Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors?
Abstract
Simple Summary
Abstract
1. Introduction
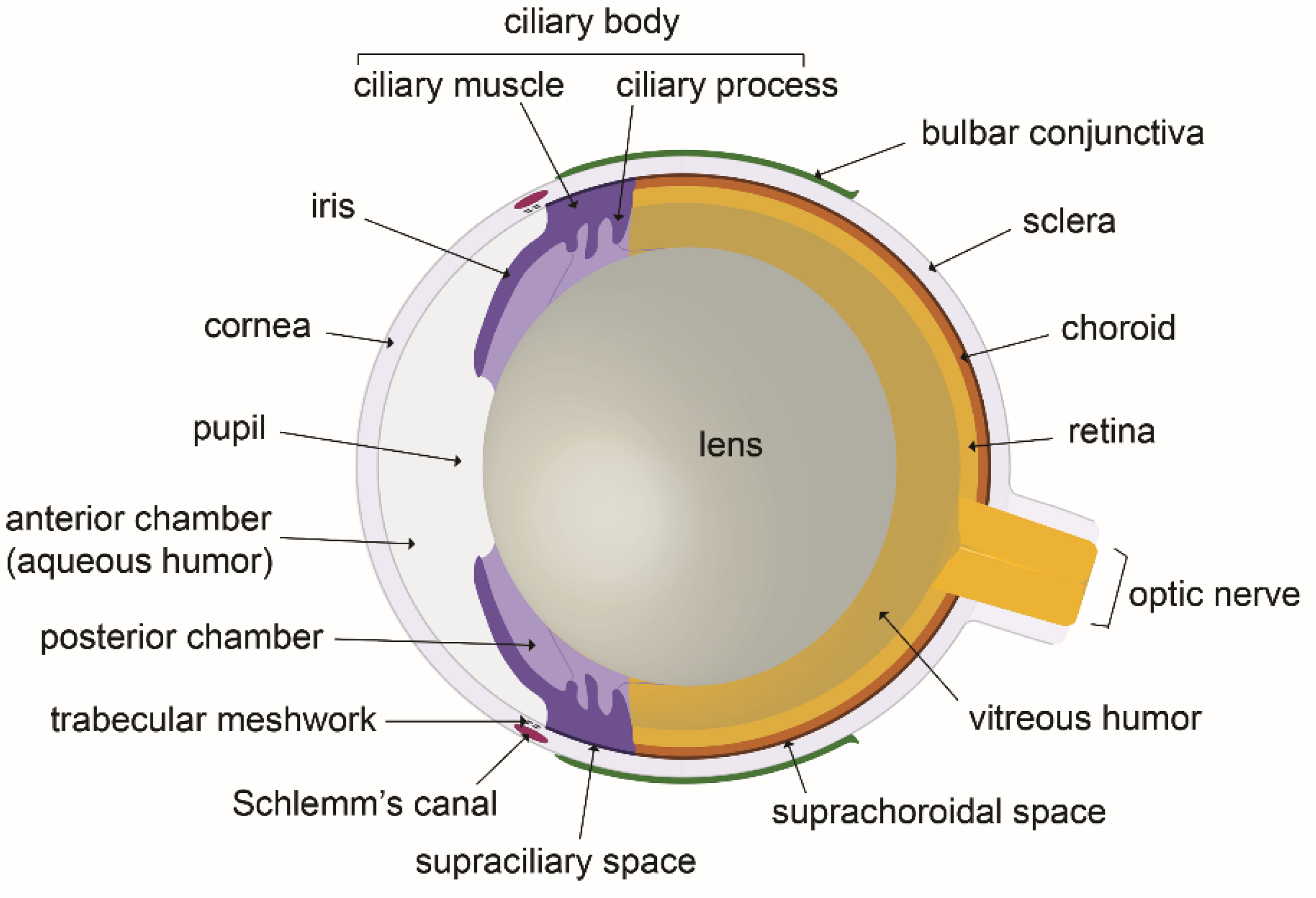
2. The Fluid Compartments of the Eye and the Ocular Fluids Dynamic
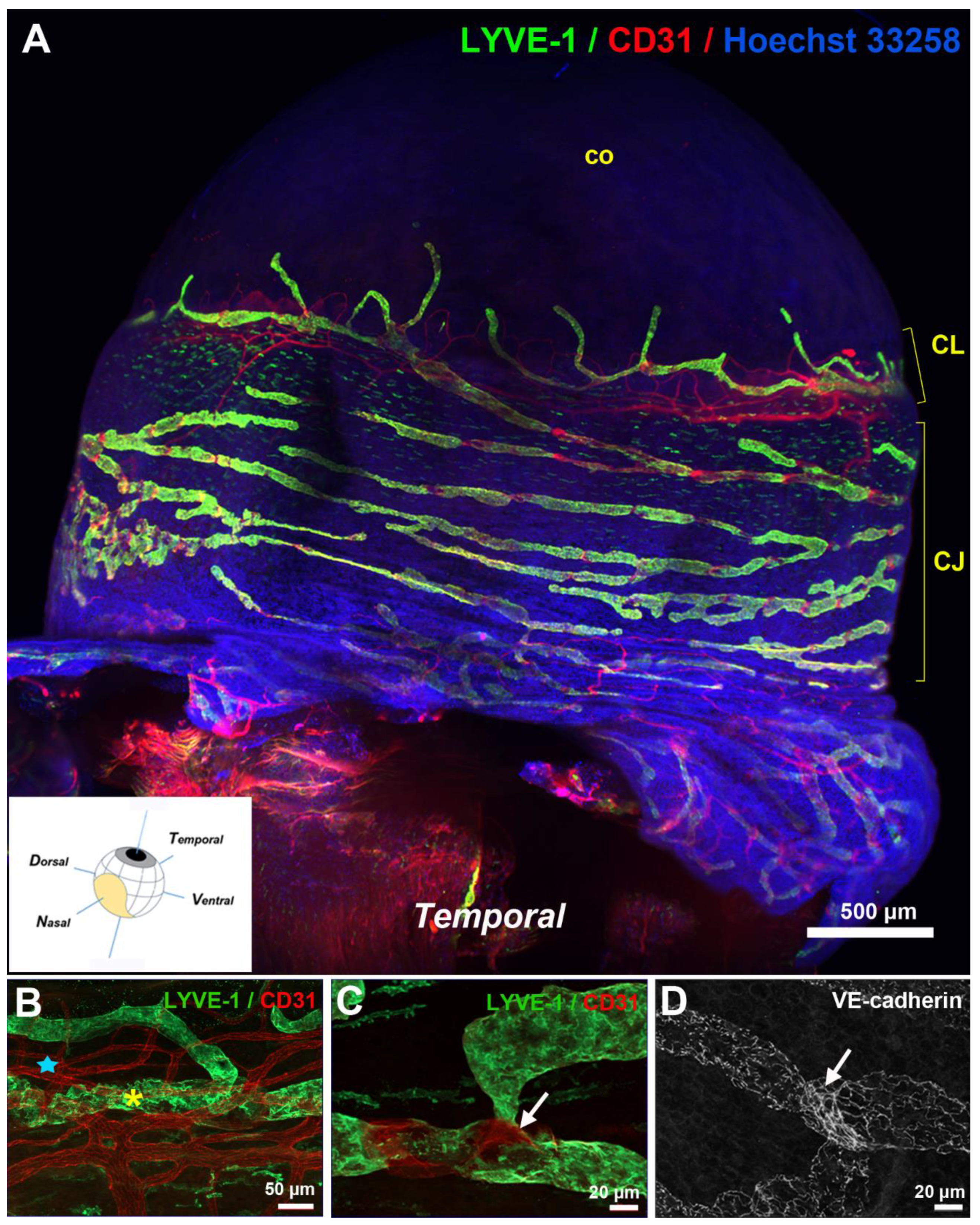
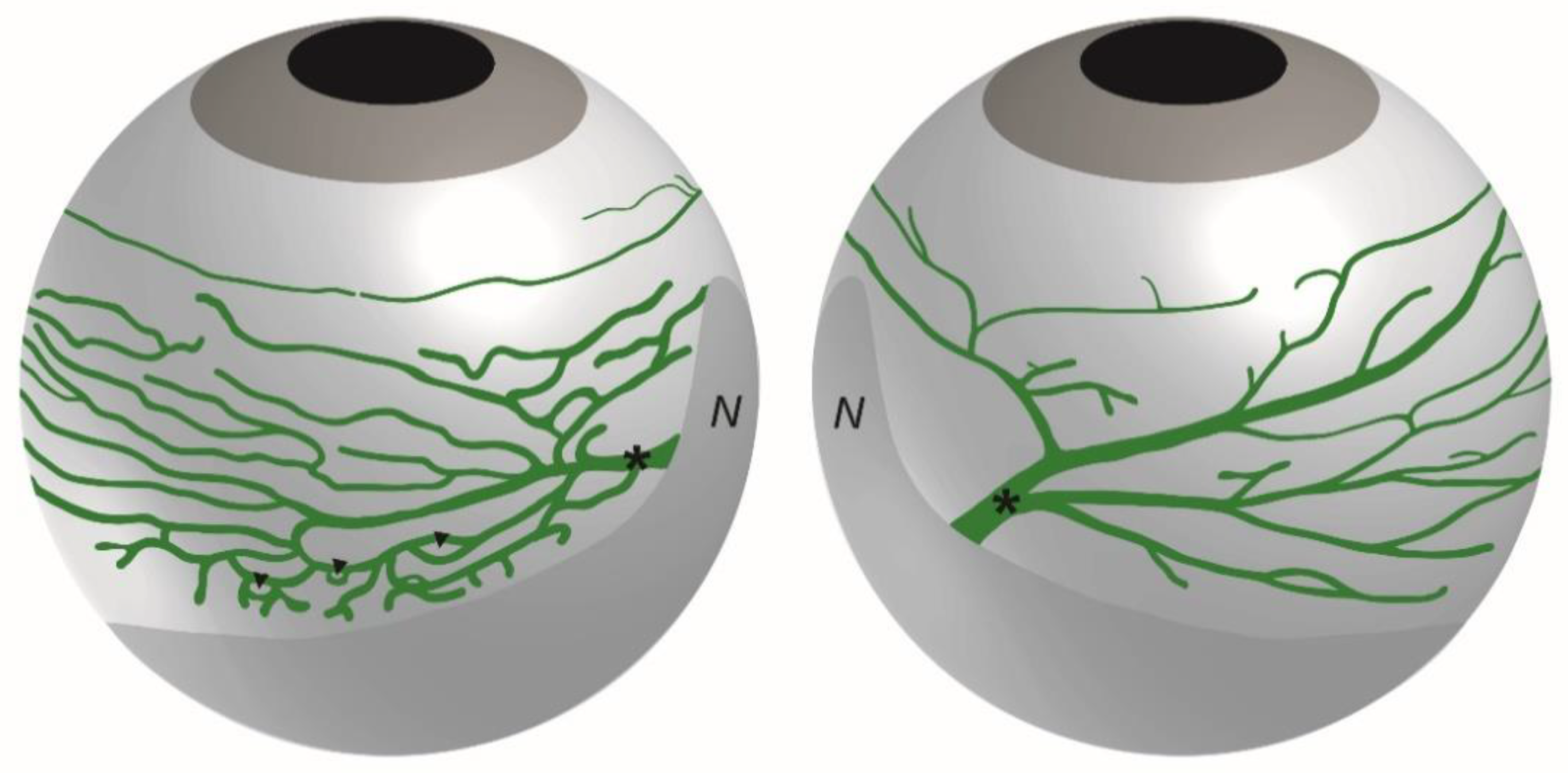
3. Anatomy of the Eye Lymphatic Vascular System
4. Molecular Mechanisms Regulating Eye Lymphatic Vessel Development
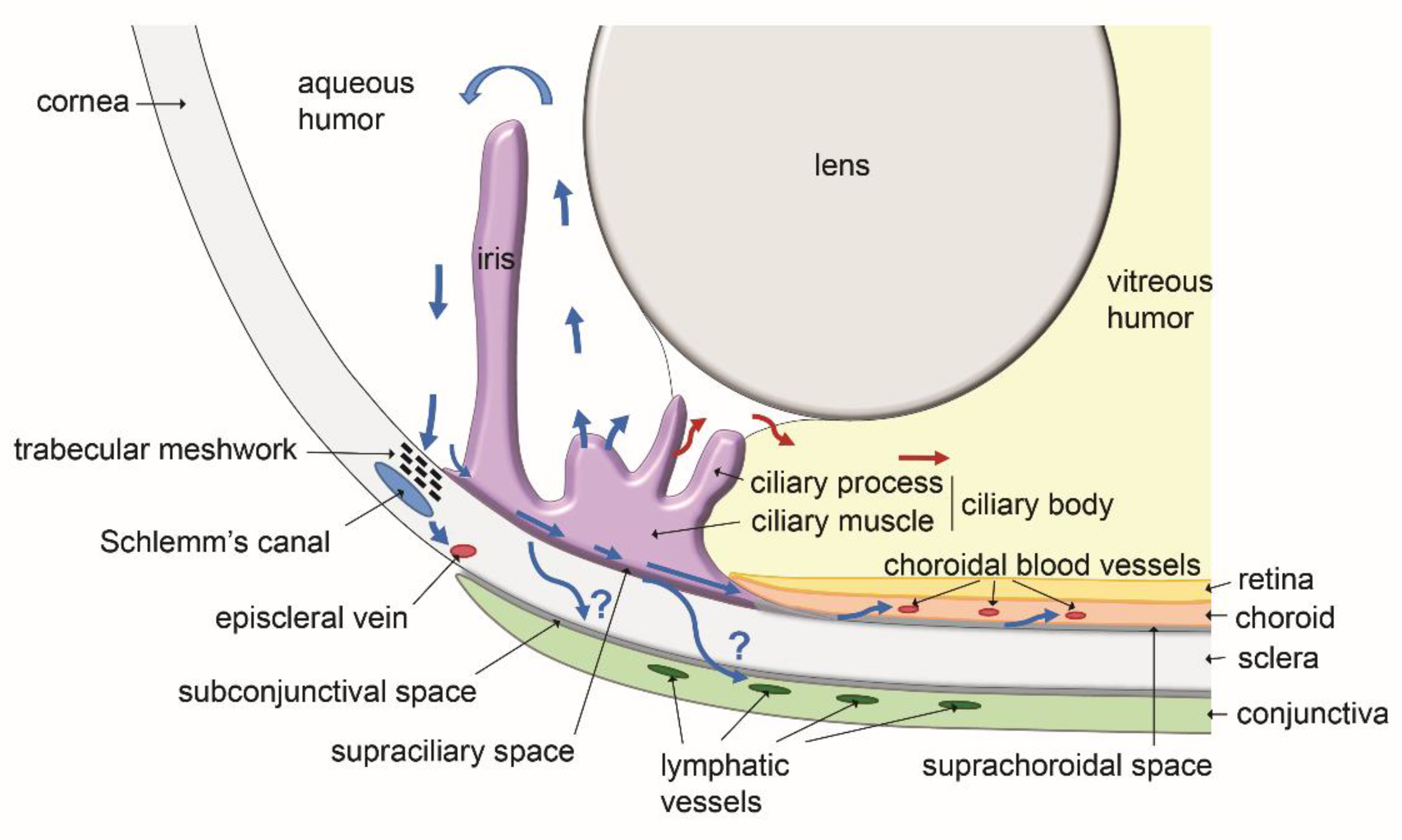
5. Functional Role of Eye Lymphatics in Aqueous Humor Drainage and Intraocular Pressure
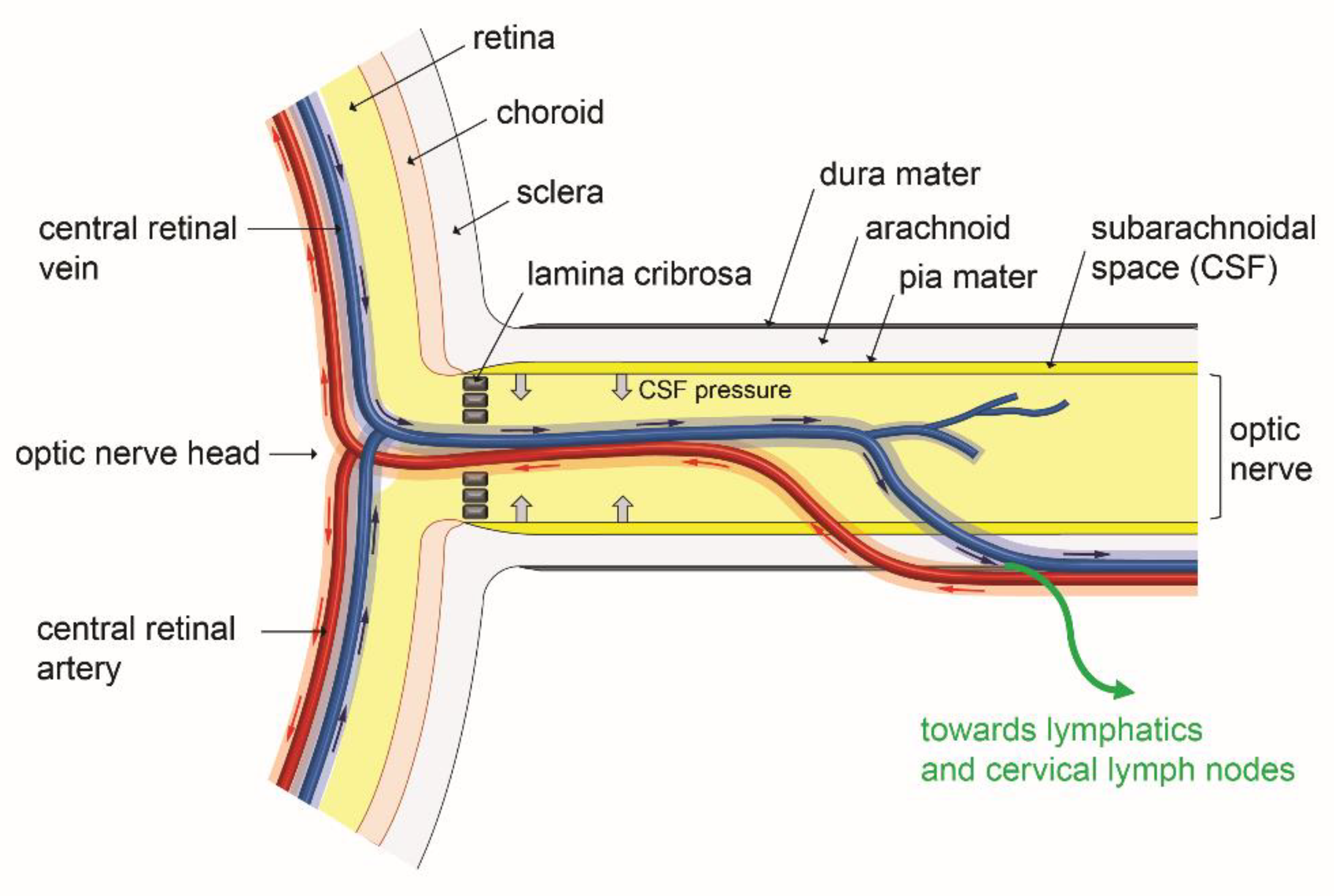
6. Interaction of an Ocular Glymphatic System with IOP and Retinal Fluid Clearance
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, T.V.; Koh, G.Y. Biological functions of lymphatic vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef] [PubMed]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- To, C.-H.; Kong, C.-W.; Chan, C.-Y.; Shahidullah, M.; Do, C.-W. The mechanism of aqueous humour formation. Clin. Exp. Optom. 2002, 85, 335–349. [Google Scholar] [PubMed]
- Nakao, S.; Hafezi-Moghadam, A.; Ishibashi, T. Lymphatics and Lymphangiogenesis in the Eye. J. Ophthalmol. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Hos, D.; Schlereth, S.L.; Bock, F.; Heindl, L.M.; Cursiefen, C. Antilymphangiogenic therapy to promote transplant survival and to reduce cancer metastasis: What can we learn from the eye? Semin. Cell Dev. Biol. 2015, 38, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Gruntzig, J.; Hollmann, F. Lymphatic vessels of the eye-old questions-new insights. Ann. Anat. 2019, 221, 1–16. [Google Scholar] [CrossRef]
- Kiel, J.; Hollingsworth, M.; Rao, R.; Chen, M.; Reitsamer, H. Ciliary blood flow and aqueous humor production. Prog. Retin. Eye Res. 2011, 30, 1–17. [Google Scholar] [CrossRef]
- Sacco, R.; Guidoboni, G.; Jerome, J.W.; Bonifazi, G.; Marazzi, N.M.; Vercellin, A.C.V.; Lang, M.S.; Harris, A. A Theoretical Approach for the Electrochemical Characterization of Ciliary Epithelium. Life 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Santos, F.M.; Rocha, A.S.; Castro-De-Sousa, J.P.; Queiroz, J.A.; Passarinha, L.A.; Tomaz, C.T. Vitreous humor in the pathologic scope: Insights from proteomic approaches. Proteom. Clin. Appl. 2014, 9, 187–202. [Google Scholar] [CrossRef]
- Nawaz, M.; Rezzola, S.; Cancarini, A.; Russo, A.; Costagliola, C.; Semeraro, F.; Presta, M. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog. Retin. Eye Res. 2019, 72, 100756. [Google Scholar] [CrossRef]
- Huang, A.S.; Francis, B.A.; Weinreb, R.N. Structural and functional imaging of aqueous humour outflow: A review. Clin. Exp. Ophthalmol. 2018, 46, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Petrash, J.M. Aging and Age-Related Diseases of the Ocular Lens and Vitreous Body. Investig. Opthalmol. Vis. Sci. 2013, 54, ORSF54–ORSF59. [Google Scholar] [CrossRef]
