Google Scholar] [CrossRef]
- Globe Newswire. Dietary Supplements Market Size, Share & Trends Analysis Report by Ingredient (Vitamins, Minerals), By Form, By Application, By End User, By Distribution Channel, By Region, and Segment Forecasts, 2020–2027. 2020. Available online: https://www.globenewswire.com/news-release/2020/03/05/1995948/0/en/Dietary-Supplements-Market-Size-Share-Trends-Analysis-Report-by-Ingredient-By-Form-By-Application-By-End-User-By-Distribution-Channel-By-Region-And-Segment-Forecasts-2020-2027.html (accessed on 21 January 2021).
- Portuguese Republic. Produtos-Fronteira Entre Suplementos Alimentares e Medicamentos 2009. Available online: https://www.infarmed.pt/documents/15786/17838/PRODUTOS+FRONTEIRA+SULEMENTOS+MEDICAMENTOS.pdf/d0cd8e0f-fad8-474b-85b4-b32c01fac5e9 (accessed on 25 January 2021).
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. The frontier between nutrition and pharma: The international regulatory framework of functional foods, food supplements and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2020, 60, 1738–1746. [Google Scholar] [CrossRef]
- China F.S.L. of the P.R. of Peoples Republic of China’ s Food Safety Law. 2015. Available online: https://www.hfgip.com/sites/default/files/law/food_safety_-_16.02.2016.pdf (accessed on 24 January 2021).
- Health Promotion Act. Act No. 103 of 2002 of Government of Japan. Available online: https://www.caa.go.jp/policies/policy/food_labeling/health_promotion/pdf/health_promotion_190509_0001.pdf (accessed on 24 January 2021).
- Therapeutic Goods Act 1989. Available online: https://www.legislation.gov.au/Details/C2019C00066 (accessed on 15 June 2021).
- Natural Health Products Regulations (SOR/2003-196). Available online: https://www.ecolex.org/details/legislation/natural-health-products-regulations-sor2003-196-lex-faoc115017/ (accessed on 24 January 2021).
- Díaz, L.D.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Rogerson, D.; Maçãs, D.; Milner, M.; Liu, Y.; Klonizakis, M. Contrasting effects of short-term Mediterranean and vegan diets on microvascular function and cholesterol in younger adults: A comparative pilot study. Nutrients 2018, 10, 1897. [Google Scholar] [CrossRef] [PubMed]
- Schuschke, D.A. Dietary copper in the physiology of the microcirculation. J. Nutr. 1997, 127, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Tyml, K. Vitamin C and microvascular dysfunction in systemic inflammation. Antioxidants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, G.; Tucker, A.T.; Harwood, S.M.; Pearse, R.M.; Raftery, M.J.; Yaqoob, M.M. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin D deficiency: An exploratory, double blind, randomised controlled trial. PLoS ONE 2014, 9, e99461. [Google Scholar] [CrossRef]
- Ralevic, V.; Milla, P.J.; Bumstock, G. Effects of chronic vitamin E deficiency on vascular function—A study of sympathetic nerves, smooth muscle and endothelium of the mesenteric arterial bed of the rat. Br. J. Pharmacol. 1995, 116, 2983–2988. [Google Scholar] [CrossRef][Green Version]
- Rorije, N.M.G.; Rademaker, E.; Schrooten, E.M.; Wouda, R.D.; Van Der Heide, J.J.H.; Van Den Born, B.J.H.; Vogt, L. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: A randomized cross-over trial. J. Hypertens. 2019, 37, 1254–1261. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef]
- Silva, H. The vascular effects of isolated isoflavones—A focus on the determinants of blood pressure regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef]
- Redman, D.A. Ruscus aculeatus (Butcher’s Broom) as a Potential Treatment for Orthostatic Hypotension, with a Case Report. J. Altern. Complementary Med. 2000, 6, 539–549. [Google Scholar] [CrossRef]
- De Almeida Cyrino, F.Z.G.; Balthazar, D.S.; Sicuro, F.L.; Bouskela, E. Effects of venotonic drugs on the microcirculation: Comparison between Ruscus extract and micronized diosmine. Clin. Hemorheol. Microcirc. 2018, 68, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bouskela, E.; Cyrino, F.Z.; Marcelon, G. Effects of Ruscus aculeatus on the internal diameter of arterioles and venules of the hamster cheek pouch microcirculation. J. Cardiovasc. Pharmacol. 1993, 22, 221–224. [Google Scholar] [CrossRef]
- Miller, V.M.; Rud, K.S.; Gloviczki, P. Pharmacological assessment of adrenergic receptors in human varicose veins. Int. Angiol. 2011, 19, 176–183. [Google Scholar]
- Lascasas-Porto, C.L.; Milhomens, A.L.M.; Virgini-Magalhães, C.E.; Fernandes, F.F.A.; Sicuro, F.L.; Bouskela, E. Use of microcirculatory parameters to evaluate clinical treatments of chronic venous disorder (CVD). Microvasc. Res. 2008, 76, 66–72. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Jost, V.; Wolna, P.; Lücker, P.W.; Müller, A.; Theurer, C.; Patz, B.; Grützner, K.I. Efficacy and safety of a Butcher’s broom preparation (Ruscus aculeatus L. extract) compared to placebo in patients suffering from chronic venous insufficiency. Arzneim. Forsch. Drug Res. 2002, 52, 243–250. [Google Scholar] [CrossRef]
