Simple Summary
In the mating, reproduction, and phylogenetic reconstruction of various insect taxa, the morphological characteristics of the male reproductive system, spermatogenesis, and sperm ultrastructure are important. We investigated these morphological characteristics of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae), which is one of the most destructive pests of Populus alba var. pyramidalis (Bunge) using light microscopy, scanning electron microscopy, and transmission electron microscopy. We also compared these morphological characteristics with that found in other Curculionidae.
Abstract
The male reproductive system, sperm structure, and spermatogenesis of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae), which is one of the most destructive pests of Populus alba var. pyramidalis (Bunge), were investigated using light microscopy, scanning electron microscopy, and transmission electron microscopy. The male reproductive system of T. klimeschi is composed of testes, seminal vesicles, tubular accessory glands, multilobulated accessory glands, vasa deferentia, and a common ejaculatory duct. In spermatogenesis, two phenomena are apparent: The nuclear chromatin condenses into two different patterns, and an oval preacrosomal vesicle is present at the flank of the Golgi apparatus. The sperm are short, measuring 76.7 ± 1.8 μm in length, and are 508.1 ± 12.9 nm in width. The sperm are composed of a three-layer acrosomal complex, a cylindrical nucleus, two mitochondrial derivatives, a 9 + 9 + 2 axoneme, and two accessory bodies with a large “puff”-like expansion. Mature sperm are individually stored in seminal vesicles. During spermiogenesis, the similarities in the nuclear chromatin condensation characteristics of Curculioninae and Scolytinae are indicative of their close phylogenetic relationship. It appears that the preacrosomal vesicle being flanked by the Golgi apparatus is a characteristic of spermatogenesis in Curculionidae.
1. Introduction
Curculionidae, with about 48,000 valid species, is the largest family of known organisms [1]. Many pest species, such as Scolytus schevyrewi, Scolytus seulensis, Scolytus rugulosus, Scolytus multistriatus, Trypophloeus populi, Lepyrus japonicuse, Sympiezomias velatus, and Cryptorhynchus lapathi, attack poplar trees and other hardwood, causing significant economic and ecological problems worldwide [2,3,4,5,6]
Trypophloeus klimeschi, belonging to Scolytinae, Curculionidae [7], is one of the most destructive pests of Populus alba var. pyramidalis (Bunge). It was first recorded in the Kyrgyz Republic, which borders Xinjiang Uygur Autonomous Region in China [7,8,9]. Following an outbreak in the Dunhuang in recent years [2,10,11,12], this beetle has caused huge economic and ecological losses. T. klimeschi first invades branch shoots and then gradually spreads to the main trunk. The beetle bores and feeds in the phloem to form a gallery in which the eggs are deposited. The injured branches turn yellow and wither, and dense holes are formed in the trunk surface, causing the injured trees to wither and rapidly die [2]. The prevention and control of T. klimeschi must involve the control of population number [13]. Reproductive ability is one of the important factors that affect population number. Long-term field investigation has shown that T. klimeschi is a monogamous insect and mates once before laying eggs, and the number of eggs laid is as high as 30 [2]. Such a strong reproductive ability is the basis for its rapid spread. In order to better understand its mating and reproduction, morphological information of the male reproductive system, the sperm structure, and spermiogenesis is essential [14].
The morphological characteristics of the male reproductive system, spermatogenesis, and sperm ultrastructure have been considered important in taxonomy of various insect taxa [15,16,17,18,19,20,21,22,23]. The Scolytinae subfamily includes 6000 species belonging to 200 genera [24], and more studies of these areas are needed for its phylogenetic reconstruction considering the huge number of species. Only a few species of these areas have been studied, such as Dendroctonus rnonticolae, Hypothenemus hampei, Dendroctonus armandi, and Hylurgus ligniperda [13,25,26,27,28]. The male reproductive tract of most Scolytinae is composed of testes, seminal vesicles, accessory glands, vasa deferentia, and ejaculatory duct [13,25,26,27,28]. Their sperm usually contain a nucleus, a 9 + 9 + 2 axoneme, two accessory bodies, two mitochondrial derivatives, and one “puff”-like expansion [13,19]. However, there seem to be exceptions among these species. For example, Hypothenemus hampei has no accessory glands [26], and the sperm of D. armandi also contains a special large spongy body [13]. Although these studies revealed important information regarding the phylogenetic reconstruction of Scolytinae, these structures may vary significantly, even between closely related species [18]. There is still an urgent need for more research on the male reproductive system, sperm, and spermatogenesis of this subfamily.
Due to the wide variety and rapid spread of bark beetles, the lack of phylogenetic studies may lead to untimely identification and inadequate proliferation detection of their species. At present, only adult integument morphology, larval morphology, antenna receptor morphology, and life history can provide support for the systematic status of T. klimeschi [2,8,9]. Ning et al. predicts that T. klimeschi will quickly spread to more places in the future [29]. Therefore, the aim of this research was to study the reproductive system, spermatogenesis, and sperm ultrastructure of T. klimeschi using light microscopy, scanning electron microscopy, and transmission electron microscopy. This study provides key information for future research into mating, reproduction, and the systematic status of T. klimeschi, more evidence for phylogenetic reconstruction of Scolytinae, and potentially useful information for subsequent pest control.
