Modulation of Stem Cell Progeny by Probiotics during Regeneration of Gastric Mucosal Erosions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design
2.3. Macroscopic Assessment of Gastric Erosions
2.4. Microscopic Assessments of Gastric Mucosal Tissues
2.5. Quantification
2.6. Statistical Analyses
3. Results
3.1. Effects of DSF on Gastric Erosions Induced by a Single Gavage of ASA-Containing Ethanol
3.1.1. Probiotics Attenuate Induction of Gastric Mucosal Erosions
3.1.2. DSF-Pretreatment Stimulates Gastric Stem/Progenitor Cell Proliferation
3.1.3. DSF-Pretreatment Restores Normal Level of H+,K+-ATPase in Parietal Cells
3.1.4. DSF-Pretreatment Increases Lectin- and Immuno-Labeling of Mucous Cells
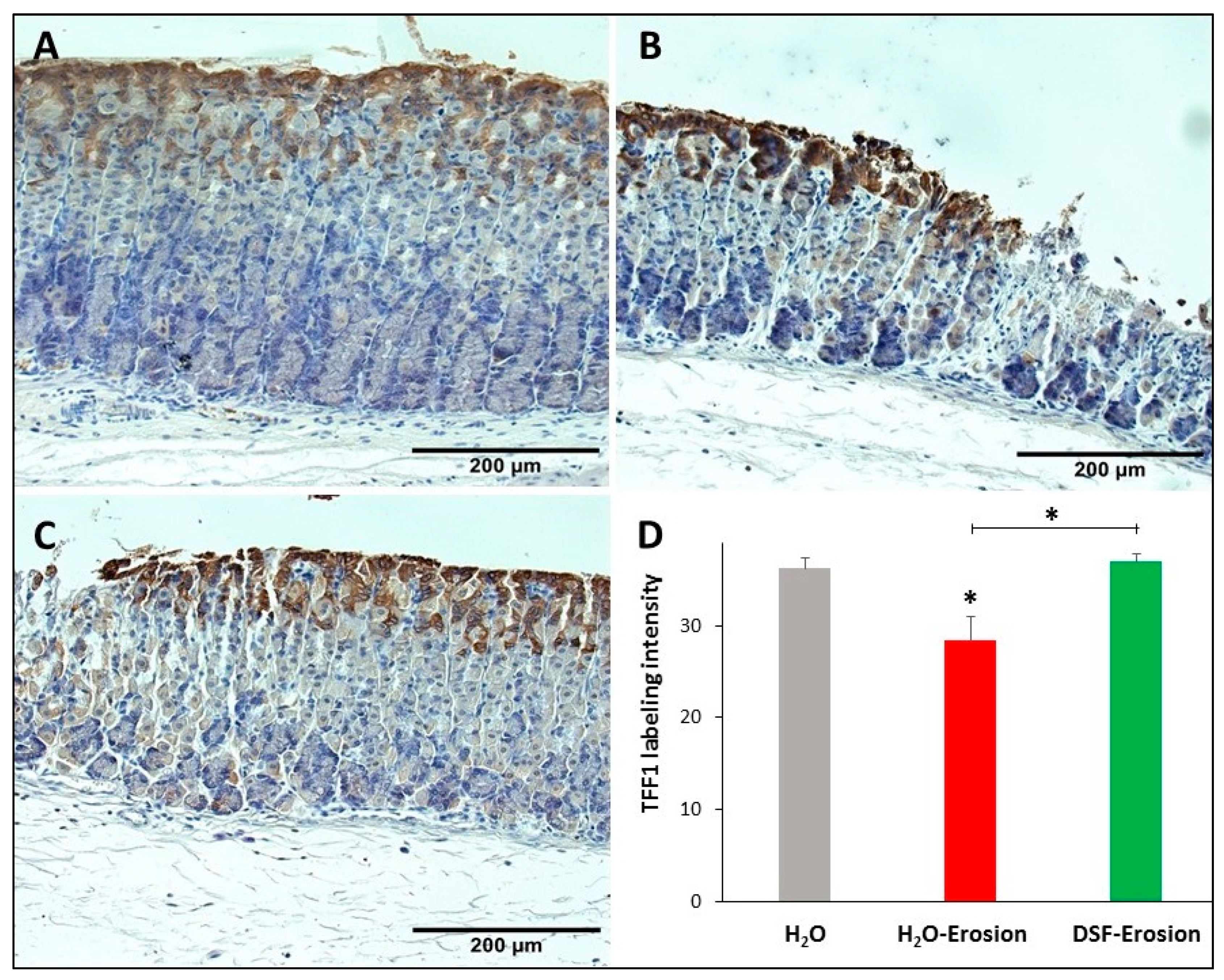
3.1.5. DSF Pretreatment Reduces Pepsinogen Production by Zymogenic Cells
3.1.6. DSF Pretreatment Increases Ghrelin-Secreting Enteroendocrine Cells
3.2. Effects of DSF on Gastric Erosion Induced by Multiple ASA Gavages
4. Discussion
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarnawski, A.; Ahluwalia, A.; Jones, M.K. Gastric cytoprotection beyond prostaglandins: Cellular and molecular mechanisms of gastroprotective and ulcer healing actions of antacids. Curr. Pharm. 2013, 19, 126–132. [Google Scholar] [CrossRef]
- Al-Awadhi, H.; John, R.; Al-Marzooqi, F.; Vincze, A.; Branicki, F.; Karam, S.M. Sequential alterations in gastric biopsies and tumor tissues support the multistep process of carcinogenesis. Histol. Histopath 2011, 26, 1153–1164. [Google Scholar] [CrossRef]
- Zanotti, G.; Cendron, L. Structural Aspects of Helicobacter pylori Antibiotic Resistance. Adv. Exp. Med. Biol. 2019, 1149, 227–241. [Google Scholar] [CrossRef]
- Karam, S.M.; Forte, J.G. Inhibiting gastric H(+)-K(+)-ATPase activity by omeprazole promotes degeneration and production of parietal cells. Am. J. Physiol. 1994, 266, G745–G758. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.S.; Chan, E.W.; Wong, A.; Chen, L.; Wong, I.; Leung, W.K. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: A population-based study. Gut 2018, 67, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Bi, W.P.; Man, H.B.; Man, M.Q. Efficacy and safety of herbal medicines in treating gastric ulcer: A review. World J. Gastroenterol. 2014, 20, 17020–17028. [Google Scholar] [CrossRef]
- Khoder, G.; Al-Menhali, A.A.; Al-Yassir, F.; Karam, S.M. Potential role of probiotics in the management of gastric ulcer. Exp. Ther. Med. 2016, 12, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmerman, H.M.; Koning, C.J.; Mulder, L.; Rombouts, F.M.; Beynen, A.C. Monostrain, multistrain and multispecies probiotics-A comparison of functionality and efficacy. Int. J. Food Microbiol. 2004, 96, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ki Cha, B.; Mun Jung, S.; Hwan Choi, C.; Song, I.D.; Woong Lee, H.; Joon Kim, H.; Hyuk, J.; Kyung Chang, S.; Kim, K.; Chung, W.S.; et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Suo, H.; Zhao, X.; Qian, Y.; Sun, P.; Zhu, K.; Li, J.; Sun, B. Lactobacillus fermentum Suo Attenuates HCl/Ethanol Induced Gastric Injury in Mice through Its Antioxidant Effects. Nutrients 2016, 8, 155. [Google Scholar] [CrossRef]