- Pouget, G.; Ducros, L.; Marcelon, G. Effect of Ruscus Extract on Peripheral Lymphatic Vessel Pressure and Flow. In Return Circulation and Norepinephrine: An Update, Proceedings of the 3rd International Symposium held in Cairo, Egypt, 12–17 March 1990; Vanhoutte, P.M., Ed.; John Libbey Eurotext: Montrouge, France, 1991. [Google Scholar]
- Thomas, P.A.; Mukassabi, T.A. Biological flora of the British Isles: Ruscus aculeatus. J. Ecol. 2014, 102, 1083–1100. [Google Scholar] [CrossRef]
- Sadarmin, P.P.; Timperley, J. An unusual case of Butcher’s broom precipitating diabetic ketoacidosis. J. Emerg. Med. 2013, 45, e63–e65. [Google Scholar] [CrossRef] [PubMed]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32–41. [Google Scholar]
- Wang, J.; Xiong, X.; Feng, B. Effect of crataegus usage in cardiovascular disease prevention: An evidence-based approach. Evid. Based Complement. Altern. Med. 2013, 2013, 149363. [Google Scholar] [CrossRef]
- Nazhand, A.; Lucarini, M.; Durazzo, A.; Zaccardelli, M.; Cristarella, S.; Souto, S.B.; Silva, A.M.; Severino, P.; Souto, E.B.; Santini, A. Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests 2020, 11, 564. [Google Scholar] [CrossRef]
- Bubik, M.F.; Willer, E.A.; Bihari, P.; Jürgenliemk, G.; Ammer, H.; Krombach, F.; Zahler, S.; Vollmar, A.M.; Fürst, R. A novel approach to prevent endothelial hyperpermeability: The Crataegus extract WS® 1442 targets the cAMP/Rap1 pathway. J. Mol. Cell. Cardiol. 2012, 52, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Willer, E.A.; Malli, R.; Bondarenko, A.I.; Zahler, S.; Vollmar, A.M.; Graier, W.F.; Fürst, R. The vascular barrier-protecting hawthorn extract WS 1442 raises endothelial calcium levels by inhibition of SERCA and activation of the IP3 pathway. J. Mol. Cell. Cardiol. 2012, 53, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Brixius, K.; Willms, S.; Napp, A.; Tossios, P.; Ladage, D.; Bloch, W.; Mehlhorn, U.; Schwinger, R.H.G. Crataegus special extract WS® 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc. Drugs Ther. 2006, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kang, K.W.; Kim, K.W.; Kim, N.D. Procyanidins in crataegus extract evoke endothelium-dependent vasorelaxation in rat aorta. Life Sci. 2000, 67, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rieckeheer, E.; Schwinger, R.H.G.; Bloch, W.; Brixius, K. Hawthorn special extract WS® 1442 increases red blood cell NO-formation without altering red blood cell deformability. Phytomedicine 2011, 19, 20–24. [Google Scholar] [CrossRef]
- Miller, L.G. Herbal Medicinals. Arch. Intern. Med. 1998, 158, 2200. [Google Scholar] [CrossRef]
- Veveris, M.; Koch, E.; Chatterjee, S.S. Crataegus special extract WS® 1442 improves cardiac function and reduces infarct size in a rat model of prolonged coronary ischemia and reperfusion. Life Sci. 2004, 74, 1945–1955. [Google Scholar] [CrossRef]
- Al Makdessi, S.; Sweidan, H.; Dietz, K.; Jacob, R. Protective effect of Crataegus oxyacantha against reperfusion arrhythmias after global no-flow ischemia in the rat heart. Basic Res. Cardiol. 1999, 94, 71–77. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Devaraj, S.N. Cardioprotective effect of tincture of Crataegus on isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2010, 56, 921–926. [Google Scholar] [CrossRef]
- Kashyap, C.P.; Arya, V.; Thakur, N. Ethnomedicinal and phytopharmacological potential of Crataegus oxyacantha Linn.—A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1194–S1199. [Google Scholar] [CrossRef]
- Schmidt, U.; Kuhn, U.; Ploch, M.; Hübner, W.D. Efficacy of the Hawthorn (Crataegus) preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Händel, M. Crataegus, toxicology and pharmacology, Part I: Toxicity. Planta Med. 1981, 43, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.; Händel, M. Crataegus, toxicology and pharmacology, Part II: Pharmacodynamics. Planta Med. 1981, 43, 209–239. [Google Scholar] [CrossRef]
- Ammon, H.P.; Händel, M. Crataegus, toxicology and pharmacology, Part III: Pharmacodynamics and pharmacokinetics. Planta Med. 1981, 43, 313–322. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 75, 546–556. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; De Sanctis, M.T.; Incandela, L.; Cacchio, M.; Bavera, P.; Ippolito, E.; Bucci, M.; Griffin, M.; Geroulakos, G.; et al. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: A prospective, placebo-controlled, randomized trial. Angiology 2001, 52, 15–18. [Google Scholar] [CrossRef]
- Incandela, L.; Cesarone, M.R.; Cacchio, M.; Sanctis, M.T. De Total Triterpenic Fraction of Centella asiatica in Chronic Venous Insufficiency and in High-Perfusion Microangiopathy. Angiology 2001, 52, 9–13. [Google Scholar] [CrossRef]