2. Materials and Methods
2.1. Insects
T. klimeschi (larvae and pupae) collected from the bark of infested P. alba var. pyramidalis in Dunhuang City (40°06′50.61″ N, 94°36′10.24″ E), Gansu Province, China, were reared in 24-hole plates with feed containing P. alba var. pyramidalis bark powder in an artificial climate incubator (14L: 10D, 25 ± 1 °C, 65 ± 5% relative humidity) [2]. On the 1st, 8th, and 16th day after eclosion, 30 males were taken for later use in experiments. For anatomical analyses, the reproductive systems of 30 males—10 T. klimeschi each at 1, 8, and 16 days after eclosion—were observed with an OLYMPUS SZ2-ILST stereomicroscope and photographed with an OLYMPUS DP25 camera.
2.2. Scanning Electron Microscopy (SEM)
The reproductive systems of the 10 males in each age group were dissected and fixed in a solution containing 2.5% glutaraldehyde in 0.1 M phosphate (pH 7.2) for 12 h at 4 °C [2]. The samples were washed in phosphate-buffered saline (PBS), pH 7.2; dehydrated through a graded series of alcohol and isoamyl acetate; critical point dried with liquid CO2; and sputter coated with gold. Samples were then examined using a HITACHI S-4800 scanning electron microscope at 15 kV.
2.3. Transmission Electron Microscopy (TEM)
The 10 fixed samples of reproductive systems for each age group were rinsed with PBS, and post-fixation was performed in 1% osmium tetroxide for 1 h at 4 °C. After four 15 min washes in the same buffer, the samples were dehydrated through a graded ethanol series and embedded in 14381-UC LR WHITE. Semithin sections were obtained with a glass knife on a LEICA RM2265 microtome, stained with toluidine blue, and observed with an OLYMPUS BX43F microscope. Ultrathin sections (70 nm thick) were obtained with a diamond knife on an ultramicrotome (LEICA ULTRACUT UCT), routinely stained with uranyl acetate and lead citrate, and observed with a HITACHI HT7700 transmission electron microscope.
3. Results
3.1. Gross Morphology of the Male Reproductive System
From the light microscopy images, we observed that the male internal reproductive tract of T. klimeschi is composed of two units (Figure 1A). Each unit comprises a bilobed testis—a seminal vesicle inserted in the depression of the testis—two accessory glands (multilobulated gland and tubular gland), and the vas deferens (Figure 1A). The tubular accessory gland is connected to the seminal vesicle, while the multilobulated accessory gland surrounds the vas deferens (Figure 1A). Two units fuse at their posterior ends, flowing into an ejaculatory duct (Figure 1A). On the first day after eclosion, the seminal vesicles are only thin tubules (Figure 1B), which become thicker with maturation (Figure 1A).
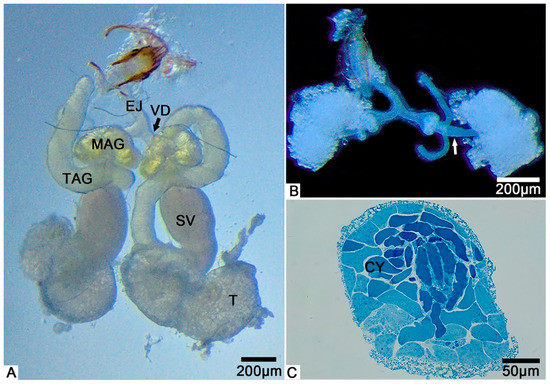
Figure 1.
The male reproductive system of T. klimeschi: (A) The male reproductive system on the 16th day after eclosion. (B) On the first day after eclosion, the seminal vesicles are only thin tubules (white arrow). (C) The cross section of the testis showing many cysts (CY). Testis (T); seminal vesicle (SV); tubular accessory gland (TAG); multilobulated accessory gland (MAG); vas deferens (VD); ejaculatory duct (EJ).
The cysts were observed to completely fill the testes (Figure 1C). A cyst is a saccular structure composed of cyst cells (Figure 2A), within which spermatogenesis occurs. During spermatogenesis, spermatogonia undergo repeated mitotic divisions and give rise to spermatocytes. These spermatocytes undergo successive meiotic divisions and give rise to spermatids, which, after morphological change (spermiogenesis), form spermatozoa. In each cyst, 350–512 (n = 10) spermatozoa were observed, resulting from nine cell division cycles. Mature sperm were observed to fill the seminal vesicles.
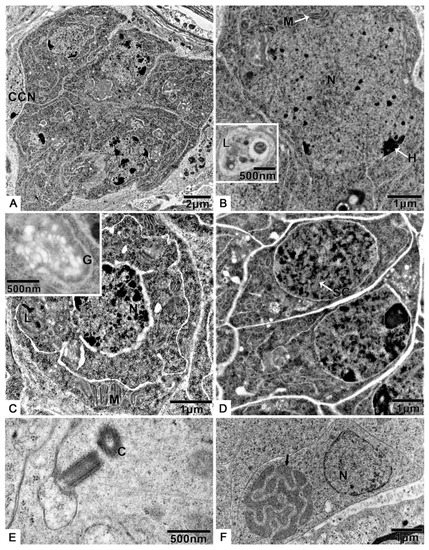
Figure 2.