- Singh, P.K.; Deol, P.K.; Kaur, I.P. Entrapment of Lactobacillus acidophilus into alginate beads for the effective treatment of cold restraint stress induced gastric ulcer. Food Funct. 2012, 3, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; De Simone, C.; Chadee, K. The probiotic mixture VSL#3 accelerates gastric ulcer healing by stimulating vascular endothelial growth factor. PLoS ONE 2013, 8, e58671. [Google Scholar] [CrossRef] [Green Version]
- De Simone, C. Letter: What gastroenterologists should know about VSL#3. Aliment. Pharmacol. Ther. 2018, 47, 698–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef]
- Kim, H.J.; Vazquez Roque, M.I.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Rizzello, F.; Helwig, U.; Poggioli, G.; Schreiber, S.; Talbot, I.C.; Nicholls, R.J.; Gionchetti, P.; Campieri, M.; Kamm, M.A. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004, 53, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P.; et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935.e2. [Google Scholar] [CrossRef] [PubMed]
- Mardini, H.E.; Grigorian, A.Y. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: A meta-analysis. Inflamm. Bowel Dis. 2014, 20, 1562–1567. [Google Scholar] [CrossRef]
- Wang, C.S.; Li, W.B.; Wang, H.Y.; Ma, Y.M.; Zhao, X.H.; Yang, H.; Qian, J.M.; Li, J.N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018, 24, 4254–4262. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.N.; Buret, A.; McKnight, W.; Miller, M.J.; Wallace, J.L. Bacteria rapidly colonize and modulate healing of gastric ulcers in rats. Am. J. Physiol. 1998, 275, G425–G432. [Google Scholar] [CrossRef]
- Lam, E.K.Y.; Tai, E.K.K.; Koo, M.W.L.; Wong, H.P.S.; Wu, W.K.K.; Yu, L.; So, W.H.L.; Woo, P.C.Y.; Cho, C.H. Enhancement of gastric mucosal integrity by Lactobacillus rhamnosus GG. Life Sci. 2007, 80, 2128–2136. [Google Scholar] [CrossRef]
- Uchida, M.; Shimizu, K.; Kurakazu, K. Yogurt containing Lactobacillus gasseri OLL 2716 (LG21 yogurt) accelerated the healing of acetic acid-induced gastric ulcer in rats. Biosci. Biotechnol. Biochem. 2010, 74, 1891–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Kaur, I.P. Synbiotic (probiotic and ginger extract) loaded floating beads: A novel therapeutic option in an experimental paradigm of gastric ulcer. J. Pharm. Pharmacol. 2012, 64, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.C.; Hou, P.P.; Wang, X.Y.; Zhao, C.H.; Cheng, B.J.; Wang, Y.L.; Hao, H.W.; Zhang, T.H.; Ye, H.Q. Pretreatment with Lactobacillus reuteri F-9-35 attenuates ethanol-induced gastric injury in rats. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Chenoll, E.; Casinos, B.; Bataller, E.; Astals, P.; Echevarría, J.; Iglesias, J.R.; Balbarie, P.; Ramón, D.; Genovés, S. Novel probiotic Bifidobacterium bifidum CECT 7366 strain active against the pathogenic bacterium Helicobacter pylori. Appl. Environ. Microbiol. 2011, 77, 1335–1343. [Google Scholar] [CrossRef] [Green Version]
- Karam, S.M.; Straiton, T.; Hassan, W.M.; Leblond, C.P. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells 2003, 21, 322–336. [Google Scholar] [CrossRef]
- Khoder, G.; Al-Yassir, F.; Al Menhali, A.; Saseedharan, P.; Sugathan, S.; Tomasetto, C.; Karam, S.M. Probiotics Upregulate Trefoil Factors and Downregulate Pepsinogen in the Mouse Stomach. Int. J. Mol. Sci. 2019, 20, 3901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Li, X.; Yu, H.; Zhu, S.; He, Y.; Komatsu, K.; Guo, D.; Li, X.; Wang, J.; Luo, H.; et al. Gastroprotective effect of araloside A on ethanol- and aspirin-induced gastric ulcer in mice: Involvement of H+/K+-ATPase and mitochondrial-mediated signaling pathway. J. Nat. Med. 2019, 73, 339–352. [Google Scholar] [CrossRef]