- Wang, Q.; Thau, A.; Levin, A.V.; Lee, D. Ocular hypotony: A comprehensive review. Surv. Ophthalmol. 2019, 64, 619–638. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; McLaren, J.W.; Overby, D. Unconventional aqueous humor outflow: A review. Exp. Eye Res. 2017, 158, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Lee, C.-J.; Gardiner, B.S. No flow through the vitreous humor: How strong is the evidence? Prog. Retin. Eye Res. 2020, 78, 100845. [Google Scholar] [CrossRef]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fer-nandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharma-cokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Cursiefen, C.; Schlötzer-Schrehardt, U.; Küchle, M.; Sorokin, L.; Breiteneder-Geleff, S.; Alitalo, K.; Jackson, D. Lymphatic vessels in vascularized human corneas: Immunohistochemical investigation using LYVE-1 and podoplanin. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2127–2135. [Google Scholar]
- Ecoiffier, T.; Yuen, N.; Chen, L. Differential Distribution of Blood and Lymphatic Vessels in the Murine Cornea. Investig. Opthalmol. Vis. Sci. 2010, 51, 2436–2440. [Google Scholar] [CrossRef]
- Cao, R.; Lim, S.; Ji, H.; Zhang, Y.; Yang, Y.; Honek, J.; Hedlund, E.-M.; Cao, Y. Mouse corneal lymphangiogenesis model. Nat. Protoc. 2011, 6, 817–826. [Google Scholar] [CrossRef]
- van der Merwe, E.L.; Kidson, S.H. The three-dimensional organisation of the post-trabecular aqueous outflow pathway and limbal vasculature in the mouse. Exp. Eye Res. 2014, 125, 226–235. [Google Scholar] [CrossRef]
- Wu, Y.; Seong, Y.J.; Li, K.; Choi, D.; Park, E.; Daghlian, G.; Jung, E.; Bui, K.; Zhao, L.; Madhavan, S.; et al. Organogenesis and distribution of the ocular lymphatic vessels in the anterior eye. JCI Insight 2020, 5, 5. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.H. Novel aspects of corneal an-giogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Dohlman, T.H.; Dana, R. Corneal Lymphatics: Role in Ocular Inflammation as Inducer and Responder of Adaptive Immunity. J. Clin. Cell Immunol. 2014, 5, 1000256. [Google Scholar] [CrossRef]
- Kang, G.J.; Ecoiffier, T.; Truong, T.; Yuen, N.; Li, G.; Lee, N.; Zhang, L.; Chen, L. Intravital Imaging Reveals Dynamics of Lymphangiogenesis and Valvulogenesis. Sci. Rep. 2016, 6, 19459. [Google Scholar] [CrossRef] [PubMed]
- Subileau, M.; Acar, N.; Carret, A.; Bretillon, L.; Vilgrain, I.; Bailly, S.; Vittet, D. Eye lymphatic defects induced by bone morphogenetic protein 9 deficiency have no functional consequences on intraocular pressure. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Ecoiffier, T.; Sadovnikova, A.; Yuen, N.; Chen, L. Conjunctival Lymphatic Response to Corneal Inflammation in Mice. J. Ophthalmol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Chen, L. High resolution three-dimensional imaging of the ocular surface and intact eyeball using tissue clearing and light sheet microscopy. Ocul. Surf. 2020, 18, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Perkins, E. The nictitating membranes of primates. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1968, 162, 53–69. [Google Scholar] [CrossRef]