The spermatogenesis of T. klimeschi: (A) spermatogonia cyst; (B) spermatogonia; (C) primary spermatocytes; (D) secondary spermatocytes; (E) centrioles in spermatocytes; (F) spermatids. Cyst cell nucleus (CCN); centrioles (C); heterochromatin (H); mitochondria (M); Golgi apparatus (G); lysosomes (L); nucleus (N); synaptonemal complexes (SC); fusing mitochondria (black arrow).
3.2. Spermatogenesis
3.2.1. From Spermatogonia to Spermatids
Transmission electron microscopy allowed us to determine that the spermatogonia are irregularly shaped cells (Figure 2A,B, Table S1), 5.6 ± 0.4 μm (n = 10) in diameter, with a large, irregularly shaped nucleus (3.5 ± 0.7 μm, n = 10). The nucleus contains homogeneously distributed granular chromatin and irregularly distributed electron-dense heterochromatin (Figure 2A,B). The cytoplasm contains many mitochondria and large lysosomes (Figure 2B).
The spermatocytes (Figure 2C, Table S1) originate from mitosis of spermatogonia and have a diameter of 4.8 ± 0.3 μm (n = 10). The cells are characterized by synaptonemal complexes in the round nucleus 3.1 ± 0.4 μm (n = 10) in diameter (Figure 2D, Table S1). Lysosomes, mitochondria with well-defined cristae, Golgi apparatus (Figure 2C), and two orthogonally arranged centrioles (Figure 2E) can be observed in the cytoplasm.
3.2.2. Spermiogenesis
Germ cells undergo many changes during spermiogenesis: The nuclear chromatin becomes more concentrated, and the electron density is enhanced (Figure 3A,B). The lateroposterior end of the nucleus forms a concavity where the basal body is located, and the axoneme elongates from here (Figure 3A,B). A round preacrosomal vesicle, 448.3 ± 14.5 nm (n = 10) (Table S1) in diameter, is visible (Figure 3C). At the initial stage of spermatid differentiation, the preacrosomal vesicle is near the nebenkern and Golgi apparatus (Figure 3C). In the early spermatid, when the nebenkern becomes two mitochondrial derivatives, the preacrosomal vesicle is found near the Golgi apparatus (Figure 3D) and nucleus (Figure 3E). In more detail, the preacrosomal vesicle flanks and is near to the concave face of the Golgi apparatus (Figure 3D). In this stage, the preacrosomal vesicle has a dark-stained mantle and a light core (Figure 3E); both are surrounded by a membrane (Figure 3F). As the cell elongates, the preacrosomal vesicle flattens (Figure 3G). At a more mature stage, the bell-shaped nucleus is obvious, and its nuclear chromatin shows two distinct regions (Figure 3H): one homogeneously compact, the other fibrillar. At this stage of development, all sperm components are surrounded by microtubules (Figure 3H). Shortly after this, the centriolar adjunct is visible on both sides of the basal body (Figure 3I). However, there are no centriolar adjuncts in the mature sperm.
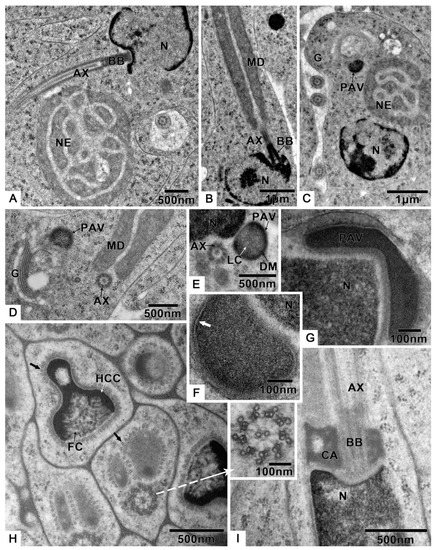
Figure 3.
Spermiogenesis of T. klimeschi: (A) The axoneme starts elongating from the concavity of nucleus. (B) Mitochondrial derivatives are located on both sides of the axoneme and parallel to the axoneme. (C) Spermatid at initial stage of differentiation showing that the preacrosomal vesicle is near to the nebenkern and Golgi apparatus. (D,E) In the early spermatid (nebenkern becomes two mitochondrial derivatives), the preacrosomal vesicle and axoneme are near to the Golgi apparatus (D) and nucleus (E). The preacrosomal vesicle has a dark-stained mantle and light core (E). (F) The preacrosomal vesicle under high magnification and its membrane structure (white arrow). (G) As the nucleus elongates, the preacrosomal vesicle becomes flat. (H) The spermatid components are surrounded by microtubules (black arrow). The bell-shaped nucleus with different chromatin aggregation states: homogeneously compact chromatin and fibrillar chromatin. The cross section of the axoneme under high magnification (white arrow). (I) The centriolar adjunct appears in the spermatid stage. Nucleus (N); axoneme (AX); preacrosomal vesicle (PAV); nebenkern (NE); Golgi apparatus (G); dark-stained mantle (DM); light core (LC); homogeneously compact chromatin (HCC); fibrillar chromatin (FC); mitochondrial derivatives (MD); centriolar adjunct (CA); basal body (BB).