- Bian, D.; Liu, M.; Li, Y.; Xia, Y.; Gong, Z.; Dai, Y. Madecassoside, a Triterpenoid Saponin Isolated from Centella asiatica Herbs, Protects Endothelial Cells Against Oxidative Stress. J. Biochem. Mol. Toxicol. 2012, 26, 399–406. [Google Scholar] [CrossRef]
- Raghavendra, M.; Maiti, R.; Kumar, S.; Trigunayat, A.; Mitra, S.; Acharya, S. Role of Centella asiatica on cerebral post-ischemic reperfusion and long-term hypoperfusion in rats. Int. J. Green Pharm. 2009, 3, 88–96. [Google Scholar] [CrossRef]
- Chandrika, U.G.; Prasad Kumara, P.A.A.S. Gotu Kola (Centella asiatica): Nutritional Properties and Plausible Health Benefits, 1st ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 76, ISBN 9780128036068. [Google Scholar]
- Izu, R.; Aguirre, A.; Gil, N.; Diaz-Perez, J.L. Allergic contact dermatitis from a cream containing Centella asiatica extract. Contact Dermat. 1992, 26, 192–213. [Google Scholar] [CrossRef]
- Yunianto, I.; Das, S.; Noor, M.M. Antispermatogenic and antifertility effect of Pegaga (Centella asiatica L) on the testis of male Sprague-Dawley rats. Clin. Ther. 2006, 161, 235–239. [Google Scholar]
- Oruganti, M.; Roy, B.K.; Singh, K.K.; Prasad, R.; Kumar, S. Safety assemment of Centella asiatica in albino rats. Pharmacogn. J. 2010, 2, 5–13. [Google Scholar] [CrossRef]
- Jorge, O.A.; Jorge, A.D. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev. Esp. Enferm. Dig. 2005, 97, 115–124. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y. Current Evaluation of the Millennium Phytomedicine-Ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and Regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, J.B.; Kim, K.J.; Chang, S.J.; Ryoo, S.W.; Jeon, B.H. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol. Sin. 2002, 23, 1152–1156. [Google Scholar]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective effects of ginsenosides against cerebral ischemia. Molecules 2019, 24, 1108. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-H.; Kim, T.-S.; Chung, H. The Protective Effect of Orally Ingested Korean Red Ginseng on the Noise Induced Hearing Loss in Mice. J. Ginseng Res. 2009, 33, 104–110. [Google Scholar]
- Sin, G.C.; Lee, S.H.; Nam, B.H.; Park, C.I.; Nam, K.Y. Effect of Korean Red Ginseng Saponin on Cochlear Damage Induced by Noise Exposure. Korean J. Otorhinolaryngol. Neck Surg. 2000, 43, 804–807. [Google Scholar]
- Fujita, K.; Hakuba, N.; Hata, R.; Morizane, I.; Yoshida, T.; Shudou, M.; Sakanaka, M.; Gyo, K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci. Lett. 2007, 415, 113–117. [Google Scholar] [CrossRef]
- Yang, F.; Ma, Q.; Matsabisa, M.G.; Chabalala, H.; Braga, F.C.; Tang, M. Panax notoginseng for cerebral Ischemia: A systematic review. Am. J. Chin. Med. 2020, 48, 1331–1351. [Google Scholar] [CrossRef]
- Sun, T.; Wang, P.; Deng, T.; Tao, X.; Li, B.; Xu, Y. Effect of Panax notoginseng Saponins on Focal Cerebral Ischemia-Reperfusion in Rat Models: A Meta-Analysis. Front. Pharmacol. 2021, 11, 572304. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Wang, S.X.; Chai, L.J.; Zhang, Y.; Guo, H.; Hu, L.M. Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress. Acta Pharmacol. Sin. 2018, 39, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Sun, K.; Wang, C.S.; Guo, J.; Xue, X.; Liu, Y.Y.; Zheng, J.; Fan, J.Y.; Liao, F.L.; Han, J.Y. Improving effect of post-treatment with Panax notoginseng saponins on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Clin. Hemorheol. Microcirc. 2008, 40, 119–131. [Google Scholar] [CrossRef]
- Sun, K.; Wang, C.S.; Guo, J.; Horie, Y.; Fang, S.P.; Wang, F.; Liu, Y.Y.; Liu, L.Y.; Yang, J.Y.; Fan, J.Y.; et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007, 81, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, S.Y.; Kang, C.W.; Yoon, I.S.; Lee, J.H.; Jeong, S.M.; Lee, B.H.; Lee, J.H.; Pyo, M.K.; Choi, S.H.; et al. Ginseng saponins diminish adverse vascular effects associated with chronic methionine-induced hyperhomocysteinemia. Biol. Pharm. Bull. 2006, 29, 2425–2431. [Google Scholar] [CrossRef]
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2005, 41, 861–868. [Google Scholar] [CrossRef]
- Lan, T.H.; Xu, Z.W.; Wang, Z.; Wu, Y.L.; Wu, W.K.; Tan, H.M. Ginsenoside Rb1 prevents homocysteine-induced endothelial dysfunction via PI3K/Akt activation and PKC inhibition. Biochem. Pharmacol. 2011, 82, 148–155. [Google Scholar] [CrossRef]
- Yun, T.K.; Lee, Y.S.; Lee, Y.H.; Kim, S.I.; Yun, H.Y. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J. Korean Med. Sci. 2001, 16, S6–S18. [Google Scholar] [CrossRef]
- Duda, R.B.; Zhong, Y.; Navas, V.; Li, M.Z.C.; Toy, B.R.; Alavarez, J.G. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J. Surg. Oncol. 1999, 72, 230–239. [Google Scholar] [CrossRef]