- Moore, C.P.; Constantinescu, G.M. Surgery of the Adnexa. Veter Clin. N. Am. Small Anim. Pr. 1997, 27, 1011–1066. [Google Scholar] [CrossRef]
- Burri, P.H.; Hlushchuk, R.; Djonov, V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev. Dyn. 2004, 231, 474–488. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Carrasco, J.L. Intussusceptive lymphangiogenesis in the sinuses of developing human foetal lymph nodes. Ann. Anat. Anat. Anz. 2019, 226, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.D.P.; Carrasco, J.L.; Sáez, F.J.; González-Gómez, M.; Madrid, J.F. Intussusceptive Lymphangiogenesis in Lymphatic Malformations/Lymphangiomas. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 302, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Ulvmar, M.H.; Mäkinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 2016, 111, 310–321. [Google Scholar] [CrossRef]
- Jafree, D.J.; Long, D.A.; Scambler, P.J.; Ruhrberg, C. Mechanisms and cell lineages in lymphatic vascular development. Angiogenesis 2021, 24, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Yücel, Y.H.; Johnston, M.G.; Ly, T.; Patel, M.; Drake, B.; Gümüş, E.; Fraenkl, S.A.; Moore, S.; Tobbia, D.; Armstrong, D.; et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp. Eye Res. 2009, 89, 810–819. [Google Scholar] [CrossRef]
- Birke, K.; Kerjaschki, N.; Birke, M.T.; Lütjen-Drecoll, E. Expression of Podoplanin and Other Lymphatic Markers in the Human Anterior Eye Segment. Investig. Opthalmol. Vis. Sci. 2010, 51, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kaser-Eichberger, A.; Schrödl, F.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Brückner, D.; Laimer, M.; Schlereth, S.L.; et al. Topography of Lymphatic Markers in Human Iris and Ciliary Body. Investig. Opthalmol. Vis. Sci. 2015, 56, 4943–4953. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Reid, D.M.; Forrester, J.V. LYVE-1–Positive Macrophages Are Present in Normal Murine Eyes. Investig. Opthalmol. Vis. Sci. 2007, 48, 2162–2171. [Google Scholar] [CrossRef]
- Schroedl, F.; Brehmer, A.; Neuhuber, W.L.; Kruse, F.E.; May, C.A.; Cursiefen, C. The Normal Human Choroid Is Endowed with a Significant Number of Lymphatic Vessel Endothelial Hyaluronate Receptor 1 (LYVE-1)-Positive Macrophages. Investig. Opthalmol. Vis. Sci. 2008, 49, 5222–5286. [Google Scholar] [CrossRef] [PubMed]