During spermiogenesis, the spherical-shaped mitochondria, which are found dispersed in the cytoplasm, begin to gather (Figure 4A). Then, the formation of two mitochondrial derivatives begins via stages that are twinned (Figure 4B), five-layer (Figure 4C) and two-layer nebenkern (Figure 4D,E). These two mitochondrial derivatives become slender (Figure 4F) as the spermatid and axoneme elongate (Figure 3B).
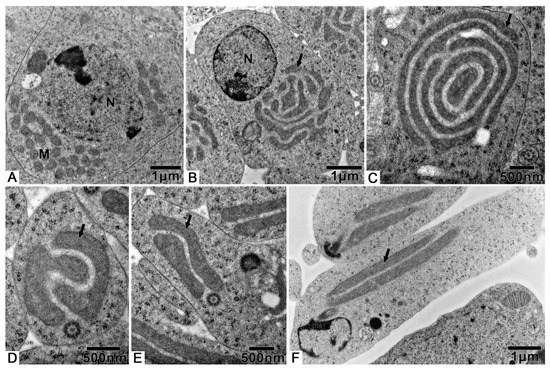
Figure 4.
Changes of mitochondria during spermiogenesis of T. klimeschi: (A) Mitochondria (M) gathered together; (B–F) changing mitochondria (black arrow). Nucleus (N).
3.3. Spermatozoa
The spermatozoa of T. klimeschi are slender (Figure 5A, Table S1), measuring 76.7 ± 1.8 μm (n = 10) in length and 508.1 ± 13.0 nm (n = 10) in width, and composed of an acrosomal complex, a nucleus (Figure 6A), an axoneme, two mitochondrial derivatives, and two accessory bodies (Figure 6F). The mitochondrial derivatives do not embrace the axoneme but rather run approximately parallel to it (Figure 5B). Mature sperm are stored in the seminal vesicles and its cross section, showing that the sperm of T. klimeschi are arranged very closely on the 16th day after eclosion (Figure 5C).
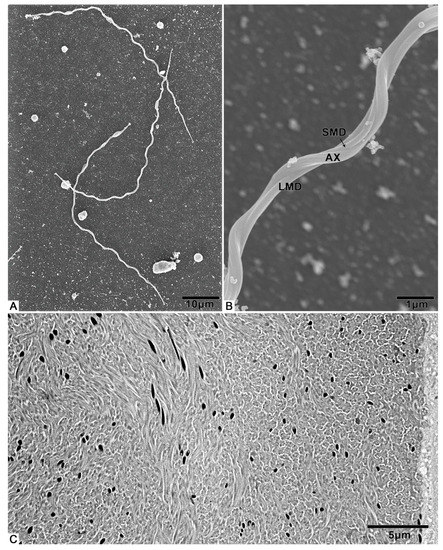
Figure 5.
SEM and TEM micrographs of T. klimeschi spermatozoa: (A) The appearance of spermatozoa; (B) Detailed view of spermatozoa; (C) TEM micrograph of spermatozoa in seminal vesicles. The black, round, and short strips are the sperm nuclei. Axoneme (AX); large mitochondrial derivative (LMD); small mitochondrial derivative (SMD).
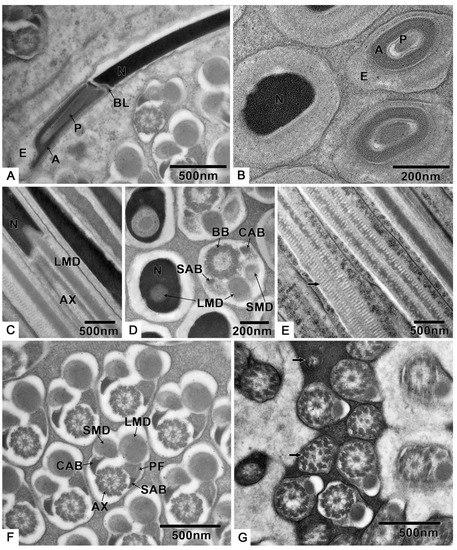
Figure 6.
The spermatozoa structure of T. klimeschi: (A,B) The longitudinal and cross sections of 3-layered acrosome complex. (C,D) The longitudinal and cross sections showing the junction of the nucleus and flagellar components. (E) The mitochondrial cristae (black arrow). (F) Cross section of the middle of the spermatozoa. (G) Cross section showing the disorganized axoneme (black arrow) at the end of the spermatozoa. Extra acrosomal layer (E); acrosomal vesicle (A); perforatorium (P); nucleus (N); basal lamina (BL); axoneme (AX); large mitochondrial derivative (LMD); small mitochondrial derivative (SMD); crescent accessory body (CAB); basal body (BB); small accessory body (SAB); puff-like expansion (PF).
The three-layer acrosomal complex (Figure 6A,B, Table S1), measuring 1.2 ± 0.0 μm (n = 10) in length, is made up of a dense extra acrosomal granular layer, a cup-like acrosomal vesicle, and a rod-like conical perforatorium. The acrosomal complex lies on a slightly concave nuclear face and is separated from the nucleus by a thick basal lamina (Figure 6A).
