Bioactive Olivacine Derivatives—Potential Application in Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Olivacine
3. The Most Active Olivacine Derivatives
3.1. Structure–Activity Relationship (SAR) Analysis In Vitro
3.1.1. 9-Hydroxyolivacine 2
3.1.2. Azo-Olivacine Derivatives 4, 15, 16
3.2. SAR In Vivo
3.3. Clinical Trials
3.4. Influence on the Effectiveness of Radiotherapy
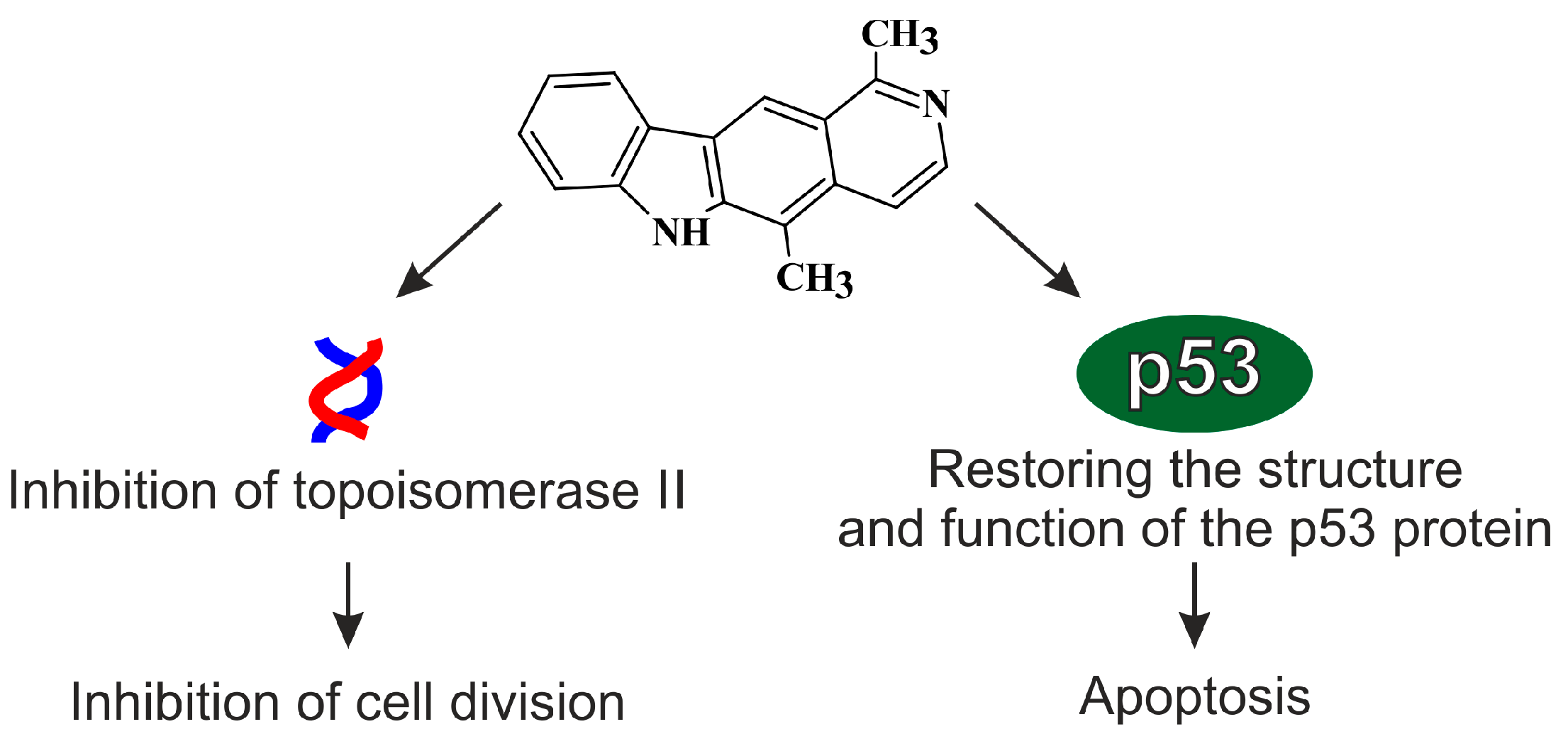
4. Mechanisms of Antitumor Activity of Olivacine and Olivacine Derivative
4.1. Inhibition of the Function of Topoisomerase II (Topo II)
4.2. p53 Protein
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwin, S.; Smith, A.F.; Horning, E.C. Alkaloids of Ochrosia elliptica Labill. J. Am. Chem. Soc. 1959, 81, 1903–1908. [Google Scholar] [CrossRef]
- Schmutz, J.; Hunziker, F. Aspidosperma-alkaloids. 3. The alkaloids of Aspidosperma olivaceum M. Arg. Pharm. Acta Helv. 1958, 33, 341–347. [Google Scholar]
- Mosher, C.W.; Crews, O.P.; Acton, E.M.; Goodman, L. Preparation and Antitumor Activity of Olivacine and Some New Analogs. J. Med. Chem. 1966, 9, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Dalton, L.; Demerac, S.; Elmes, B.; Loder, J.; Swan, J.; Teitei, T. Synthesis of the tumour-inhibitory alkaloids, ellipticine, 9-methoxyellipticine, and related pyrido[4,3-b]carbazoles. Aust. J. Chem. 1967, 20, 2715. [Google Scholar] [CrossRef]
- Kohn, K.W.; Waring, M.J.; Glaubiger, D.; Friedman, C.A. Intercalative binding of ellipticine to DNA. Cancer Res. 1975, 35, 71–76. [Google Scholar] [PubMed]
- Chu, Y.; Hsu, M. ta Ellipticine increase the superhelical density of intracellular SV40 DNA by intercalation. Nucleic Acids Res. 1992, 20, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Stiborova, M.; Rupertova, M.; Schmeiser, H.H.; Frei, E. Molecular mechanisms of antineoplastic action of an anticancer drug ellipticine. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2006, 150, 13–23. [Google Scholar] [CrossRef]
- Monnot, M.; Mauffret, O.; Simon, V.; Lescot, E.; Psaume, B.; Saucier, J.M.; Charra, M.; Belehradek, J.; Fermandjian, S. DNA-drug recognition and effects on topoisomerase II-mediated cytotoxicity. A three-mode binding model for ellipticine derivatives. J. Biol. Chem. 1991, 266, 1820–1829. [Google Scholar] [CrossRef]
- Fossé, P.; René, B.; Charra, M.; Paoletti, C.; Saucier, J.M. Stimulation of topoisomerase II-mediated DNA cleavage by ellipticine derivatives: Structure-activity relationship. Mol. Pharmacol. 1992, 42, 590–595. [Google Scholar]
- Froelich-Ammon, S.J.; Patchan, M.W.; Osheroff, N.; Thompson, R.B. Topoisomerase II binds to ellipticine in the absence or presence of DNA: Chaeacterization of enzyme-drug interactions by fluorescence spectroscopy. J. Biol. Chem. 1995, 270, 14998–15004. [Google Scholar] [CrossRef]