- Schrödl, F.; Kaser-Eichberger, A.; Trost, A.; Strohmaier, C.; Bogner, B.; Runge, C.; Motloch, K.; Brückner, D.; Laimer, M.; Heindl, L.M.; et al. Lymphatic Markers in the Adult Human Choroid. Investig. Opthalmol. Vis. Sci. 2015, 56, 7406. [Google Scholar] [CrossRef]
- Schlereth, S.L.; Neuser, B.; Caramoy, A.; Grajewski, R.S.; Koch, K.R.; Schrödl, F.; Cursiefen, C.; Heindl, L.M. Enrichment of Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE1)-Positive Macrophages Around Blood Vessels in the Normal Human Sclera. Investig. Opthalmol. Vis. Sci. 2014, 55, 865–872. [Google Scholar] [CrossRef]
- Trost, A.; Runge, C.; Bruckner, D.; Kaser-Eichberger, A.; Bogner, B.; Strohmaier, C.; Reitsamer, H.; Schroedl, F. Lymphatic markers in the human optic nerve. Exp. Eye Res. 2018, 173, 113–120. [Google Scholar] [CrossRef] [PubMed]
- González-Loyola, A.; Petrova, T.V. Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 2021, 169, 63–78. [Google Scholar] [CrossRef]
- Eklund, L.; Kangas, J.; Saharinen, P. Angiopoietin–Tie signalling in the cardiovascular and lymphatic systems. Clin. Sci. 2017, 131, 87–103. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Pawlak, J.B.; Caron, K.M. Lymphatic Programing and Specialization in Hybrid Vessels. Front. Physiol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Aspelund, A.; Tammela, T.; Antila, S.; Nurmi, H.; Leppanen, V.M.; Zarkada, G.; Stanczuk, L.; Francois, M.; Makinen, T.; Sa-harinen, P.; et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J. Clin. Investig. 2014, 124, 3975–3986. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-Y.; Lee, J.; Park, I.; Choi, D.; Lee, S.; Song, S.; Hwang, Y.; Hong, K.Y.; Nakaoka, Y.; Makinen, T.; et al. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J. Clin. Investig. 2014, 124, 3960–3974. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.R.; Heinen, S.; Jeansson, M.; Ghosh, A.K.; Fatima, A.; Sung, H.-K.; Onay, T.; Chen, H.; Yamaguchi, S.; Economides, A.N.; et al. A lymphatic defect causes ocular hypertension and glaucoma in mice. J. Clin. Investig. 2014, 124, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Elamaa, H.; Kihlström, M.; Kapiainen, E.; Kaakinen, M.; Miinalainen, I.; Ragauskas, S.; Cerrada-Gimenez, M.; Mering, S.; Nätynki, M.; Eklund, L. Angiopoietin-4-dependent venous maturation and fluid drainage in the peripheral retina. eLife 2018, 7, 7. [Google Scholar] [CrossRef]
- Kubo, H.; Cao, R.; Brakenhielm, E.; Makinen, T.; Cao, Y.; Alitalo, K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 2002, 99, 8868–8873. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chauhan, S.K.; Dana, R. Blockade of Prolymphangiogenic Vascular Endothelial Growth Factor C in Dry Eye Disease. Arch. Ophthalmol. 2012, 130, 84–89. [Google Scholar] [CrossRef]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef]