- Tomasetto, C.; Karam, S.M.; Ribieras, S.; Masson, R.; Lefèbvre, O.; Staub, A.; Alexander, G.; Chenard, M.P.; Rio, M.C. Identification and characterization of a novel gastric peptide hormone: The motilin-related peptide. Gastroenterology 2000, 119, 395–405. [Google Scholar] [CrossRef]
- Karam, S.M.; Tomasetto, C.; Rio, M.C. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut 2004, 53, 1408–1415. [Google Scholar] [CrossRef] [Green Version]
- Vitellio, P.; Celano, G.; Bonfrate, L.; Gobbetti, M.; Portincasa, P.; De Angelis, M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients 2019, 11, 886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhang, J.; Dong, E.; Zhang, X.; Tao, Y.; Zhong, J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development Through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef]
- Russo, F.; Linsalata, M.; Orlando, A. Probiotics against neoplastic transformation of gastric mucosa: Effects on cell proliferation and polyamine metabolism. World J. Gastroenterol. 2014, 20, 13258–13272. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Mattar, A.F.; Teitelbaum, D.H.; Drongowski, R.A.; Yongyi, F.; Harmon, C.M.; Coran, A.G. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 2002, 18, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Franco, C.; Keller, K.; De Simone, C.; Chadee, K. The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G315–G322. [Google Scholar] [CrossRef]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Tamura, M.; Matsui, H.; Kaneko, T.; Hyodo, I. Alcohol is an oxidative stressor for gastric epithelial cells: Detection of superoxide in living cells. J. Clin. Biochem. Nutr. 2013, 53, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef]
- Gioacchini, G.; Ciani, E.; Pessina, A.; Cecchini, C.; Silvi, S.; Rodiles, A.; Merrifield, D.L.; Olivotto, I.; Carnevali, O. Correction to: Effects of Lactogen 13, a New Probiotic Preparation, on Gut Microbiota and Endocrine Signals Controlling Growth and Appetite of Oreochromis niloticus Juveniles. Microb. Ecol. 2018, 76, 1075. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.P.; Souza, L.K.M.; Araújo, T.S.L.; Araújo, S.; Nogueira, K.M.; Sousa, F.B.M.; Silva, R.O.; Pacífico, D.M.; Martins, C.S.; Brito, G.A.C.; et al. Lactobacillus reuteri DSM 17938 Protects against Gastric Damage Induced by Ethanol Administration in Mice: Role of TRPV1/Substance P Axis. Nutrients 2019, 11, 208. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kissoon-Singh, V.; Coria, A.L.; Moreau, F.; Chadee, K. Probiotic mixture VSL#3 reduces colonic inflammation and improves intestinal barrier function in Muc2 mucin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G34–G45. [Google Scholar] [CrossRef]
- Nienhüser, H.; Kim, W.; Malagola, E.; Ruan, T.; Valenti, G.; Middelhoff, M.; Bass, A.; Der, C.J.; Hayakawa, Y.; Wang, T.C. Mist1+ gastric isthmus stem cells are regulated by Wnt5a and expand in response to injury and inflammation in mice. Gut 2021, 70, 654–665. [Google Scholar] [CrossRef]