- Peng, Y.H.; Li, C.G.; Chen, L.H.; Sebti, S.; Chen, J.D. Rescue of mutant p53 transcription function by ellipticine. Oncogene 2003, 22, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Sugikawa, E.; Hosoi, T.; Yazaki, N.; Gamanuma, M.; Nakanishi, N.; Ohashi, M. Mutant p53 mediated induction of cell cycle arrest and apoptosis at G1 phase by 9-hydroxyellipticine. Anticancer Res. 1999, 19, 3099–3108. [Google Scholar]
- Schwaller, M.A.; Allard, B.; Lescot, E.; Moreau, F. Protonophoric activity of ellipticine and isomers across the energy-transducing membrane of mitochondria. J. Biol. Chem. 1995, 270, 22709–22713. [Google Scholar] [CrossRef] [PubMed]
- Hägg, M.; Berndtsson, M.; Mandic, A.; Zhou, R.; Shoshan, M.C.; Linder, S. Induction of endoplasmic reticulum stress by ellipticine plant alkaloids. Mol. Cancer Ther. 2004, 3, 489–497. [Google Scholar] [PubMed]
- Stiborová, M.; Bieler, C.A.; Wiessler, M.; Frei, E. The anticancer agent ellipticine on activation by cytochrome P450 forms covalent DNA adducts. Biochem. Pharmacol. 2001, 62, 1675–1684. [Google Scholar] [CrossRef]
- Frei, E.; Bieler, C.A.; Arlt, V.M.; Wiessler, M.; Stiborová, M. Covalent binding of the anticancer drug ellipticine to DNA in V79 cells transfected with human cytochrome P450 enzymes. Biochem. Pharmacol. 2002, 64, 289–295. [Google Scholar] [CrossRef]
- Stiborová, M.; Stiborová-Rupertová, M.; Bořek-Dohalská, L.; Wiessler, M.; Frei, E. Rat microsomes activating the anticancer drug ellipticine to species covalently binding to deoxyguanosine in DNA are a suitable model mimicking ellipticine bioactivation in humans. Chem. Res. Toxicol. 2003, 16, 38–47. [Google Scholar] [CrossRef]
- Stiborová, M.; Sejbal, J.; Bořek-Dohalská, L.; Aimová, D.; Poljaková, J.; Forsterová, K.; Rupertová, M.; Wiesner, J.; Hudeček, J.; Wiessler, M.; et al. The anticancer drug ellipticine forms covalent DNA adducts, mediated by human cytochromes P450, through metabolism to 13-hydroxyellipticine and ellipticine N2-oxide. Cancer Res. 2004, 64, 8374–8380. [Google Scholar] [CrossRef]
- Stiborová, M.; Breuer, A.; Aimová, D.; Stiborová-Rupertová, M.; Wiessler, M.; Frei, E. DNA adduct formation by the anticancer drug ellipticine in rats determined by 32P postlabeling. Int. J. Cancer 2003, 107, 885–890. [Google Scholar] [CrossRef]
- Kotrbová, V.; Aimová, D.; Brezinová, A.; Janouchová, K.; Poljaková, J.; Frei, E.; Stiborová, M. Cytochromes P450 reconstituted with NADPH: P450 reductase mimic the activating and detoxicating metabolism of the anticancer drug ellipticine in microsomes. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 2), 18–22. [Google Scholar]
- Aimová, D.; Svobodová, L.; Kotrbová, V.; Mrázová, B.; Hodek, P.; Hudeček, J.; Václavíková, R.; Frei, E.; Stiborová, M. The anticancer drug ellipticine is a potent inducer of rat cytochromes P450 1A1 and 1A2, thereby modulating its own metabolism. Drug Metab. Dispos. 2007, 35, 1926–1934. [Google Scholar] [CrossRef]
- Malonne, H.; Atassi, G. DNA topoisomerase targeting drugs: Mechanisms of action and perspectives. Anticancer Drugs 1997, 8, 811–822. [Google Scholar] [CrossRef]