- Hos, D.; Bucher, F.; Regenfuss, B.; Dreisow, M.-L.; Bock, F.; Heindl, L.M.; Eming, S.A.; Cursiefen, C. IL-10 Indirectly Regulates Corneal Lymphangiogenesis and Resolution of Inflammation via Macrophages. Am. J. Pathol. 2016, 186, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, G.; Sessa, R.; Kang, G.J.; Shi, M.; Ge, S.; Gong, A.J.; Wen, Y.; Chintharlapalli, S.; Chen, L. Angiopoietin-2 Blockade Promotes Survival of Corneal Transplants. Investig. Opthalmol. Vis. Sci. 2017, 58, 79–86. [Google Scholar] [CrossRef]
- Garcia de Vinuesa, A.; Abdelilah-Seyfried, S.; Knaus, P.; Zwijsen, A.; Bailly, S. BMP signaling in vascular biology and dys-function. Cytokine Growth Factor. Rev. 2016, 27, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Fukushima, Y.; Maruyama, K.; Hirashima, M.; Nishida, K.; Nishikawa, S.-I.; Uemura, A. EphrinB2–EphB4 Signals Regulate Formation and Maintenance of Funnel-Shaped Valves in Corneal Lymphatic Capillaries. Investig. Opthalmol. Vis. Sci. 2013, 54, 4102–4108. [Google Scholar] [CrossRef] [PubMed]
- Betterman, K.L.; Harvey, N.L. The lymphatic vasculature: Development and role in shaping immunity. Immunol. Rev. 2016, 271, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Dana, R. Corneal Lymphangiogenesis: Implications in Immunity. Semin. Ophthalmol. 2009, 24, 135–138. [Google Scholar] [CrossRef]
- Ji, Y.W.; Lee, J.L.; Kang, H.G.; Gu, N.; Byun, H.; Yeo, A.; Noh, H.; Kim, S.; Choi, E.Y.; Song, J.S.; et al. Corneal lymphan-giogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul. Surf. 2018, 16, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Bukowiecki, A.; Horstmann, J.; Bock, F.; Bucher, F.; Heindl, L.M.; Siebelmann, S.; Steven, P.; Dana, R.; Eming, S.A.; et al. Transient ingrowth of lymphatic vessels into the physiologically avascular cornea regulates corneal edema and trans-parency. Sci. Rep. 2017, 7, 7227. [Google Scholar] [CrossRef][Green Version]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.-H.; Han, K.-Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and lymphangiogenesis in corneal transplantation–A review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef]
- Chennakesavalu, M.; Somala, S.R.R.; Dommaraju, S.R.; Peesapati, M.P.; Guo, K.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Corneal lymphangiogenesis as a potential target in dry eye disease-a systematic review. Surv. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Camelo, S.; Kezic, J.; Shanley, A.; Rigby, P.; McMenamin, P.G. Antigen from the Anterior Chamber of the Eye Travels in a Soluble Form to Secondary Lymphoid Organs via Lymphatic and Vascular Routes. Investig. Opthalmol. Vis. Sci. 2006, 47, 1039–1046. [Google Scholar] [CrossRef]
- Tam, A.L.C.; Gupta, N.; Zhang, Z.; Yücel, Y.H. Quantum dots trace lymphatic drainage from the mouse eye. Nanotechnology 2011, 22, 425101. [Google Scholar] [CrossRef] [PubMed]