The nucleus is close to the acrosomal complex (Figure 6A,B) with a circular cross section, which is indicated by a longitudinal section that is 6.0 ± 0.2 μm (n = 10) in length and 289.9 ± 22.6 nm (n = 10) in diameter (Table S1).
The longitudinal and cross sections (Figure 6C,D) show that the flagella components are inserted into the nucleus, while the large mitochondrial derivative is the deepest. The 9 + 9 + 2 axoneme (Figure 6F, Table S1) has a diameter of 282.9 ± 8.1 nm (n = 10). The large mitochondrial derivative with distinct cristae (Figure 6E), 236.0 ± 18.9 nm (n = 10) in diameter is always thinner than the axoneme and becomes thinner along the flagellum before completely disappearing (Figure 6G).
The two accessory bodies are different in shape (Figure 6F). From the cross section of the middle part of the sperm, we know that the crescent accessory body is smaller than one-quarter of the axoneme size, and the other is small and mostly triangular (sometimes crescent-shaped) with a large, compact, “puff”-like expansion.
At the end of the sperm, the two accessory bodies are the first to disappear, followed by the smaller mitochondrial derivative, larger mitochondrial derivative, and, finally, the disorganized disappearance of the axoneme (Figure 6G).
4. Discussion
In terms of general morphology, the male reproductive tract of T. klimeschi is composed of two testes, two seminal vesicles, two tubular accessory glands, two multilobulated accessory glands, two vasa deferentia, and an ejaculatory duct, and this composition is identical to that reported for other Curculionidae [13,25,26,27,30,31,32].
T. klimeschi has two types of accessory gland. The multilobulated accessory glands of some Scolytinae—for instance, D. armandi [13], Scolytus destructor [28], H. ligniperda [27], and D. rnonticolae [25]—correspond to the prostate glands of some species of other Curculionidae subfamilies, such as Hyperodes bonariensis (Coleoptera: Curculionidae: Cylindrorhininae) [31], Listronotus bonariensis (Coleoptera: Curculionidae: Cylindrorhininae) [33], and Tanymecus dilaticollis (Coleoptera: Curculionidae: Entiminae) [32]. Because the two structures have a similar appearance (they are both circular and multilobulated) and position (they are both next to tubular accessory glands and surround vasa deferentia), it is possible that they have the same structure. However, H. hampei (Coleoptera: Curculionidae: Scolytinae) [26] does not have this accessory gland.
In T. klimeschi, spermatogenesis occurs in cysts, as is the case for most insects [34,35,36,37,38]. The number of sperm per cyst is less than 512 as a result of nine cycles (29) of cell division. In Coleoptera, the number of divisions is between 4 to 10 [13,21,34,39]. According to the hypothesis of Virkki [40], insects of more archaic orders have more sperm cells per bundle than their more modern counterparts; if so, T. klimeschi may be a primitive coleopteran species.
It seems that two different patterns of nuclear chromatin condensation during spermiogenesis (one showing the chromatin more homogeneously condensed and another showing the chromatin with a fibrillar aspect) is a shared feature of Curculionidae [13,21,30,34,41]. However, the process of condensation is different and can generally be divided into two types: one having a honeycomb chromatin stage, such as for Sitophilus zeamais (Coleoptera: Curculionidae: Dryophthorinae), Sitophilus oryzae (Coleoptera: Curculionidae: Dryophthorinae) [21], and Rhynchophorus ferrugineus (Coleoptera: Curculionidae: Rhynchophorinae) [41], and the other not, such as for Anthonomus grandis (Coleoptera: Curculionidae: Curculioninae) [30], and D. armandi [13]. T. klimeschi belongs to the latter. In addition, another feature of the latter is its bell-shaped nucleus [13,34]. This result supports the theory that there is a close relationship between Curculioninae and Scolytinae, and Rhynchophorinae and Dryophthorinae [19,42].
In insects, as in animals in general, the acrosome is a product of the Golgi apparatus [43]. The position of the acrosome changes as the sperm matures. However, in the early spermatid, for many insects, the preacrosomal vesicle is situated on the concave face of the Golgi apparatus, between its innermost cisternae and the spermatid nucleus, such as in Euschistus (Hemiptera: Pentatomidae) [43], some Orthoptera [44], Acheta domesticus (Orthopteroidea, Ensifera) [45], Sciara coprophila (Diptera, Sciaridae) [46,47], and Machilontus sp. (Archaeognatha: Meinertellidae) [48]. However, in Conocephalus (Orthoptera: Tettigoniidae) [43], the preacrosomal vesicle is situated on the convex side of the Golgi apparatus, which is situated between the preacrosomal vesicle and the nucleus. In this study, the preacrosomal vesicle is situated on the flank of the Golgi apparatus and near to the concave face of the Golgi apparatus. The preacrosomal vesicle was also found in the same position in some Curculionidae, for example, S. oryzae [21] and R. ferrugineus [49]. However, unlike the bean-shaped preacrosomal vesicle of S. oryzae and the round preacrosomal vesicle of R. ferrugineus, the vesicle of T. klimeschi is oval-shaped. This special position of the preacrosomal vesicle may be a characteristic of Curculionidae.