- Le Mée, S.; Pierré, A.; Markovits, J.; Atassi, G.; Jacquemin-Sablon, A.; Saucier, J.M. S16020-2, a new highly cytotoxic antitumor olivacine derivative: DNA interaction and DNA topoisomerase II inhibition. Mol. Pharmacol. 1998, 53, 213–220. [Google Scholar] [CrossRef]
- Haider, N.; Sotelo, E. 1,5-dimethyl-6H-pyridazino[4,5-b]carbazole, a 3-aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef]
- Pichard-Garcia, L.; Weaver, R.J.; Eckett, N.; Scarfe, G.; Fabre, J.-M.; Lucas, C.; Maurel, P. The olivacine derivative S 16020 (9-hydroxy-5,6-dimethyl-N-[2-(dimethylamino)ethyl)-6H-pyrido(4,3-b)-carbazole-1-carboxamide) induces CYP1A and its own metabolism in human hepatocytes in primary culture. Drug Metab. Dispos. 2004, 32, 80–88. [Google Scholar] [CrossRef]
- Piasny, J.; Wiatrak, B.; Dobosz, A.; Tylińska, B.; Gębarowski, T. Antitumor Activity of New Olivacine Derivatives. Molecules 2020, 25, 2512. [Google Scholar] [CrossRef]
- Gębarowski, T.; Wiatrak, B.; Gębczak, K.; Tylińska, B.; Gąsiorowski, K. Effect of new olivacine derivatives on p53 protein level. Pharmacol. Rep. 2020, 72, 214–224. [Google Scholar] [CrossRef]
- Walderley, M.G.L.; Shepherd, G.J.; Melhem, T.S.; Giulietti, A.M. Flora Fanerogâmica do Estado de São Paulo 4; Instituto de Botânica: São Paulo, Brazil, 2005. [Google Scholar]
- Krentkowski, F.L.; Duarte, M.R. Morpho-anatomical analysis of Aspidosperma olivaceum and A. polyneuron, Apocynaceae. Rev. Bras. Farmacogn. 2012, 22, 937–945. [Google Scholar] [CrossRef]
- Frausin, G.; de Freitas Hidalgo, A.; Lima, R.B.S.; Kinupp, V.F.; Ming, L.C.; Pohlit, A.M.; Milliken, W. An ethnobotanical study of anti-malarial plants among indigenous people on the upper Negro River in the Brazilian Amazon. J. Ethnopharmacol. 2015, 174, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Dolabela, M.F.; Oliveira, S.G.; Peres, J.M.; Nascimento, J.M.S.; Póvoa, M.M.; Oliveira, A.B. In vitro antimalarial activity of six Aspidosperma species from the state of Minas Gerais (Brazil). An. Acad. Bras. Cienc. 2012, 84, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, L.F.; Montoia, A.; Amorim, R.C.N.; Melo, M.R.; Henrique, M.C.; Nunomura, S.M.; Costa, M.R.F.; Andrade Neto, V.F.; Costa, D.S.; Dantas, G.; et al. Comparative in vitro and in vivo antimalarial activity of the indole alkaloids ellipticine, olivacine, cryptolepine and a synthetic cryptolepine analog. Phytomedicine 2012, 20, 71–76. [Google Scholar] [CrossRef]
- Touaty, P.; Simon, M. Effects of olivacine on the metabolism of proteins and nucleic acids in Escherichia coli. Biochim. Biophys. Acta 1982, 697, 313–321. [Google Scholar] [CrossRef]
- Tylińska, B.; Redzicka, A. Synthesis of olivacine. Wiadomości Chem. 2015, 69, 11–12. [Google Scholar]