- Guignier, B.; Bourahla, K.; Bekaert, V.; Brasse, D.; Gaucher, D.; Speeg-Schatz, C.; Bourcier, T. Scintigraphic study of the lymphatic drainage of the anterior chamber of the mouse eye and its pathophysiological implications. J. Fr. Ophtalmol. 2013, 36, 836–842. [Google Scholar] [CrossRef]
- Tam, A.L.C.; Gupta, N.; Zhang, Z.; Yücel, Y.H. Latanoprost Stimulates Ocular Lymphatic Drainage: An In Vivo Nanotracer Study. Transl. Vis. Sci. Technol. 2013, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Yücel, Y.H.; Cardinell, K.; Khattak, S.; Zhou, X.; Lapinski, M.; Cheng, F.; Gupta, N. Active Lymphatic Drainage From the Eye Measured by Noninvasive Photoacoustic Imaging of Near-Infrared Nanoparticles. Investig. Opthalmol. Vis. Sci. 2018, 59, 2699–2707. [Google Scholar] [CrossRef]
- Camelo, S.; Lajavardi, L.; Bochot, A.; Goldenberg, B.; Naud, M.C.; Fattal, E.; Behar-Cohen, F.; de Kozak, Y. Drainage of flu-orescent liposomes from the vitreous to cervical lymph nodes via conjunctival lymphatics. Ophthalmic. Res. 2008, 40, 145–150. [Google Scholar] [CrossRef]
- Cardinell, K.; Gupta, N.; Koivisto, B.D.; Kumaradas, J.C.; Zhou, X.; Irving, H.; Luciani, P.; Yucel, Y.H. A novel photoacous-tic-fluorescent contrast agent for quantitative imaging of lymphatic drainage. Photoacoustics 2021, 21, 100239. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Morgan, W.H.; Sun, X.; Su, E.-N.; Cringle, S.J.; Yu, P.K.; House, P.; Guo, W.; Yu, X. The critical role of the conjunctiva in glaucoma filtration surgery. Prog. Retin. Eye Res. 2009, 28, 303–328. [Google Scholar] [CrossRef]
- Costagliola, C.; Dell’Omo, R.; Agnifili, L.; Bartollino, S.; Fea, A.M.; Uva, M.G.; Zeppa, L.; Mastropasqua, L. How many aqueous humor outflow pathways are there? Surv. Ophthalmol. 2020, 65, 144–170. [Google Scholar] [CrossRef]
- Bock, F.; Onderka, J.; Braun, G.; Schneider, A.C.; Hos, D.; Bi, Y.; Bachmann, B.O.; Cursiefen, C. Identification of Novel En-dogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6554–6560. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, L.; Ye, E.-A.; Wang, A.; Li, G.; Chen, L. Aqueous humor induces lymphatic regression on the ocular surface. Ocul. Surf. 2020, 18, 505–510. [Google Scholar] [CrossRef]
- Kim, M.; Johnston, M.G.; Gupta, N.; Moore, S.; Yücel, Y.H. A model to measure lymphatic drainage from the eye. Exp. Eye Res. 2011, 93, 586–591. [Google Scholar] [CrossRef]
- Yücel, Y.H.; Cheng, F.; Cardinell, K.; Zhou, X.; Irving, H.; Gupta, N. Age-related decline of lymphatic drainage from the eye: A noninvasive in vivo photoacoustic tomography study. Exp. Eye Res. 2020, 194, 108029. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, K.I.; Jeoung, J.W.; Park, K.H. Intraocular Pressure-Lowering Effect of Latanoprost Is Hampered by Defective Cervical Lymphatic Drainage. PLoS ONE 2017, 12, e0169683. [Google Scholar] [CrossRef]