- Lee, H.; Park, D.; Yoon, M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet-induced obese C57BL/6J mice. Food Chem. Toxicol. 2013, 53, 402–408. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M.; Shik Shin, S.; Yoon, M. Ginseng treatment reverses obesity and related disorders by inhibiting angiogenesis in female db/db mice. J. Ethnopharmacol. 2014, 155, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, W.; Li, S.; Nagarkatti, P.; Nagarkatti, M.; Windust, A.; Wang, X.L.; Tang, D.; Cui, T. American ginseng inhibits vascular smooth muscle cell proliferation via suppressing Jak/Stat pathway. J. Ethnopharmacol. 2012, 144, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, Y.; Ahn, S.; Doo, H.; Lee, B. Interaction Between Warfarin and Panax ginseng in Ischemic Stroke Patients. J. Altern. Complement. Med. 2008, 14, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.M.; Hong, S.J.; Choi, S.C.; Park, J.H.; Kim, J.S.; Lim, D.S. Red ginseng extract improves coronary flow reserve and increases absolute numbers of various circulating angiogenic cells in patients with first ST-segment elevation acute myocardial infarction. Phyther. Res. 2011, 25, 239–249. [Google Scholar] [CrossRef]
- Sengupta, S.; Toh, S.A.; Sellers, L.A.; Skepper, J.N.; Koolwijk, P.; Leung, H.W.; Yeung, H.W.; Wong, R.N.S.; Sasisekharan, R.; Fan, T.P.D. Modulating angiogenesis: The yin and the yang in ginseng. Circulation 2004, 110, 1219–1225. [Google Scholar] [CrossRef]
- Lee, K.-H.; Wang, H.-K.; Itokawa, H.; Morris-Natschke, S.L. Current perspectives on Chinese medicines and dietary supplements in China, Japan and the United States. J. Food Drug Anal. 2000, 8, 219–228. [Google Scholar] [CrossRef]
- Coon, J.T.; Ernst, E. Panax ginseng A Systematic Review of Adverse Effects and Drug Interactions. Drug Saf. 2002, 25, 323–344. [Google Scholar] [CrossRef]
- Vogler, B.K.; Pittler, M.H.; Ernst, E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur. J. Clin. Pharmacol. 1999, 55, 567–575. [Google Scholar] [CrossRef]
- Ernst, E. The Risk–Benefit Profile of Commonly Used Herbal Therapies: Ginkgo, St. John’s Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann. Intern. Med. 2002, 136, 42–53. [Google Scholar] [CrossRef]
- Wilkinson, J.A.; Brown, A.M.G. Horse chestnut—Aesculus hippocastanum: Potential applications in cosmetic skin-care products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef]
- Baróniková, S.; Apers, S.; Vanden Berghe, D.; Cos, P.; Vermeulen, P.; Van Daele, A.; Pieters, L.; Van Marck, E.; Vlietinck, A. An ex-vivo angiogenesis assay as a screening method for natural compounds and herbal drug preparations. Planta Med. 2004, 70, 887–892. [Google Scholar] [CrossRef]
- Arnould, T.; Janssens, D.; Michiels, C. Effect of aescine on hypoxia-induced activation of human endothelial cells. Eur. J. Pharmacol. 1996, 315, 227–233. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E.; Del Maschio, A. Platelet-neutrophil interactions. Possible relevance in the pathogenesis of thrombosis and inflammation. Haematologica 1991, 76, 491–499. [Google Scholar]
- Longiave, D.; Omini, C.; Nicosia, S.; Berti, F. The mode of action of aescin on isolated veins: Relationship with PGF2 alpha. Pharmacol. Res. Commun. 1978, 10, 145–152. [Google Scholar] [CrossRef]
- Sirtori, C.R. Aescin: Pharmacology, pharmacokinetics and therapeutic profile. Pharmacol. Res. 2001, 44, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Padioleau, F. Veinotonic effect, vascular protection, antiinflammatory and free radical scavenging properties of horse chestnut extract. Arzneimittelforschung 1994, 44, 25–35. [Google Scholar]
- Facino, R.M.; Carini, M.; Stefani, R.; Aldini, G.; Saibene, L. Anti-elastase and anti-hyaluronidase activities of saponins and sapogenins from Hedera helix, Aesculus hippocastanum, and Ruscus aculeatus: Factors contributing to their efficacy in the treatment of venous insufficiency. Arch. Pharm. 1995, 328, 720–724. [Google Scholar] [CrossRef]
- Suter, A. Treatment of patients with venous insufficiency with fresh plant horse chestnut seed extract. Adv. Ther. 2006, 23, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.J.; Pincombe, J.; Foster, G. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. J. Wound Care 2006, 15, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Horse chestnut seed extract for venous insufficiency. Altern. Ther. Women’s Health 2007, 9, 25–27. [Google Scholar]
- Ezberci, F.; Ünal, E. Aesculus hippocastanum (Aescin, Horse Chestnut) in the Management of Hemorrhoidal Disease: Review. Turk. J. Colorectal Dis. 2018, 28, 54–57. [Google Scholar] [CrossRef]
- Hu, S.; Belcaro, G.; Dugall, M.; Hosoi, M.; Togni, S.; Maramaldi, G.; Giacomelli, L. Aescin-based topical formulation to prevent foot wounds and ulcerations in diabetic microangiopathy. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4337–4342. [Google Scholar] [PubMed]
- European Medicines Agency. European Union Herbal Monograph on Aesculus hippocastanum L., Semen. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/european-union-herbal-monograph-aesculus-hippocastanum-l-semen-final-revision-1_en.pdf (accessed on 10 June 2021).