- Maftouh, M.; Besselievre, R.; Monsarrat, B.; Lesca, P.; Meunier, B.; Husson, H.P.; Paoletti, C. Synthesis and cytotoxic activity of hydroxylated derivatives of olivacine in relation with their biotransformation. J. Med. Chem. 1985, 28, 708–714. [Google Scholar] [CrossRef]
- Rivalle, C.; Wendling, F.; Tambourin, P.; Lhoste, J.M.; Bisagni, E.; Chermann, J.C. Antitumor amino-substituted pyrido[3’,4’:4,5]pyrrolo[2,3-g]isoquinolines and pyrido[4,3-b]carbazole derivatives: Synthesis and evaluation of compounds resulting from new side chain and heterocycle modifications. J. Med. Chem. 1983, 26, 181–185. [Google Scholar] [CrossRef]
- Ducrocq, C.; Wendling, F.; Chermann, J.C.; Tourbez-Perrin, M.; Rivalle, C.; Tambourin, P.; Pochon, F.; Bisagni, E. Structure-activity relationship in a series of newly synthesized 1-amino-substituted ellipticine derivatives. J. Med. Chem. 1980, 23, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Jasztold-Howorko, R.; Bisagni, E.; Chermann, J.-C. Synthesis and biological activity of N-(N,N-dialkylaminoalkyl)-1-aminomethyl-5-methyl-9-methoxy(6H)pyrido[4,3-b]carbazoles (ellipticines). Eur. J. Med. Chem. Chim. Ther. 1984, 19, 541–544. [Google Scholar]
- Guillonneau, C.; Pierré, A.; Charton, Y.; Guilbaud, N.; Kraus-Berthier, L.; Léonce, S.; Michel, A.; Bisagni, E.; Atassi, G. Synthesis of 9-O-substituted derivatives of 9-hydroxy-5, 6-dimethyl-6H-pyrido[4,3-b]carbazole-1-carboxylic acid (2-(dimethylamino)ethyl)amide and their 10- and 11-methyl analogues with improved antitumor activity. J. Med. Chem. 1999, 42, 2191–2203. [Google Scholar] [CrossRef]
- Jasztold-Howorko, R.; Landras, C.; Pierré, A.; Atassi, G.; Guilbaud, N.; Kraus-Berthier, L.; Léonce, S.; Rolland, Y.; Prost, J.F.; Bisagni, E. Synthesis and evaluation of 9-hydroxy-5-methyl-(and 5,6-dimethyl)-6H-pyrido[4,3-b]carbazole-1-N-[(dialkylamino)alkyl]carboxamides, a new promising series of antitumor olivacine derivatives. J. Med. Chem. 1994, 37, 2445–2452. [Google Scholar] [CrossRef]
- Landras, C.; Jasztold-Howorko, R.; Pierré, A.; Léonce, S.; Guilbaud, N.; Kraus-Berthier, L.; Guillonneau, C.; Rolland, Y.; Atassi, G.; Bisagni, E. Synthesis and biological evaluation of 6-(9-hydroxy-5-methyl (and 5,6-dimethyl)-6H-pyrido[4,3-b]carbazol-1-yl)picolinic amides as new olivacine derivatives. Chem. Pharm. Bull. (Tokyo) 1996, 44, 2169–2172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tylińska, B.; Jasztold-Howorko, R.; Kowalczewska, K.; Szczaurska-Nowak, K.; Gębarowski, T.; Wietrzyk, J. Design, synthesis and analysis of anticancer activity of new SAR-based S16020 derivatives. Acta Pol. Pharm.—Drug Res. 2018, 75, 1313–1320. [Google Scholar] [CrossRef]
- Jasztold-Howorko, R.; Tylińska, B.; Biaduń, B.; Gebarowski, T.; Gasiorowski, K. New pyridocarbazole derivatives. Synthesis and their in vitro anticancer activity. Acta Pol. Pharm. 2013, 70, 823–832. [Google Scholar]