- Lindsey, J.D.; Hofer, A.; Wright, K.N.; Weinreb, R.N. Partitioning of the aqueous outflow in rat eyes. Investig. Opthalmol. Vis. Sci. 2009, 50, 5754–5758. [Google Scholar] [CrossRef] [PubMed]
- Lohrberg, M.; Wilting, J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 2016, 366, 667–677. [Google Scholar] [CrossRef]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef]
- Ashok, A.; Singh, N.; Chaudhary, S.; Bellamkonda, V.; Kritikos, A.E.; Wise, A.S.; Rana, N.; McDonald, D.; Ayyagari, R. Retinal Degeneration and Alzheimer’s Disease: An Evolving Link. Int. J. Mol. Sci. 2020, 21, 7290. [Google Scholar] [CrossRef]
- Chen, L. Ocular lymphatics: State-of-the-art review. Lymphology 2009, 42, 66–76. [Google Scholar] [PubMed]
- Denniston, A.K.; Keane, P.A. Paravascular Pathways in the Eye: Is There an ‘Ocular Glymphatic System’? Investig. Ophthalmol. Vis. Sci. 2015, 56, 3955–3956. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Murcia-Belmonte, V.; Erskine, L. Wiring the Binocular Visual Pathways. Int. J. Mol. Sci. 2019, 20, 3282. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.; Rouhani, S.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nat. Cell Biol. 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Valenza, M.; Facchinetti, R.; Steardo, L.; Scuderi, C. Altered Waste Disposal System in Aging and Alzheimer’s Disease: Focus on Astrocytic Aquaporin-4. Front. Pharmacol. 2020, 10, 1656. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef]
- Bacyinski, A.; Xu, M.; Wang, W.; Hu, J. The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front. Neuroanat. 2017, 11, 101. [Google Scholar] [CrossRef]
- Smith, A.J.; Verkman, A.S. CrossTalk opposing view: Going against the flow: Interstitial solute transport in brain is diffusive and aquaporin-4 independent. J. Physiol. 2019, 597, 4421–4424. [Google Scholar] [CrossRef]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nat. Cell Biol. 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.; Ringstad, G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Forstera, B.; Peng, S.; Shi, M.; Ladron-de-Guevara, A.; et al. An ocular glymphatic clearance system removes beta-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, 536. [Google Scholar] [CrossRef]
- Niwa, M.; Aoki, H.; Hirata, A.; Tomita, H.; Green, P.G.; Hara, A. Retinal Cell Degeneration in Animal Models. Int. J. Mol. Sci. 2016, 17, 110. [Google Scholar] [CrossRef]
- Hayreh, S.S. Occlusion of the central retinal vessels. Br. J. Ophthalmol. 1965, 49, 626–645. [Google Scholar] [CrossRef]
- Thrane, V.R.; Hynnekleiv, L.; Wang, X.; Thrane, A.S.; Krohn, J.; Nedergaard, M. Twists and turns of ocular glymphatic clearance–new study reveals surprising findings in glaucoma. Acta Ophthalmol. 2021, 99, e283–e284. [Google Scholar] [CrossRef]