The spermatozoa of T. klimeschi are linear and slender and are similar to the general description for other Curculionidae sperm [13,21,30,50]. Compared to spermatozoa of other Curculionidae, which are 110 to 300 μm in length [19], they are short, with a length of about 77 μm, though they share a similar structure to most [13,19,21,22,30,50,51]: a three-layer acrosome with a cup-like acrosome vesicle; a 9 + 9 + 2 axoneme; two mitochondrial derivatives of different sizes; a large mitochondrial derivative, which is always thinner than the axoneme in, for example, D. armandi [13], Kissophagus hederae (Coleoptera: Curculionidae: Scolytinae), and Ernoporus fagi (Coleoptera: Curculionidae: Scolytinae) [19]; two accessory bodies; and one large, compact, “puff”-like expansion.
At present, two types of insect spermatozoa storage have been characterized: in bundles and individually [18,52,53,54,55]. Bundled spermatozoa are maintained in primary arrangement (the arrangement in the testes), and their heads are inserted into the extracellular matrix. However, there seems to be no description of how spermatozoa are individually stored. In this study, according to the cross section of the seminal vesicle of T. klimeschi (Figure 5C), we determined that the sperm are arranged very tightly, but the sperm heads are not always gathered together as is the case for the heads of sperm stored in bundles [55]. Instead, the sperm heads are dispersed similarly to when they are stored individually [53]. Therefore, based on this observation, we believe that the sperm of T. klimeschi are individually stored in seminal vesicles.
5. Conclusions
In conclusion, the general morphology of the male reproductive tract, spermatogenesis, and the structure of the spermatozoa of T. klimeschi is, for the most part, similar to that of the majority of the Scolytinae. However, the spermatozoa of T. klimeschi is, at approximately 77 μm, very short. During spermiogenesis, the similarity in the nuclear chromatin condensation characteristics of Scolytinae and Curculioninae is indicative of their close phylogenetic relationship. The preacrosomal vesicle flanking the Golgi apparatus seems to be a characteristic of spermatogenesis in Curculionidae. This study provides novel information on the reproductive biology of the Curculionidae family and develops the existing taxonomic and phylogenetic research on Curculionidae.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology10070583/s1. Table S1: Size of different cells and organelles.
Author Contributions
Conceptualization, J.G.; methodology, J.G. and G.G.; software, J.W.; formal analysis, J.G.; investigation, J.G., J.W. and G.G.; writing—original draft preparation, J.G.; writing—review and editing, J.W.; supervision, H.C.; funding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (31870636) and the National Key Research and Development Program of China (2017YFD0600104-4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anderson, R.S. Weevils and plants: Phylogenetic versus ecological mediation of evolution of host plant associations in curculioninae (coleoptera: Curculionidae). Memoirs Èntomol. Soc. Can. 1993, 125, 197–232. [Google Scholar] [CrossRef]
- Gao, G. Occurrence and Host Selection Mechanism of Trypophloeus klimeschi Eggers. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2018. [Google Scholar]
- Marchetti, S.B.; Worrall, J.J.; Eager, T. Secondary insects and diseases contribute to sudden aspen decline in southwestern Colorado, USA. Can. J. For. Res. 2011, 41, 2315–2325. [Google Scholar] [CrossRef]
- Legalov, A.A. Revised checklist of weevils (Coleoptera: Curculionoidea excluding Scolytidae and Platypodidae) from Siberia and the Russian Far East. Acta Biol. Sib. 2020, 6, 437–549. [Google Scholar] [CrossRef]
- Cave, G.L.; Redlin, S.C. Importation of Chinese Penjing into the United States with Particular Reference to Ehretia microphylla; United States Department of Agriculture: Riverdale, MD, USA, 1996.