- Guillonneau, C.; Nault, A.; Raimbaud, E.; Léonce, S.; Kraus-Berthier, L.; Pierré, A.; Goldstein, S. Cytotoxic and antitumoral properties in a series of new, ring D modified, olivacine analogues. Bioorg. Med. Chem. 2005, 13, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Tylińska, B.; Jasztold-Howorko, R.; Mastalarz, H.; Szczaurska-Nowak, K.; Materek, P.; Wietrzyk, J. Synthesis of new 1-phenyl-6H-pyrido[4,3-b]carbazole derivatives with potential cytostatic activity. Acta Pol. Pharm. 2011, 68, 31–37. [Google Scholar] [PubMed]
- Tylińska, B.; Jasztold-Howorko, R.; Mastalarz, H.; Kłopotowska, D.; Filip, B.; Wietrzyk, J. Synthesis and structure-activity relationship analysis of new olivacine derivatives. Acta Pol. Pharm. 2010, 67, 495–502. [Google Scholar] [PubMed]
- Jasztold-Howorko, R.; Pelczynska, M.; Nasulewicz, A.; Wietrzyk, J.; Opolski, A. Synthesis of 5,6-dimethyl-9-methoxy-1-phenyl-6H-pyrido[4,3-b]carbazole derivatives and their cytotoxic activity. Arch. Pharm. (Weinheim) 2005, 338, 556–561. [Google Scholar] [CrossRef]
- Jasztold-Howorko, R.; Croisy, A.; Carrez, D.; Jaroszewicz, I.; Nasulewicz, A.; Pełczyńska, M.; Opolski, A. Synthesis, structure, and cytostatic properties of new olivacine derivatives. Arch. Pharm. (Weinheim) 2004, 337, 599–604. [Google Scholar] [CrossRef]
- Jasztold-Howorko, R.; Doroszkiewicz, W.; Bugla-Płoskońska, G.; Croisy, A.; Carrez, D. The synthesis and biological properties of a 1-(2-methylpyridin-4-yl) olivacine derivative. Sci. Pharm. 2005, 73, 101–112. [Google Scholar] [CrossRef][Green Version]
- Jasztold-Howorko, R.; Croisy, A.; Carrez, D. New 1-substituted 9-hydroxy-5,6-dimethyl-6H-pyrido [4,3-b]carbazole derivatives and their cytotoxicity. Acta Pol. Pharm. 2005, 62, 265–270. [Google Scholar]
- Tylińska, B.; Ryng, S.; Gębarowski, T.; Gąsiorowski, K. Hypoxia- selective cytoxicity of olivacine analogues. Synthesis and biological screening. Acta Pol. Pharm. Drug Res. 2017, 74, 1753–1759. [Google Scholar]
- Schmidt, U.; Theumer, G.; Jäger, A.; Kataeva, O.; Wan, B.; Franzblau, S.; Knölker, H.-J. Synthesis and Activity against Mycobacterium tuberculosis of Olivacine and Oxygenated Derivatives. Molecules 2018, 23, 1402. [Google Scholar] [CrossRef] [PubMed]
- Vilarem, M.J.; Charcosset, J.Y.; Primaux, F.; Gras, M.P.; Calvo, F.; Larsen, C.J. Differential effects of ellipticine and aza-analogue derivatives on cell cycle progression and survival of BALB/c 3T3 cells released from serum starvation or thymidine double block. Cancer Res. 1985, 45, 3906–3911. [Google Scholar] [PubMed]
- Bisagni, E.; Hung, N.C. Premiere synthese de methyl-4-5h- pyrido [3’,4’:4,5]pyrrolo [3,2-c]pyridines, analogues tricycliques des aza-9 ellipticines. Tetrahedron 1986, 42, 2311–2318. [Google Scholar] [CrossRef]
- Vilarem, M.J.; Riou, J.F.; Multon, E.; Gras, M.P.; Larsen, C.J. The in vitro involvement of topoisomerase II in the activity of aza-ellipticine analogues is not correlated with drug activity on isolated nuclei. Biochem. Pharmacol. 1986, 35, 2087–2095. [Google Scholar] [CrossRef]