- Daz-Gonzlez, M.; Rocasalbas, G.; Francesko, A.; Tourio, S.; Torres, J.L.; Tzanov, T. Inhibition of deleterious chronic wound enzymes with plant polyphenols. Biocatal. Biotransform. 2012, 30, 102–110. [Google Scholar] [CrossRef]
- Mackay, D. Hemorrhoids and Varicose Veins: A Review of Treatment Options. Altern. Med. Rev. 2001, 6, 126–140. [Google Scholar]
- Gami, B. Hemorrhoids—A common ailment among adults, causes & treatment: A review. Int. J. Pharm. Pharm. Sci. 2011, 3, 5–12. [Google Scholar]
- Trüeb, R.M. North American Virginian Witch Hazel (Hamamelis virginiana): Based Scalp Care and Protection for Sensitive Scalp, Red Scalp, and Scalp Burn-Out. Int. J. Trichology 2014, 6, 100–103. [Google Scholar] [CrossRef]
- Qinna, N.A. Safety profile of suppository Hamamelis virginiana leaf extract. J. Med. Plants Res. 2013, 7, 2669–2679. [Google Scholar] [CrossRef]
- Korting, H.C.; Schäfer-Korting, M.; Hart, H.; Laux, P.; Schmid, M. Anti-inflammatory activity of hamamelis distillate applied topically to the skin—Influence of vehicle and dose. Eur. J. Clin. Pharmacol. 1993, 44, 315–318. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Hamamelis virginiana L., Cortex, Hamamelis virginiana L. Folium, Hamamelis virginiana L., Folium et Cortex aut Ramunculus Destillatum; European Medicines Agency: London, UK, 2009.
- Arroyo Garcia, R.A.; Revill, E. The Current Status of Wild Grapevine Populations (Vitis vinifera ssp sylvestris) in the Mediterranean Basin. In The Mediterranean Genetic Code—Grapevine and Olive; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Ardid-Ruiz, A.; Harazin, A.; Barna, L.; Walter, F.R.; Bladé, C.; Suárez, M.; Deli, M.A.; Aragonès, G. The effects of Vitis vinifera L. phenolic compounds on a blood-brain barrier culture model: Expression of leptin receptors and protection against cytokine-induced damage. J. Ethnopharmacol. 2020, 247, 112253. [Google Scholar] [CrossRef]
- Lin, L.; Wang, P.; Wang, Y.; Huang, Y.; Jiang, L.; Wang, X. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol. Med. Rep. 2015, 13, 1070–1076. [Google Scholar] [CrossRef]
- Agarwal, C.; Singh, R.P.; Dhanalakshmi, S.; Agarwal, R. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol. Rep. 2004, 11, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Marabini, L.; Lombardo, G.; Cornaghi, L.; Piazza, S.; Marinovich, M.; Donetti, E. Protective effect by Vitis vinifera L. extract after UVA irradiation in human endothelial cells EAhy.926: Genotoxical and morphological analysis. Ital. J. Anat. Embryol. 2018, 123, 134. [Google Scholar]
- Naseri, M.K.G.; Hamidi, M.N.; Heidari, A. Vasorelaxatory Effect of Vitis vinifera Extract on Rat Aorta Mohammad. Iran. J. Pharm. Res. 2005, 2, 93–99. [Google Scholar]
- Nematbakhsh, M.; Zolfaghari, B.; Eshraghi, F.; Safari, T.; Pezeshki, Z.; Sorooshzadeh, M.-A.S. The effects of unripe grape extract on systemic blood pressure, nitric oxide production, and response to angiotensin II administration. Pharmacogn. Res. 2013, 5, 60–64. [Google Scholar] [CrossRef]
- Fernandes, F.; Pereira, A.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crop. Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Seo, M.G.; Jo, M.J.; Hong, N.I.; Kim, M.J.; Shim, K.S.; Shin, E.; Lee, J.J.; Park, S.J. Anti-Inflammatory and Anti-Vascular Leakage Effects by Combination of Centella asiatica and Vitis vinifera L. Leaf Extracts. Evid. Based Complement. Altern. Med. 2021, 2021, 7381620. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf extract inhibits in vitro mediators of inflammation and oxidative stress involved in inflammatory-based skin diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Vitis vinifera L., Folium; European Medicines Agency: London, UK, 2019.