- Broberg, C.L.; Borden, J.H.; Humble, L.M. Distribution and abundance of Cryptorhynchus lapathi on Salix spp. in British Columbia. Can. J. For. Res. 2002, 32, 561–568. [Google Scholar] [CrossRef]
- Eggers, V.O.H. Trypophloeus klimeschi nov. spec. Entomol. Bl. 1915, 25, 7–9. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Trypophloeus klimeschi Eggers—A new insect pest to Xinjiang poplar. J. Tarim Univ. 2003, 15, 9–11. [Google Scholar]
- Cao, Y.; Luo, Z.; Wang, S.; Zhang, P. Bionomics and control of Trypophloeus klimeschi. Entomol. Knowl. 2004, 41, 36–38. [Google Scholar]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Volatile Organic Compound Analysis of Host and Non-Host Poplars for Trypophloeus klimeschi (Coleoptera: Curculionidae: Ipinae). Russ. J. Plant Physiol. 2018, 65, 916–925. [Google Scholar] [CrossRef]
- Gao, G.; Gao, J.; Hao, C.; Dai, L.; Chen, H. Biodiversity and Activity of Gut Fungal Communities across the Life History of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Int. J. Mol. Sci. 2018, 19, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Dai, L.; Gao, J.; Wang, J.; Chen, H. Electroantennogram, behavioural responses, and field trapping of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae) to eight host volatiles. Can. Èntomol. 2019, 151, 236–250. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Wei, L.-S.; Torres, M.A.; Zhang, X.; Wu, S.-P.; Chen, H. Morphology of the Male Reproductive System and Spermiogenesis ofDendroctonus armandiTsai and Li (Coleoptera: Curculionidae: Scolytinae). J. Insect Sci. 2017, 17, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberprieler, R.G.; Marvaldi, A.E.; Anderson, R.S. Weevils, weevils, weevils everywhere. Zootaxa 2007, 1668, 491–520. [Google Scholar] [CrossRef] [Green Version]
- Kuschel, G. A phylogenetic classification of Curculionoidea to families and subfamilies. Mem. Entomolol. Soc. Wash. 1995, 14, 5–33. [Google Scholar]
- Dallai, R. Overview on spermatogenesis and sperm structure of Hexapoda. Arthropod Struct. Dev. 2014, 43, 257–290. [Google Scholar] [CrossRef]
- Salazar, K.; Dias, G.; Boucher, S.; Lino-Neto, J.; Serrão, J.E. Morpho-anatomy of the male reproductive tract and spermatogenesis of the South American Spasalus silvarum Kuwert (Coleoptera: Passalidae). Zoomorphology 2016, 135, 487–497. [Google Scholar] [CrossRef]
- Schubert, L.F.; Krüger, S.; Moritz, G.B.; Schubert, V. Male reproductive system and spermatogenesis of Limodromus assimilis (Paykull 1790). PLoS ONE 2017, 12, e0180492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrini, A.G.; Magnano, L.; Magnano, A.R.; Scala, C.; Baccetti, B. Spermatozoa and phylogeny of Curculionoidea (Coleoptera). Int. J. Insect Morphol. Embryol. 1988, 17, 1–50. [Google Scholar] [CrossRef]
- Dallai, R.; Afzelius, B.A.; Lupetti, P.; Osella, G. Sperm structure of some Curculionoidea and their relationship with Chrysomeloidea. Boll. Mus. Regionale Sci. Nat. Torino. 1998, 1, 27–50. [Google Scholar]
- Name, K.P.; Dos Reis, G.P.; Báo, S.N. An ultrastructural study of spermiogenesis in two species of Sitophilus (Coleoptera: Curculionidae). Biocell 2007, 31, 229–236. [Google Scholar] [CrossRef]
- Dallai, R.; Gottardo, M.; Beutel, R.G. Structure and Evolution of Insect Sperm: New Interpretations in the Age of Phylogenomics. Annu. Rev. Èntomol. 2016, 61, 1–23. [Google Scholar] [CrossRef]
- Dallai, R.; Mercati, D.; Fanciulli, P.P.; Petrioli, A.; Lupetti, P. New findings on the sperm ultrastructure of Carabidae (Insecta, Coleoptera). Arthropod Struct. Dev. 2020, 54, 100912. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, G.; Leschen, R.A.B.; Zimmerman, E.C. Platypodidae under scrutiny. Invertebr. Syst. 2000, 14, 771–805. [Google Scholar] [CrossRef]
- Cerezke, H.F. The Morphology and Functions of the Reproductive Systems of Dendroctonus monticolae Hopk. (Coleoptera: Scolytidae). Can. Èntomol. 1964, 96, 477–500. [Google Scholar] [CrossRef]
- Rubio, G.J.D.; Bustillo, P.A.E.; Vallejo, E.L.F.; Acuña, Z.J.R.; Benavides, M.P. Alimentary canal and reproductive tract of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae, Scolytinae). Neotrop. Èntomol. 2008, 37, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Calder, A.A. Gross morphology of the soft parts of the male and female reproductive systems of Curculionoidea (Coleoptera). J. Nat. Hist. 1990, 24, 453–505. [Google Scholar] [CrossRef]
- Aslam, N.A. An assessment of some internal characters in the higher classification of the curculionidae s.l. (coleoptera). Trans. R. Entomol. Soc. Lond. 1961, 113, 417–480. [Google Scholar] [CrossRef]
- Ning, H.; Tang, M.; Chen, H. Mapping Invasion Potential of the Pest from Central Asia, Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae), in the Shelter Forests of Northwest China. Insects 2021, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Grodner, M.L. Aberrant spermatogenesis in hybrid progeny of sub-species of the boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1975, 4, 107–114. [Google Scholar] [CrossRef]