- Awada, A.; Giacchetti, S.; Gerard, B.; Eftekhary, P.; Lucas, C.; de Valeriola, D.; Poullain, M.G.; Soudon, J.; Dosquet, C.; Brillanceau, M.-H.; et al. Clinical phase I and pharmacokinetic study of S 16020, a new olivacine derivative: Report on three infusion schedules. Ann. Oncol. 2002, 13, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, C.; Le Pecq, J.B.; Dat-Xuong, N.; Juret, P.; Garnier, H.; Amiel, J.L.; Rouesse, J. Antitumor activity, pharmacology, and toxicity of ellipticines, ellipticinium, and 9-hydroxy derivatives: Preliminary clinical trials of 2-methyl-9-hydroxy ellipticinium (NSC 264-137). Recent Results Cancer Res. 1980, 74, 107–123. [Google Scholar] [CrossRef]
- Rouëssé, J.; Huertas, D.; Sancho-Garnier, H.; Le Chevalier, T.; Amiel, J.L.; Brulé, G.; Tursz, T.; Mondésir, J.M. 2 N methyl 9 hydroxy-ellipticine in treatment of metastatic breast cancers. Bull. Cancer 1981, 68, 437–441. [Google Scholar]
- Clarysse, A.; Brugarolas, A.; Siegenthaler, P.; Abele, R.; Cavalli, F.; de Jager, R.; Renard, G.; Rozencweig, M.; Hansen, H.H. Phase II study of 9-hydroxy-2N-methylellipticinium acetate. Eur. J. Cancer Clin. Oncol. 1984, 20, 243–247. [Google Scholar] [CrossRef]
- Khayat, D.; Borel, C.; Azab, M.; Paraisot, D.; Malaurie, E.; Bouloux, C.; Weil, M. Phase I study of Datelliptium chloride, hydrochloride given by 24-h continuous intravenous infusion. Cancer Chemother. Pharmacol. 1992, 30, 226–228. [Google Scholar] [CrossRef]
- Ohashi, M.; Oki, T. Overview oncologic, endocrine & metabolic: Oncologic, endocrine & metabolic: Ellipticine and related anti-cancer agents. Expert Opin. Ther. Pat. 1996, 6, 1285–1294. [Google Scholar] [CrossRef]
- Kattan, J.; Durand, M.; Droz, J.P.; Mahjoubi, M.; Marino, J.P.; Azab, M. Phase I study of retelliptine dihydrochloride (SR 95325 B) using a single two-hour intravenous infusion schedule. Am. J. Clin. Oncol. 1994, 17, 242–245. [Google Scholar] [CrossRef]
- Auclair, C.; Paoletti, C. Bioactivation of the antitumor drugs 9-hydroxyellipticine and derivatives by a peroxidase-hydrogen peroxide system. J. Med. Chem. 1981, 24, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, N.; Kraus-Berthier, L.; Saint-Dizier, D.; Rouillon, M.H.; Jan, M.; Burbridge, M.; Visalli, M.; Bisagni, E.; Pierré, A.; Atassi, G. In vivo antitumor activity of S 16020-2, a new olivacine derivative. Cancer Chemother. Pharmacol. 1996, 38, 513–521. [Google Scholar] [CrossRef]
- Léonce, S.; Perez, V.; Casabianca-Pignede, M.R.; Anstett, M.; Bisagni, E.; Pierré, A.; Atassi, G. In vitro cytotoxicity of S16020-2, a new olivacine derivative. Investig. New Drugs 1996, 14, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, N.; Kraus-Berthier, L.; Saint-Dizier, D.; Rouillon, M.H.; Jan, M.; Burbridge, M.; Pierré, A.; Atassi, G. Antitumor activity of S 16020-2 in two orthotopic models of lung cancer. Anticancer Drugs 1997, 8, 276–282. [Google Scholar] [CrossRef]