- Isah, T. Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharmacogn. Rev. 2015, 9, 140–148. [Google Scholar] [CrossRef]
- Ou, H.C.; Lee, W.J.; Lee, I.T.; Chiu, T.H.; Tsai, K.L.; Lin, C.Y.; Sheu, W.H.H. Ginkgo biloba extract attenuates oxLDL-induced oxidative functional damages in endothelial cells. J. Appl. Physiol. 2009, 106, 1674–1685. [Google Scholar] [CrossRef]
- Cheung, F.; Siow, Y.L.; Chen, W.Z.; Karmin, O. Inhibitory effect of Ginkgo biloba extract on the expression of inducible nitric oxide synthase in endothelial cells. Biochem. Pharmacol. 1999, 58, 1665–1673. [Google Scholar] [CrossRef]
- Dong, X.X.; Hui, Z.J.; Xiang, W.X.; Rong, Z.F.; Jian, S.; Zhu, C.J. Ginkgo biloba Extract Reduces Endothelial Progenitor-Cell Senescence Through Augmentation of Telomerase Activity. J. Cardiovasc. Pharmacol. 2007, 49, 111–115. [Google Scholar] [CrossRef]
- Tisato, V.; Zauli, G.; Rimondi, E.; Gianesini, S.; Brunelli, L.; Menegatti, E.; Zamboni, P.; Secchiero, P. Inhibitory effect of natural anti-inflammatory compounds on cytokines released by chronic venous disease patient-derived endothelial cells. Mediat. Inflamm. 2013, 2013, 423407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jung, F.; Mrowietz, C.; Kiesewetter, H.; Wenzel, E. Effect of Ginkgo biloba on fluidity of blood and peripheral microcirculation in volunteers. Arzneimittelforschung 1990, 40, 589–593. [Google Scholar] [PubMed]
- Mehlsen, J.; Drabæk, H.; Wiinberg, N.; Winther, K. Effects of a Ginkgo biloba extract on forearm haemodynamics in healthy volunteers. Clin. Physiol. Funct. Imaging 2002, 22, 375–378. [Google Scholar] [CrossRef]
- Boelsma, E.; Lamers, R.J.A.N.; Hendriks, H.F.J.; Van Nesselrooij, J.H.J.; Roza, L. Evidence of the regulatory effect of Ginkgo biloba extract on skin blood flow and study of its effects on urinary metabolites in healthy humans. Planta Med. 2004, 70, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Wimpissinger, B.; Berisha, F.; Garhoefer, G.; Polak, K.; Schmetterer, L. Influence of Ginkgo biloba on ocular blood flow. Acta Ophthalmol. Scand. 2007, 85, 445–449. [Google Scholar] [CrossRef]
- Lee, E.J.; Chen, H.Y.; Wu, T.S.; Chen, T.Y.; Ayoub, I.A.; Maynard, K.I. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague-Dawley rats. J. Neurosci. Res. 2002, 68, 636–645. [Google Scholar] [CrossRef]
- Park, J.W.; Kwon, H.J.; Chung, W.S.; Kim, C.Y.; Seong, G.J. Short-term effects of Ginkgo biloba extract on peripapillary retinal blood flow in normal tension glaucoma. Korean J. Ophthalmol. 2011, 25, 323–328. [Google Scholar] [CrossRef]
- Huang, S.Y.; Jeng, C.; Kao, S.C.; Yu, J.J.H.; Liu, D.Z. Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin. Nutr. 2004, 23, 615–621. [Google Scholar] [CrossRef]
- Galduróz, J.C.F.; Antunes, H.K.; Santos, R.F. Gender- and age-related variations in blood viscosity in normal volunteers: A study of the effects of extract of Allium sativum and Ginkgo biloba. Phytomedicine 2007, 14, 447–451. [Google Scholar] [CrossRef]
- Didier, A.; Droy-Lefaix, M.-T.; Aurousseau, C.; Cazals, Y. Effects of Ginkgo biloba extract (EGb 761) on cochlear vasculature in the guinea pig: Morphometric measurements and laser Doppler flowmetry. Eur. Arch. Otorhinolaryngol. 1996, 253, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Cho, Y.B.; Kim, J.S.; Cho, S.W.; Yang, H.C.; Jung, K.H.; Kim, J.Y.; Choi, C.H.; Lim, Y.; Park, H.; et al. Effect of Ginkgo biloba extract on endotoxin-induced labyrinthitis. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, J.; Beck, T.; Seibert, A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 2013, 39, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, A.; Pham, D.L.; Yousem, D.M.; Dizon, M.; Barker, P.B.; Lin, D.D.M. Effects of Ginkbo biloba on cerebral blood flow assesses by quantitative MR perfusion imaging: A pilot study. Neuroradiology 2011, 53, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, D.; Zhu, H.; Wang, X. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012, 87, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Xue, Z.Y. Effect of Western medicine therapy assisted by Ginkgo biloba tablet on vascular cognitive impairment of none dementia. Asian Pac. J. Trop. Med. 2012, 5, 661–664. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Cui, W.; Zu, X.; Du, J.; Wang, F. Ginkgo biloba extract improves coronary blood flow in healthy elderly adults: Role of endothelium-dependent vasodilation. Phytomedicine 2008, 15, 164–169. [Google Scholar] [CrossRef]