- Linsalata, M.; Russo, F.; Berloco, P.; Valentini, A.M.; Caruso, M.L.; De Simone, C.; Barone, M.; Polimeno, L.; Di Leo, A. Effects of probiotic bacteria (VSL#3) on the polyamine biosynthesis and cell proliferation of normal colonic mucosa of rats. In Vivo 2005, 19, 989–995. [Google Scholar] [PubMed]
- Ichikawa, H.; Kuroiwa, T.; Inagaki, A.; Shineha, R.; Nishihira, T.; Satomi, S.; Sakata, T. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig. Dis. Sci. 1999, 44, 2119–2123. [Google Scholar] [CrossRef]
- Mohania, D.; Kansal, V.K.; Kruzliak, P.; Kumari, A. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum modulates the formation of aberrant crypt foci, mucin-depleted foci, and cell proliferation on 1,2-dimethylhydrazine-induced colorectal carcinogenesis in Wistar rats. Rejuvenation Res. 2014, 17, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cinque, B.; La Torre, C.; Lombardi, F.; Palumbo, P.; Van der Rest, M.; Cifone, M.G. Production Conditions Affect the In Vitro Anti-Tumoral Effects of a High Concentration Multi-Strain Probiotic Preparation. PLoS ONE 2016, 11, e0163216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.J.; Do, E.J.; Kim, S.Y.; Cho, E.A.; Kim, D.H.; Pak, S.; Hwang, S.W.; Lee, H.J.; Byeon, J.S.; Ye, B.D.; et al. Combination of metformin and VSL#3 additively suppresses western-style diet induced colon cancer in mice. Eur. J. Pharmacol. 2017, 794, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Darby, T.M.; Naudin, C.R.; Luo, L.; Jones, R.M. Lactobacillus rhamnosus GG-induced Expression of Leptin in the Intestine Orchestrates Epithelial Cell Proliferation. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 627–639. [Google Scholar] [CrossRef]
- Karam, S.M. A focus on parietal cells as a renewing cell population. World J. Gastroenterol. 2010, 16, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stürmer, R.; Müller, S.; Hanisch, F.G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell Physiol. Biochem. 2014, 33, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204. [Google Scholar] [CrossRef]
- Rodríguez, C.; Medici, M.; Mozzi, F.; Font de Valdez, G. Therapeutic effect of Streptococcus thermophilus CRL 1190-fermented milk on chronic gastritis. World J. Gastroenterol. 2010, 16, 1622–1630. [Google Scholar] [CrossRef]
- Gomi, A.; Harima-Mizusawa, N.; Shibahara-Sone, H.; Kano, M.; Miyazaki, K.; Ishikawa, F. Effect of Bifidobacterium bifidum BF-1 on gastric protection and mucin production in an acute gastric injury rat model. J. Dairy Sci. 2013, 96, 832–837. [Google Scholar] [CrossRef] [Green Version]
- Otte, J.M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, G.; Matar, C.; Perdigón, G. Milk fermentation products of L. helveticus R389 activate calcineurin as a signal to promote gut mucosal immunity. BMC Immunol. 2007, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudier, E.; Michel, C.; Segain, J.P.; Cherbut, C.; Hoebler, C. The VSL# 3 probiotic mixture modifies microflora but does not heal chronic dextran-sodium sulfate-induced colitis or reinforce the mucus barrier in mice. J. Nutr. 2005, 135, 2753–2761. [Google Scholar] [CrossRef] [Green Version]
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial Porcine Gastric Mucin Preparations, also Used as Artificial Saliva, are a Rich Source for the Lectin TFF2: In Vitro Binding Studies. Chembiochem 2018, 19, 2598–2608. [Google Scholar] [CrossRef]