- Kraus-Berthier, L.; Guilbaud, N.; Jan, M.; Saint-Dizier, D.; Rouillon, M.H.; Burbridge, M.F.; Pierré, A.; Atassi, G. Experimental antitumour activity of S 16020-2 in a panel of human tumours. Eur. J. Cancer 1997, 33, 1881–1887. [Google Scholar] [CrossRef]
- Giacchetti, S.; Cornez, N.; Eftekhari, P.; Awada, A.; Cuvier, C.; Bleiberg, H.; Hidvegi, E.; Berger, E.; Gerard, B.; Giroux, B.; et al. Phase I clinical trial of the olivacine S16020. Proc. Am. Assoc. Cancer Res. 1998, 39, 324. [Google Scholar]
- Vassal, G.; Merlin, J.-L.; Terrier-Lacombe, M.-J.; Grill, J.; Parker, F.; Sainte-Rose, C.; Aubert, G.; Morizet, J.; Sévenet, N.; Poullain, M.-G.; et al. In vivo antitumor activity of S16020, a topoisomerase II inhibitor, and doxorubicin against human brain tumor xenografts. Cancer Chemother. Pharmacol. 2003, 51, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Awada, A.; Gedouin, D.; Kerger, J.; Rolland, F.; Cupissol, D.; Caponigro, F.; Comella, G.; Lopez-Pousa, J.J.; Guardiola, E.; et al. Results of randomised phase II studies comparing S16020 with methotrexate in patients with recurrent head and neck cancer. Ann. Oncol. 2003, 14, 373–377. [Google Scholar] [CrossRef]
- Malonne, H.; Farinelle, S.; Decaestecker, C.; Gordower, L.; Fontaine, J.; Chaminade, F.; Saucier, J.M.; Atassi, G.; Kiss, R. In vitro and in vivo pharmacological characterizations of the antitumor properties of two new olivacine derivatives, S16020-2 and S30972-1. Clin. Cancer Res. 2000, 6, 3774–3782. [Google Scholar] [PubMed]
- Kraus-Berthier, L.; Guilbaud, N.; Léonce, S.; Parker, T.; Genissel, P.; Guillonneau, C.; Goldstein, S.; Atassi, G.; Pierré, A. Comparison of the pharmacological profile of an olivacine derivative and a potential prodrug. Cancer Chemother. Pharmacol. 2002, 50, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shewach, D.S.; Lawrence, T.S. Radiosensitization of human solid tumor cell lines with gemcitabine. Semin. Oncol. 1996, 23, 65–71. [Google Scholar]
- Maggiorella, L.; Frascogna, V.; Poullain, M.G.; Berlion, M.; Lucas, C.; Razy, S.D.; Eschwege, F.; Bourhis, J. The olivacine S16020 enhances the antitumor effect of ionizing radiation without increasing radio-induced mucositis. Clin. Cancer Res. 2001, 7, 2091–2095. [Google Scholar] [PubMed]
- Boal, D.K.; Newburger, P.E.; Teele, R.L. Esophagitis induced by combined radiation and adriamycin. AJR. Am. J. Roentgenol. 1979, 132, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Burden, D.A.; Osheroff, N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim. Biophys. Acta 1998, 1400, 139–154. [Google Scholar] [CrossRef]
- Montecucco, A.; Biamonti, G. Cellular response to etoposide treatment. Cancer Lett. 2007, 252, 9–18. [Google Scholar] [CrossRef]


| No. | Compound | Structure | In Vitro Cell Lines IC50 µM ± SD | In Vivo | Reference Number | |||
|---|---|---|---|---|---|---|---|---|
| L1210 | A549 | MCF-7 | Dose mg/kg (Cell Lines) | Therapeutic Effect % | ||||