- Sun, B.-L.; Zhang, J.; Wang, X.-C.; Xia, Z.-L.; Yang, M.-F.; Zhang, S.-M.; Ye, W.-J.; Yuan, H. Effects of extract of Ginkgo biloba on spasms of the basilar artery and cerebral microcirculatory perfusion in rats with subarachnoid hemorrhage. Clin. Hemorheol. Microcirc. 2003, 29, 231–238. [Google Scholar]
- Mansour, S.M.; Bahgat, A.K.; El-Khatib, A.S.; Khayyal, M.T. Ginkgo biloba extract (EGb 761) normalizes hypertension in 2K, 1C hypertensive rats: Role of antioxidant mechanisms, ACE inhibiting activity and improvement of endothelial dysfunction. Phytomedicine 2011, 18, 641–647. [Google Scholar] [CrossRef]
- Kubota, Y.; Tanaka, N.; Kagota, S.; Nakamura, K.; Kunitomo, M.; Shinozuka, K.; Umegaki, K. Effects of Ginkgo biloba extract on blood pressure and vascular endothelial response by acetylcholine in spontaneously hypertensive rats. J. Pharm. Pharmacol. 2010, 58, 243–249. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Lovato, J.F.; Arnold, A.M.; Furberg, C.D.; Kuller, L.H.; Burke, G.L.; Nahin, R.L.; Lopez, O.L.; Yasar, S.; Williamson, J.D. Effect of Ginkgo biloba on blood pressure and incidence of hypertension in elderly men and women. Am. J. Hypertens. 2010, 23, 528–533. [Google Scholar] [CrossRef] [PubMed]
- MacVie, O.P.; Harney, B.A. Vitreous haemorrhage associated with Gingko biloba use in a patient with age related macular disease. Br. J. Ophthalmol. 2005, 89, 1378. [Google Scholar] [CrossRef][Green Version]
- Pedroso, J.L.; Henriques Aquino, C.C.; Escórcio Bezerra, M.L.; Baiense, R.F.; Suarez, M.M.; Dutra, L.A.; Braga-Neto, P.; Povoas Barsottini, O.G. Ginkgo biloba and cerebral bleeding: A case report and critical review. Neurologist 2011, 17, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G. Possible Subdural Hematoma Associated with Ginkgo biloba. J. Herb. Pharmacother. 2002, 2, 57–63. [Google Scholar] [CrossRef]
- Xia, S.; Fang, D. Pharmacological action and mechanisms of ginkgolide B. Chin. Med. J. 2007, 120, 922–928. [Google Scholar] [CrossRef]
- Pennisi, R.S. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med. J. Aust. 2006, 184, 583–584. [Google Scholar] [CrossRef]
- Yuste, M.; Sánchez-Estella, J.; Santos, J.C.; Alonso, M.T.; Bordel, M.T.; Gutiérrez, J.L.; Zamora, T. Síndrome de Stevens-Johnson/necrolisis epidérmica tóxica tratado con inmunoglobulinas intravenosas. Actas Dermosifiliogr. 2005, 96, 589–592. [Google Scholar] [CrossRef]
- Cianfrocca, C.; Pelliccia, F.; Auriti, A.; Santini, M. Ginkgo biloba-induced frequent ventricular arrhythmia. Ital. Heart J. 2002, 3, 689–691. [Google Scholar]
- Miwa, H.; Iijima, M.; Tanaka, S.; Mizuno, Y. Generalized convulsions after consuming a large amount of Gingko nuts. Epilepsia 2001, 42, 280–281. [Google Scholar] [CrossRef]
- Bebbington, A.; Kulkarni, R.; Roberts, P. Ginkgo biloba: Persistent bleeding after total hip arthroplasty caused by herbal self-medication. J. Arthroplast. 2005, 20, 125–126. [Google Scholar] [CrossRef]
- Varona, F.J.C.; Morales, M.P.A. Ginkgo biloba and cerebral hemorrhage. An. Med. Interna 2005, 22, 199. [Google Scholar]
- Meisel, C.; Johne, A.; Roots, I. Fatal intracerebral mass bleeding associated with Ginkgo biloba and ibuprofen. Atherosclerosis 2003, 167, 367. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, B.; Andersen, S.; Dalhoff, K. Drug-drug interactions among recently hospitalised patients—Frequent but mostly clinically insignificant. Eur. J. Clin. Pharmacol. 2005, 61, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, T.; Raj, V. Fatal seizures due to potential herb-drug interactions with Ginkgo biloba. J. Anal. Toxicol. 2005, 29, 755–758. [Google Scholar] [CrossRef]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, G.-R.; Ye, T.-X.; Yuan, Y.-J. Salvia miltiorrhiza protects endothelial cells against oxidative stress. J. Altern. 2006, 12, 5–6. [Google Scholar] [CrossRef]
- Chan, K.; Chui, S.H.; Wong, D.Y.L.; Ha, W.Y.; Chan, C.L.; Wong, R.N.S. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci. 2004, 75, 3157–3171. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ye, T.X.; Zhao, G.R.; Yuan, Y.J.; Guo, Z.X. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-α. Int. Immunopharmacol. 2005, 5, 1641–1651. [Google Scholar] [CrossRef]
- Ren, D.C.; Du, G.H.; Zhang, J.T. Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn. J. Pharmacol. 2002, 90, 276–280. [Google Scholar] [CrossRef]
- Sun, C.; Su, S.; Zhu, Y.; Guo, J.; Guo, S.; Qian, D.; Yu, L.; Gu, W.; Duan, J. Salvia miltiorrhiza stem-leaf active components of salvianolic acids and flavonoids improved the hemorheological disorder and vascular endothelial function on microcirculation dysfunction rats. Phytother. Res. 2020, 34, 1704–1720. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Dong, X.L.; Fan, X.D.; Wu, J.H.; Wang, Q.H.; Tian, X.L.; Guo, D.J.; Wong, M.S.; Qiu, T.Q.; Chan, S.W. Aqueous extract of danshen (Salvia miltiorrhiza Bunge) protects ovariectomized rats fed with high-fat diet from endothelial dysfunction. Menopause 2013, 20, 100–109. [Google Scholar] [CrossRef]