- Mestre, H.; Kostrikov, S.; Mehta, R.I.; Nedergaard, M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. 2017, 131, 2257–2274. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, N.; Zhang, M.; Ren, X.; Tang, J.; Fu, J. Concomitant enlargement of perivascular spaces and decrease in glymphatic transport in an animal model of cerebral small vessel disease. Brain Res. Bull. 2020, 161, 78–83. [Google Scholar] [CrossRef]
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad between the Immune, Vascular and Nervous System. Front. Neuroanat. 2020, 14, 17. [Google Scholar] [CrossRef]
- Verkman, A.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef]
- Schey, K.L.; Wang, Z.; Wenke, J.L.; Qi, Y. Aquaporins in the eye: Expression, function, and roles in ocular disease. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 1513–1523. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, J.; Zhang, W.; Gong, X.; Yan, S.; Zhang, K.; Luo, Z.; Sun, J.; Jiang, Q.; Lou, M. Impairment of the Glymphatic Pathway and Putative Meningeal Lymphatic Vessels in the Aging Human. Ann. Neurol. 2020, 87, 357–369. [Google Scholar] [CrossRef]
- Jonas, J.B.; Ritch, R.; Panda-Jonas, S. Cerebrospinal fluid pressure in the pathogenesis of glaucoma. Prog. Brain Res. 2015, 221, 33–47. [Google Scholar] [CrossRef]
- Mirra, S.; Marfany, G.; Garcia-Fernandez, J. Under pressure: Cerebrospinal fluid contribution to the physiological homeo-stasis of the eye. Semin. Cell Dev. Biol. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Mathieu, E.; Gupta, N.; Ahari, A.; Zhou, X.; Hanna, J.; Yücel, Y.H. Evidence for Cerebrospinal Fluid Entry into the Optic Nerve via a Glymphatic Pathway. Investig. Opthalmol. Vis. Sci. 2017, 58, 4784–4791. [Google Scholar] [CrossRef]
- Mathieu, E.; Gupta, N.; Paczka-Giorgi, L.A.; Zhou, X.; Ahari, A.; Lani, R.; Hanna, J.; Yücel, Y.H. Reduced Cerebrospinal Fluid Inflow to the Optic Nerve in Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 5876–5884. [Google Scholar] [CrossRef]
- Jacobsen, H.H.; Ringstad, G.; Jørstad, Ø.K.; Moe, M.C.; Sandell, T.; Eide, P.K. The Human Visual Pathway Communicates Directly with the Subarachnoid Space. Investig. Opthalmol. Vis. Sci. 2019, 60, 2773–2780. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subileau, M.; Vittet, D. Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors? Biology 2021, 10, 582. https://doi.org/10.3390/biology10070582
Subileau M, Vittet D. Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors? Biology. 2021; 10(7):582. https://doi.org/10.3390/biology10070582
Chicago/Turabian StyleSubileau, Mariela, and Daniel Vittet. 2021. "Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors?" Biology 10, no. 7: 582. https://doi.org/10.3390/biology10070582
APA StyleSubileau, M., & Vittet, D. (2021). Lymphatics in Eye Fluid Homeostasis: Minor Contributors or Significant Actors? Biology, 10(7), 582. https://doi.org/10.3390/biology10070582