- Goldson, S.L.; Emberson, R.M. Reproductive morphology of the Argentine stem weevil, Hyperodes bonariensis (Coleoptera: Curculionidae). N. Z. J. Zoöl. 1981, 8, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Koçakoğlu, N.Ö.; Candan, S.; Güllü, M. The histomorphological structure of the male reproductive system of maize leaf weevil Tanymecus dilaticollis Gyllenhal, 1834 (Coleoptera: Curculionidae). Microsc. Res. Tech. 2019, 82, 1345–1352. [Google Scholar] [CrossRef]
- Barker, G.M. Functional anatomy of the reproductive system ofListronotus bonariensis (Kuschel). N. Z. Èntomol. 1989, 12, 34–42. [Google Scholar] [CrossRef]
- Gassner, G.; Childress, D.; Klemetson, D.J.; Iii, G.G. Spermiogenesis in boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1975, 4, 115–125. [Google Scholar] [CrossRef]
- Gracielle, I.M.S.; Fiorillo, B.S.; Lino-Neto, J.; Báo, S.N. Morphology of the male reproductive system and spermiogenesis in Hypanthidium foveolatum (Alfken, 1930) (Hymenoptera: Apidae: Megachilinae). Micron 2009, 40, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Hua, B. RETRACTED: Sperm ultrastructure in two species of Panorpa and one Bittacus (Mecoptera). Micron 2010, 41, 622–632. [Google Scholar] [CrossRef]
- Werner, M.; Simmons, L.W. Ultrastructure of spermatozoa of Onthophagus taurus (Coleoptera, Scarabaeidae) exhibits heritable variation. Naturwissenschaften 2011, 98, 213–223. [Google Scholar] [CrossRef]
- Dias, G.; Lino-Neto, J.; Mercati, D.; Dallai, R. The sperm ultrastructure and spermiogenesis of Tribolium castaneum (Coleoptera: Tenebrionidae) with evidence of cyst degeneration. Micron 2015, 73, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Jurecic, R. Sperm cell number per bundle in Gnorimus nobilis L. (Coleoptera, Scarabaeidae). Genetica 1988, 76, 27–31. [Google Scholar] [CrossRef]
- Virkki, N. Sperm bundles and phylogenesis. Cell Tissue Res. 1969, 101, 13–27. [Google Scholar] [CrossRef]
- Paoli, F.; Dallai, R.; Cristofaro, M.; Arnone, S.; Francardi, V.; Roversi, P.F. Morphology of the male reproductive system, sperm ultrastructure and γ-irradiation of the red palm weevil Rhynchophorus ferrugineus Oliv. (Coleoptera: Dryophthoridae). Tissue Cell 2014, 46, 274–285. [Google Scholar] [CrossRef]
- Morrone, J.J.; Marvaldi, A.E. Phylogenetic systematics of weevils (Coleoptera: Curculionoidea): A reappraisal based on larval and adult morphology. Insect Syst. Evol. 2000, 31, 43–58. [Google Scholar] [CrossRef]
- Phillips, D.M. Insect sperm: Their structure and morphogenesis. J. Cell Biol. 1970, 44, 243–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatenby, J.B.; Tahmisian, T.N. Centriole adjunct, centrioles, mitochondria, and ergastoplasm in Orthopteran spermatogenesis. An electron microscope study. Cellule Rec. Trav. Originaux Cytol. Histol. Biol. Gen. 1959, 60, 105. [Google Scholar]
- Kaye, J.S. Acrosome formation in the house cricket. J. Cell Biol. 1962, 12, 411–431. [Google Scholar] [CrossRef]
- Phillips, D.M. Observations on spermiogenesis in the fungus gnat sciara coprophila. J. Cell Biol. 1966, 30, 477–497. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.M. Fine structure of sciara coprophila sperm. J. Cell Biol. 1966, 30, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanciulli, P.P.; Mercati, D.; Machida, R.; Dallai, R. Spermiogenesis and sperm ultrastructure of Machilontus sp (Insecta: Archaeognatha) with phylogenetic consideration. Micron 2015, 73, 47–53. [Google Scholar] [CrossRef]
- Alzahrani, A.M.; Abdelsalam, S.A.; Elmenshawy, O.M.; Abdel-Moneim, A.M. Ultrastructural Characteristics of Spermiogenesis inRhynchophorus ferrugineus (Coleoptera: Curculionidae). Fla. Èntomol. 2013, 96, 1463–1469. [Google Scholar] [CrossRef]
- Dallai, R.; Lino-Neto, J.; Dias, G.; Nere, P.H.A.; Mercati, D.; Lupetti, P. Fine structure of the ladybird spermatozoa (Insecta, Coleoptera, Coccinellidae). Arthropod Struct. Dev. 2018, 47, 286–298. [Google Scholar] [CrossRef]
- Jamieson, B.G.M.; Dallai, R.; Afzelius, B.A. Insects: Their Spermatozoa and Phylogeny; Science Publishers: Enfield, CT, USA, 1999; p. 555. [Google Scholar]
- Mojica, J.M.; Bruck, D.L. Sperm bundle coiling: Transporting long sperm bundles in Drosophila dunni dunni. J. Insect Physiol. 1996, 42, 303–307. [Google Scholar] [CrossRef]
- Moreira, J.; Zama, U.; Lino-Neto, J. Release, behavior and phylogenetic significance of spermatozoa in bundles in the seminal vesicle during sexual maturation in Aculeata (Hymenoptera). Braz. J. Morphol. Sci. 2004, 21, 185–189. [Google Scholar]
- Moreira, J.; Brito, P.; Mancini, K.; Dolder, H.; Lino-Neto, J. The descriptions of new microanatomical structures of the male reproductive system and sperm of Myschocyttarus cassununga (Hymenoptera: Vespidae). Micron 2012, 43, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Moreira, J.; Gomes, L.F.; Camargo-Mathias, M.I.; Lino-Neto, J. Sperm Bundles in the Seminal Vesicle of the Crematogaster victima (Smith) Adult Males (Hymenoptera: Formicidae). Neotrop. Èntomol. 2014, 43, 201–208. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).