- Lao, C.J.; Lin, J.G.; Kuo, J.S.; Chiang, S.Y.; Chen, S.C.; Liao, E.T.; Hsieh, C.L. Effect of Salvia miltiorrhiza Bunge on cerebral infarct in ischemia-reperfusion injured rats. Am. J. Chin. Med. 2003, 31, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.O. Cardiovascular effects of Danshen. Int. J. Cardiol. 2007, 121, 9–22. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, R.; Li, B.; Gu, L.Y.; Gou, H. An in vivo and in vitro study: High-dosage Danshen injection induces peripheral vascular endothelial cells injury. Hum. Exp. Toxicol. 2016, 35, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Saurabh, V.; Tomar, M.; Hasan, M.; Changan, S.; Sasi, M.; Maheshwari, C.; Prajapati, U.; Singh, S.; Prajapat, R.K.; et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants 2021, 10, 299. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Gerstgrasser, A.; Röchter, S.; Dressler, D.; Schön, C.; Reule, C.; Buchwald-Werner, S. In Vitro Activation of eNOS by Mangifera indica (CarelessTM) and Determination of an Effective Dosage in a Randomized, Double-Blind, Human Pilot Study on Microcirculation. Planta Med. 2015, 82, 298–304. [Google Scholar] [CrossRef]
- Buchwald-Werner, S.; Schön, C.; Frank, S.; Reule, C. Effects of Mangifera indica (Careless) on Microcirculation and Glucose Metabolism in Healthy Volunteers. Planta Med. 2017, 83, 824–829. [Google Scholar] [CrossRef]










| Authorized Health Claims | Qualified Health Claims |
|---|---|
| Dietary saturated fat and cholesterol and risk of coronary heart disease | Whole grain foods with moderate fat content and risk of heart disease |
| Fruit, vegetables, and grain products that contain fiber, particularly soluble fiber, and risk of coronary heart disease | Saturated fat, cholesterol, and trans fat, and reduced risk of heart disease |
| Soluble fiber from certain foods and risk of coronary heart disease | Substitution of saturated fat in diet for unsaturated fatty acids and reduced risk of heart disease |
| Soy protein and risk of coronary heart disease | B vitamins and vascular disease |
| Plant sterol/stanol esters and risk of coronary heart disease | Nuts and heart disease |
| Walnuts and heart disease | |
| Omega 3 fatty acids and coronary heart disease | |
| Monounsaturated fatty acids from olive oil and coronary heart disease | |
| Unsaturated fatty acids from canola oil and reduced risk of coronary heart disease | |
| Corn oil and corn oil-containing products and a reduced risk of heart disease |
| Nutrient, Substance, Food, or Food Category | Claim | Ref |
|---|---|---|
| Dry isoflavones soy Extract | Acts on hair bulb to support hair growth. Prevents hair from premature aging via antioxidant properties and microcirculation. | [56] |
| Vitamin B3 | Activates scalp microcirculation. | [57] |
| Bioflavonoids | It has a positive effect on microcirculatory tropism by favoring the processes that protect small venous vessels. It protects the body from the harmful action of free radicals and skin from ultraviolet rays. | [58] |
| Vitamin E acetate (D,L alpha tocopherol acetate) | It supports microcirculation and scalp oxygenation. | [59] |
| OPC Plus, containing 40 mg oligomeric procyanidins (OPC) and 40 mg berry blend per capsule | OPC Plus has been shown to increase microcirculation and may, therefore, reduce the risk of chronic venous insufficiency. | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raposo, A.; Saraiva, A.; Ramos, F.; Carrascosa, C.; Raheem, D.; Bárbara, R.; Silva, H. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology 2021, 10, 616. https://doi.org/10.3390/biology10070616
Raposo A, Saraiva A, Ramos F, Carrascosa C, Raheem D, Bárbara R, Silva H. The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology. 2021; 10(7):616. https://doi.org/10.3390/biology10070616
Chicago/Turabian StyleRaposo, António, Ariana Saraiva, Fernando Ramos, Conrado Carrascosa, Dele Raheem, Rita Bárbara, and Henrique Silva. 2021. "The Role of Food Supplementation in Microcirculation—A Comprehensive Review" Biology 10, no. 7: 616. https://doi.org/10.3390/biology10070616
APA StyleRaposo, A., Saraiva, A., Ramos, F., Carrascosa, C., Raheem, D., Bárbara, R., & Silva, H. (2021). The Role of Food Supplementation in Microcirculation—A Comprehensive Review. Biology, 10(7), 616. https://doi.org/10.3390/biology10070616