- Bossenmeyer-Pourié, C.; Kannan, R.; Ribieras, S.; Wendling, C.; Stoll, I.; Thim, L.; Tomasetto, C.; Rio, M.C. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J. Cell Biol. 2002, 157, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, K.; Hans, W.; Van Huysse, J.; Neirynck, S.; Demetter, P.; Remaut, E.; Rottiers, P.; Steidler, L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology 2004, 127, 502–513. [Google Scholar] [CrossRef]
- Karam, S.M.; Leblond, C.P. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat. Rec. 1993, 236, 297–313. [Google Scholar] [CrossRef]
- Burclaff, J.; Willet, S.G.; Sáenz, J.B.; Mills, J.C. Proliferation and differentiation of gastric mucous neck and chief cells during homeostasis and injury-induced metaplasia. Gastroenterology 2020, 158, 598–609.e5. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Jiang, J.; Yuan, Y. Pepsinogen C expression, regulation and its relationship with cancer. Cancer Cell Int. 2017, 17, 57. [Google Scholar] [CrossRef] [Green Version]
- Konturek, P.C.; Brzozowski, T.; Pajdo, R.; Nikiforuk, A.; Kwiecien, S.; Harsch, I.; Drozdowicz, D.; Hahn, E.G.; Konturek, S.J. Ghrelin-a new gastroprotective factor in gastric mucosa. J. Physiol. Pharmacol. 2004, 55, 325–336. [Google Scholar] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar] [PubMed]
- Saito, H.; Nakakita, Y.; Segawa, S.; Tsuchiya, Y. Oral administration of heat-killed Lactobacillus brevis SBC8803 elevates the ratio of acyl/des-acyl ghrelin in blood and increases short-term food intake. Benef. Microbes 2019, 10, 671–677. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Yang, Z.; Chen, F.; Zhang, Y. Effects of probiotics on ghrelin and lungs in children with acute lung injury: A double-blind randomized, controlled trial. Pediatr. Pulmonol. 2018, 53, 197–203. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Golubeva, A.V.; Zhdanov, A.V.; Wallace, S.; Arboleya, S.; Papkovsky, D.B.; El Aidy, S.; Ross, P.; Roy, B.L.; Stanton, C.; et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019, 33, 13546–13559. [Google Scholar] [CrossRef] [Green Version]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. App. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and Covid-19. Int. J. Food Sci. Nut. 2021, 72, 293–299. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Yassir, F.; Khoder, G.; Sugathan, S.; Saseedharan, P.; Al Menhali, A.; Karam, S.M. Modulation of Stem Cell Progeny by Probiotics during Regeneration of Gastric Mucosal Erosions. Biology 2021, 10, 596. https://doi.org/10.3390/biology10070596
Al-Yassir F, Khoder G, Sugathan S, Saseedharan P, Al Menhali A, Karam SM. Modulation of Stem Cell Progeny by Probiotics during Regeneration of Gastric Mucosal Erosions. Biology. 2021; 10(7):596. https://doi.org/10.3390/biology10070596
Chicago/Turabian StyleAl-Yassir, Farah, Ghalia Khoder, Subi Sugathan, Prashanth Saseedharan, Asma Al Menhali, and Sherif M. Karam. 2021. "Modulation of Stem Cell Progeny by Probiotics during Regeneration of Gastric Mucosal Erosions" Biology 10, no. 7: 596. https://doi.org/10.3390/biology10070596
APA StyleAl-Yassir, F., Khoder, G., Sugathan, S., Saseedharan, P., Al Menhali, A., & Karam, S. M. (2021). Modulation of Stem Cell Progeny by Probiotics during Regeneration of Gastric Mucosal Erosions. Biology, 10(7), 596. https://doi.org/10.3390/biology10070596