| 1 | 1,5-dimethyl-6H-pyrido[4,3-b]carbazole olivacine | 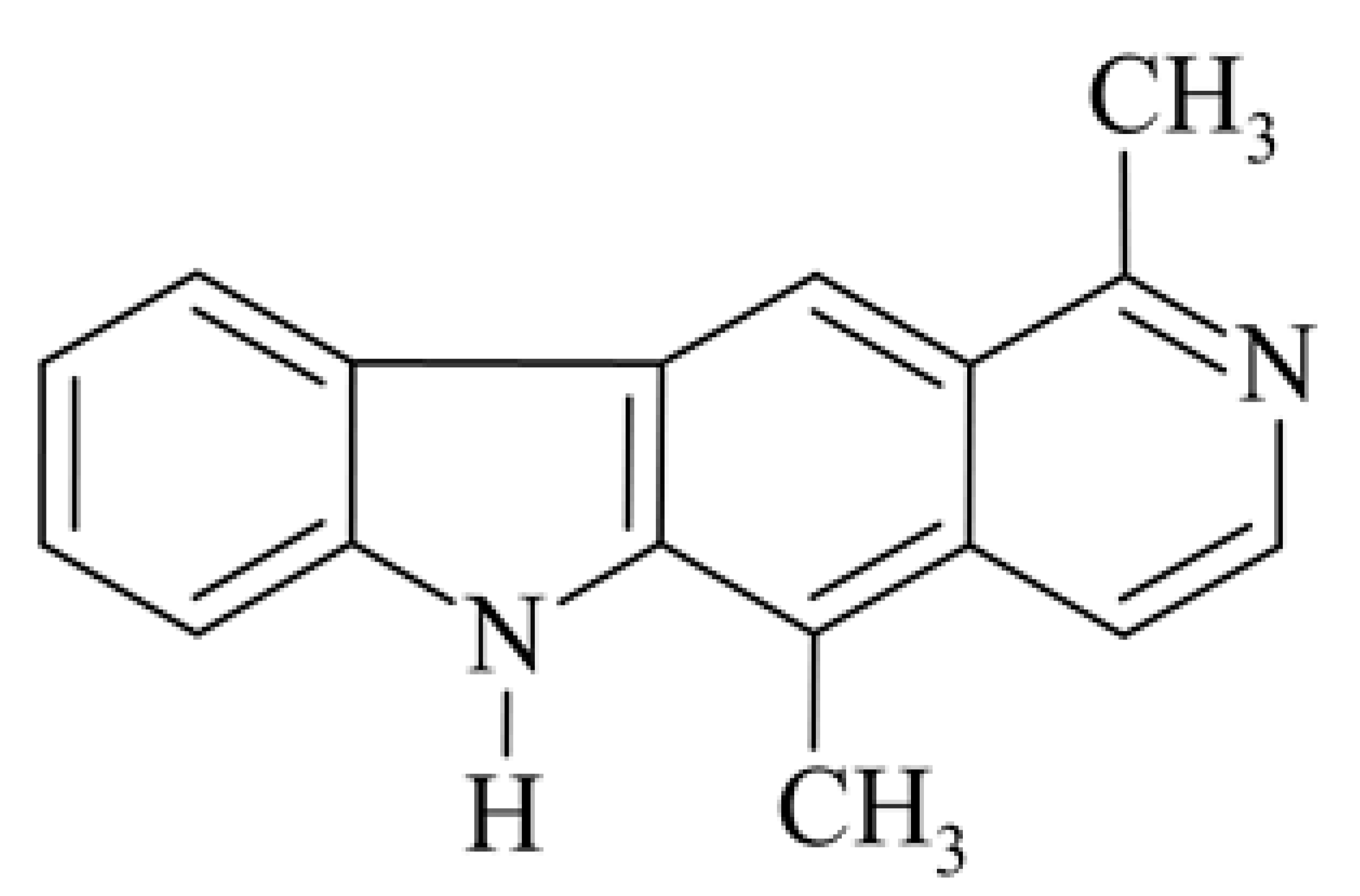 | 2.03 | 250 (L2110) 84.0 (L1220) | 35 (ILS) 141 (T/C) | [35] [3] | ||
| 2 | 9-hydroxy-1,5-dimethyl-6H-pyrido[4,3-b]carbazole 9-hydroxyolivacine | 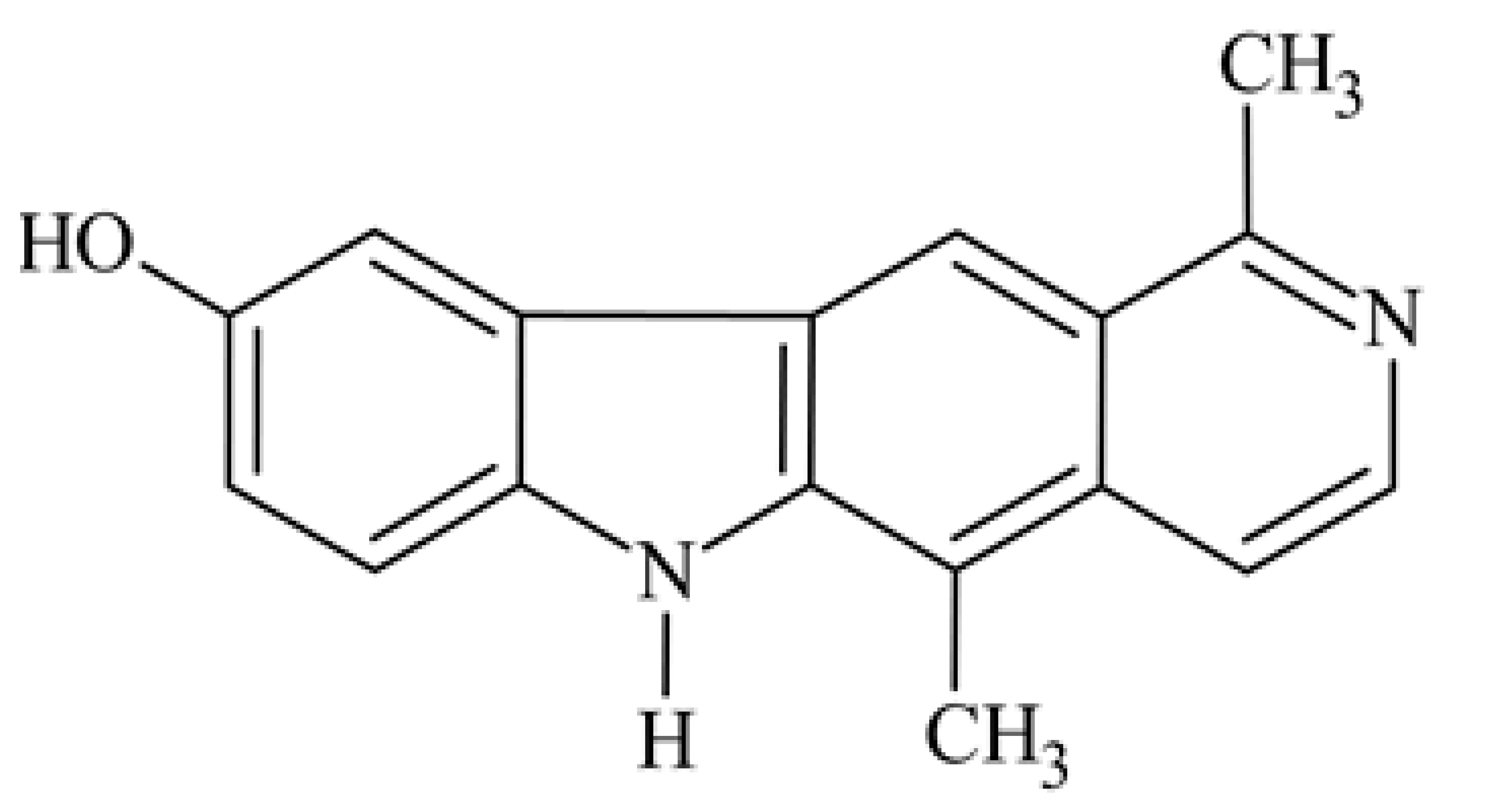 | 0.06 | [35] | ||||
| 3 | 1,5-dimethyl-6H-pyridazino[4,3-b]carbazole |  | 1.79 | 4.50 | [24] | |||
| 4 | 10-{[3-(diethylamino)propyl]amino}-6-methyl-5H-pyrido[3′4′:4,5]pyrrolo[2,3-g]isoquinoline Pazellipticine (PZN) |  | 0.02 | 20 (L1210) | 85 (ILS) | [36] | ||
| 5 | 1-{[3-(diethylamino)propyl]amino}-9-methoxy-5-methyl-6H-pyrido[4,3-b]carbazole | 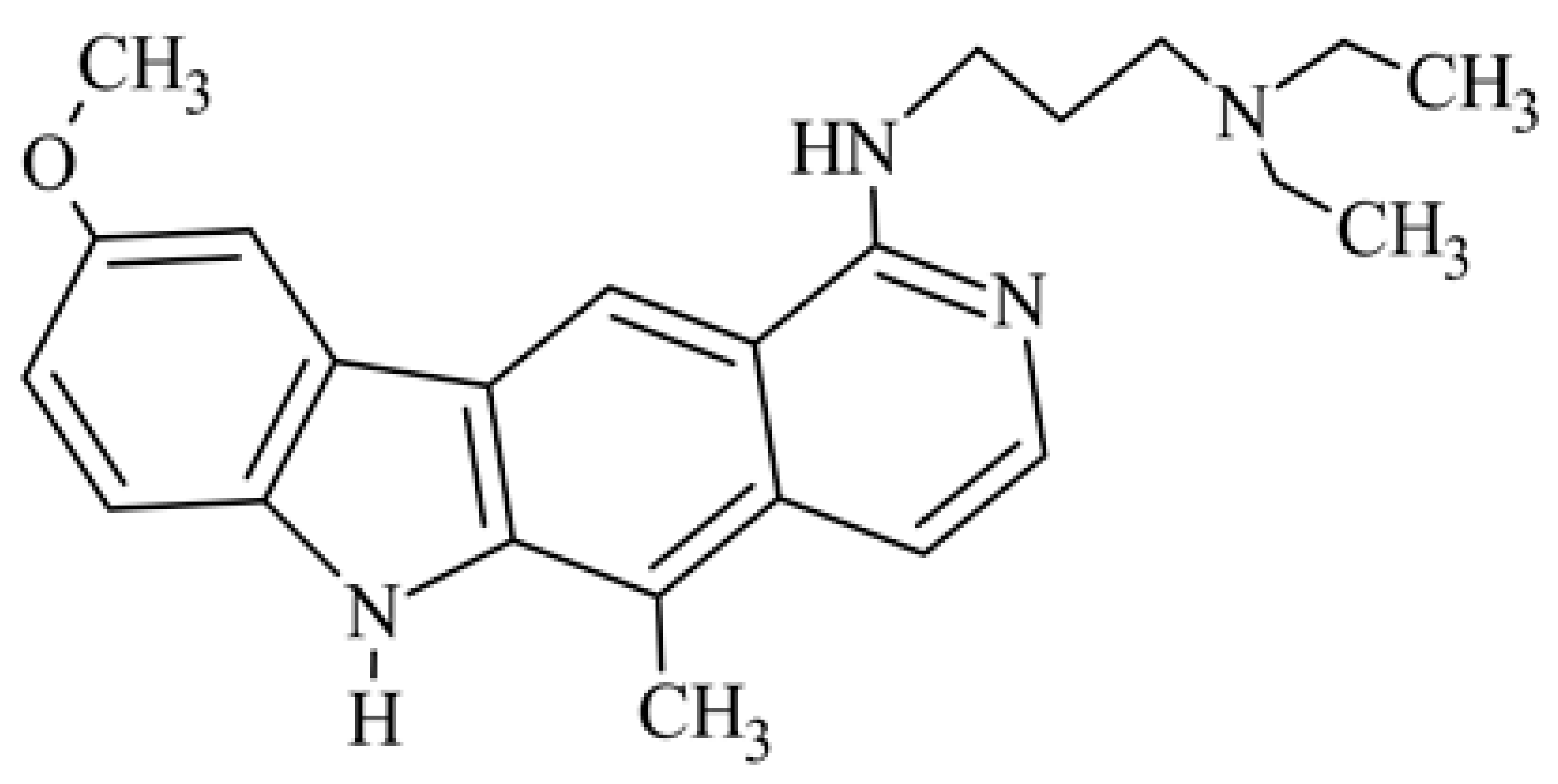 | 5 | 15 (L1210) | 24.6 (ILS) | [36] | ||
| 6 | 1-{[3-(diethylamino)propyl]amino]}-9-hydroxy-5-methyl-6H-pyrido[4,3-b]carbazole 1-{[3-(diethylamino)propyl]amino}-5-methyl-6H-pyrido[4,3-b]carbazol-9-ol | 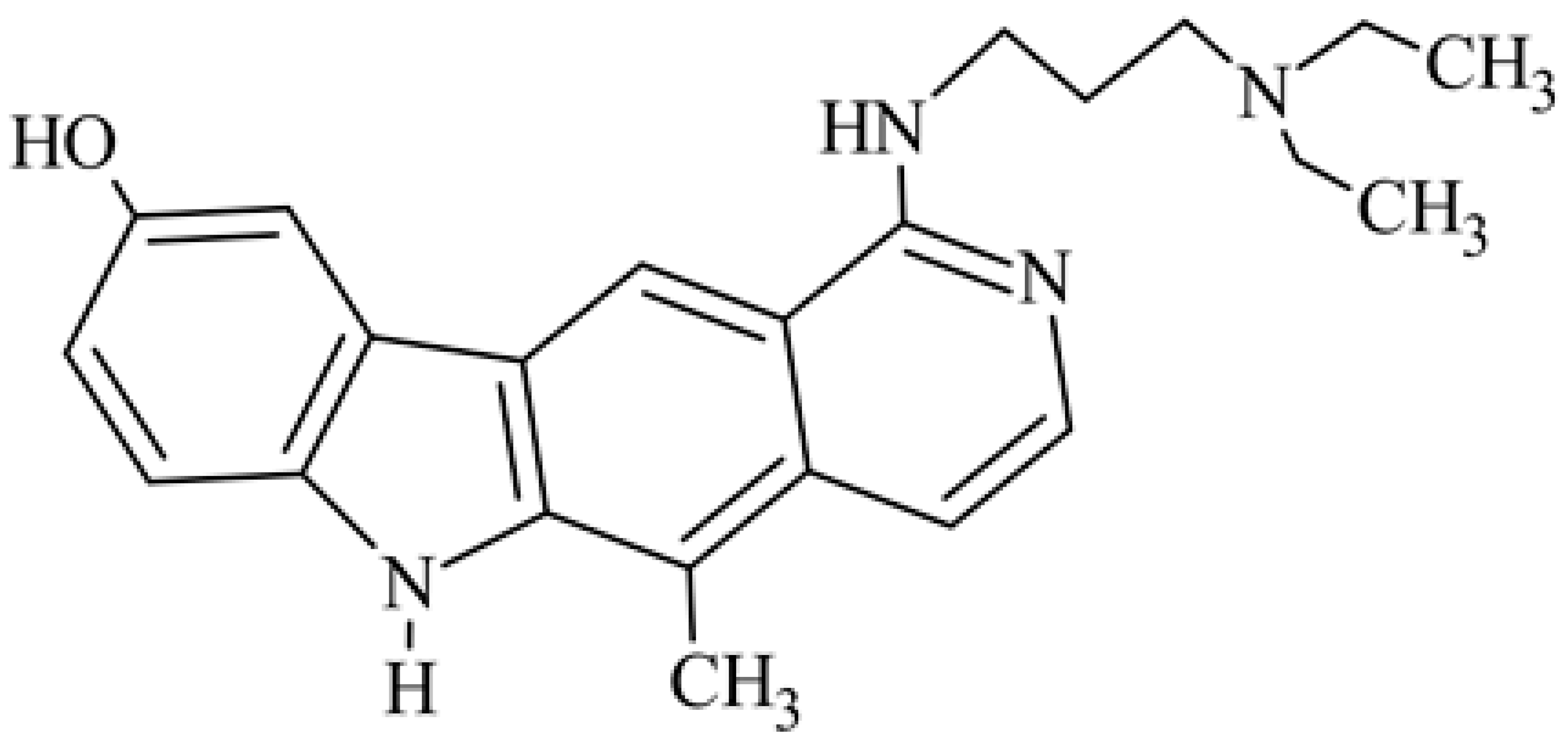 | 0.02 | 5 (L1210) | 49.5 (ILS) | [37] | ||
| 7 | 1-{{[3-(diethylamino)propyl]amino}methyl}-9-methoxy-5-methyl-6H-pyrido[4,3-b]carbazole |  | 1 | 20 10 (L1210) | 21 16 (ILS) | [38] | ||
| 8 | Pentanedioic acid mono{1-[2-(dimethylamino)ethyl]carbamoyl}-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-9-yl] ester dihydrochloride S 30972-1 | 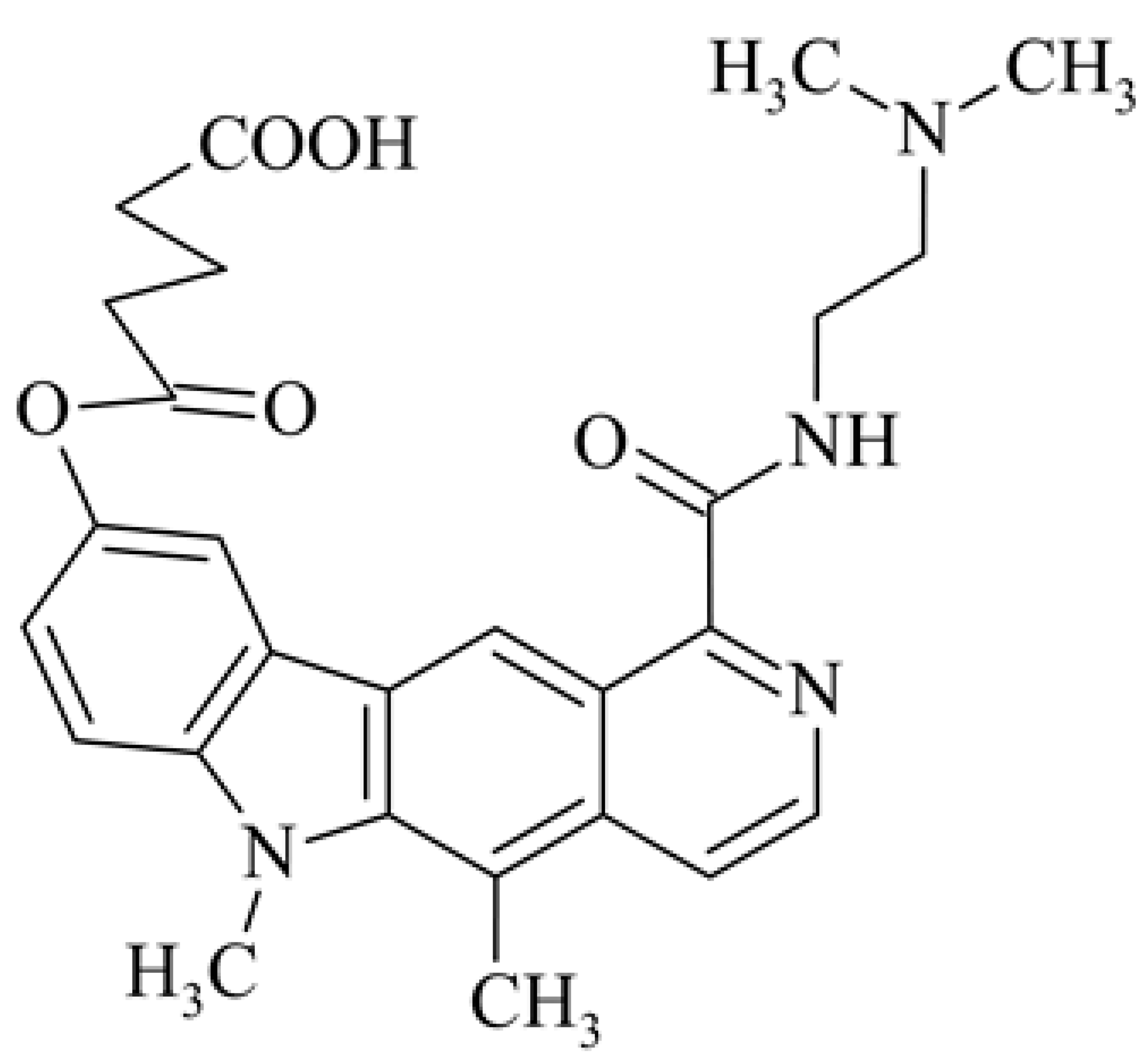 , 2 HCl , 2 HCl | 0.019 | 160-320 80 (P388) | 246->590 427->582 (T/C) | [39] | ||
| 9 | 9-hydroxy-1-{[2-(dimethylamino)ethyl]carbamoyl}-5,6-dimethyl-6H-pyrido[4,3-b]carbazole |  | 0.0041 ± 0.0006 | 90 (P388) | 238 (T/C) | [40] | ||
| 9-hydroxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazole-1-N-[2-(dimethylamino)ethyl]carboxamides S16020 | 0.0084 ± 0.0007 | 0.030 ± 0.004 | 0.075 ± 0.011 | 120 80 (P388) | 301 >631 (T/C) | [41] | ||
| 10 | 9-hydroxymethyl-1-{[2-(dimethylamino)ethyl)carbamoyl}-5,6-dimethyl-6H-pyrido[4,3-b]carbazole 9-hydroxymethyl-5,6-dimethyl-6H-pyrido[4,3-b]carbazole-1-N-[2-(dimethylami-no)ethyl]carboxamides | 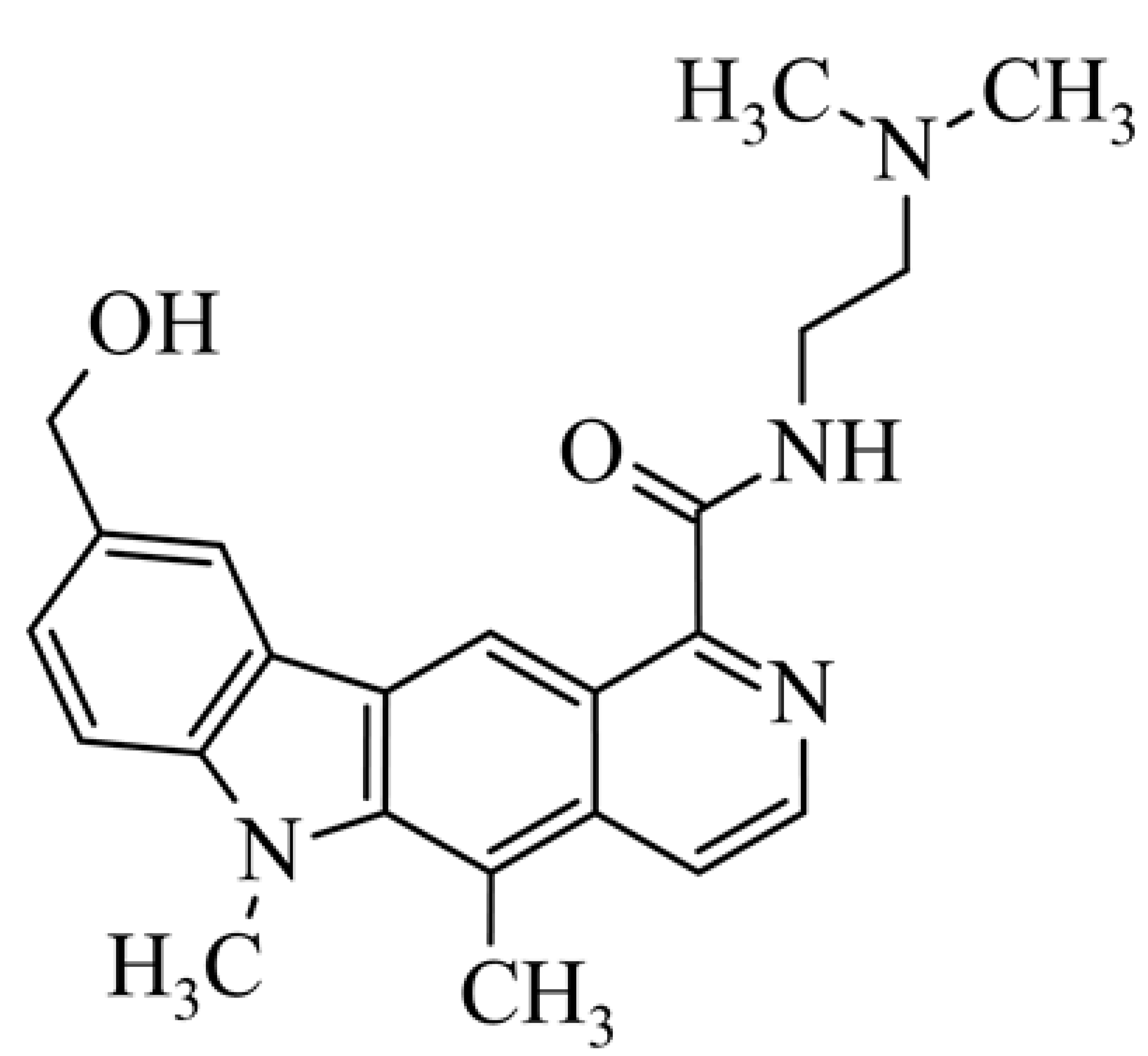 | 0.65 ± 0.3 | [42] | ||||
| 11 | 5,6-dimethyl-1-{[2-(dimethylamino)ethyl]carbamoyl}-9-(N-methylcarbamoyloxymethyl)-6H-pyrido[4,3-b]carbazole (1-{[2-(dimethylamino)ethyl]carbamoyl}-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-9-yl)methyl methylcarbamate |  | 1.25 ± 0.29 | [42] | ||||
| 12 | 9-methoxy-5,6-dimethyl-1-({[1-hydroxy-2-(hydroxymethyl)butan-2-yl]amino}methyl)-6H-pirydo[4,3-b]carbazole 2-ethyl-2-{[(5,6-dimethyl-9-methoxy-6H-pyrido[4,3-b]carbazole-1-yl)methyl]amino}propane-1,3-diol | 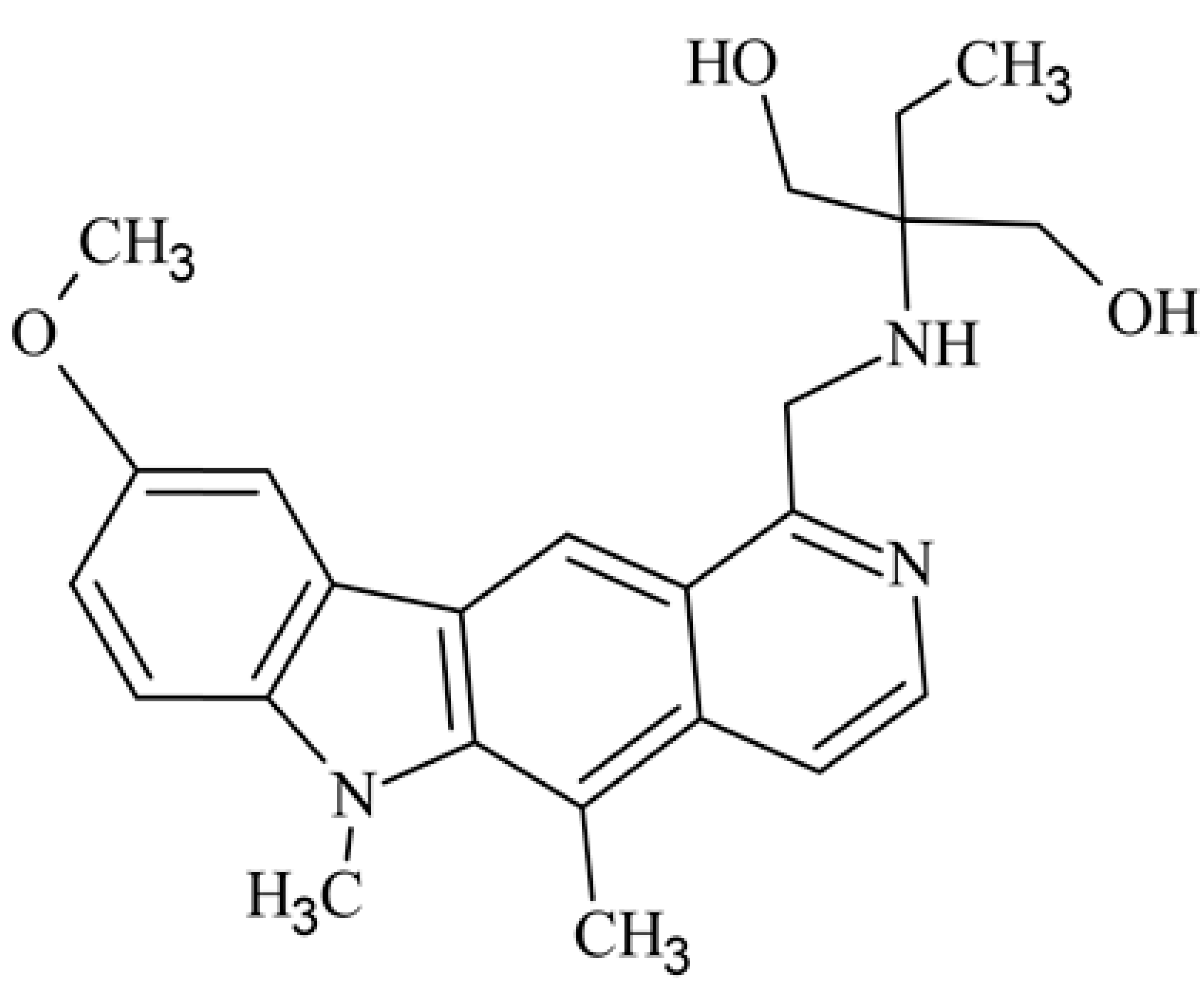 | 0.377 ± 0.159 | [43] | ||||
| 13 | 9-hydroxy-5,6-dimethyl-1-{[(1-hydroxy-2-methylpropan-2-yl)amino]methyl} -6H-pirydo[4,3-b]carbazole 5,6-dimethyl-1-{[(1-hydroxy-2-methylpropan-2-yl)amino]methyl} -6H-pirydo[4,3-b]carbazol-9-ol |  | 0.885 ± 0.071 | [43] | ||||
| 14 | 9-hydroxy-1-hydroxymethyl-5,6-dimethyl-6H-pyrido[4,3-b]carbazole 1-hydroxymethyl-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 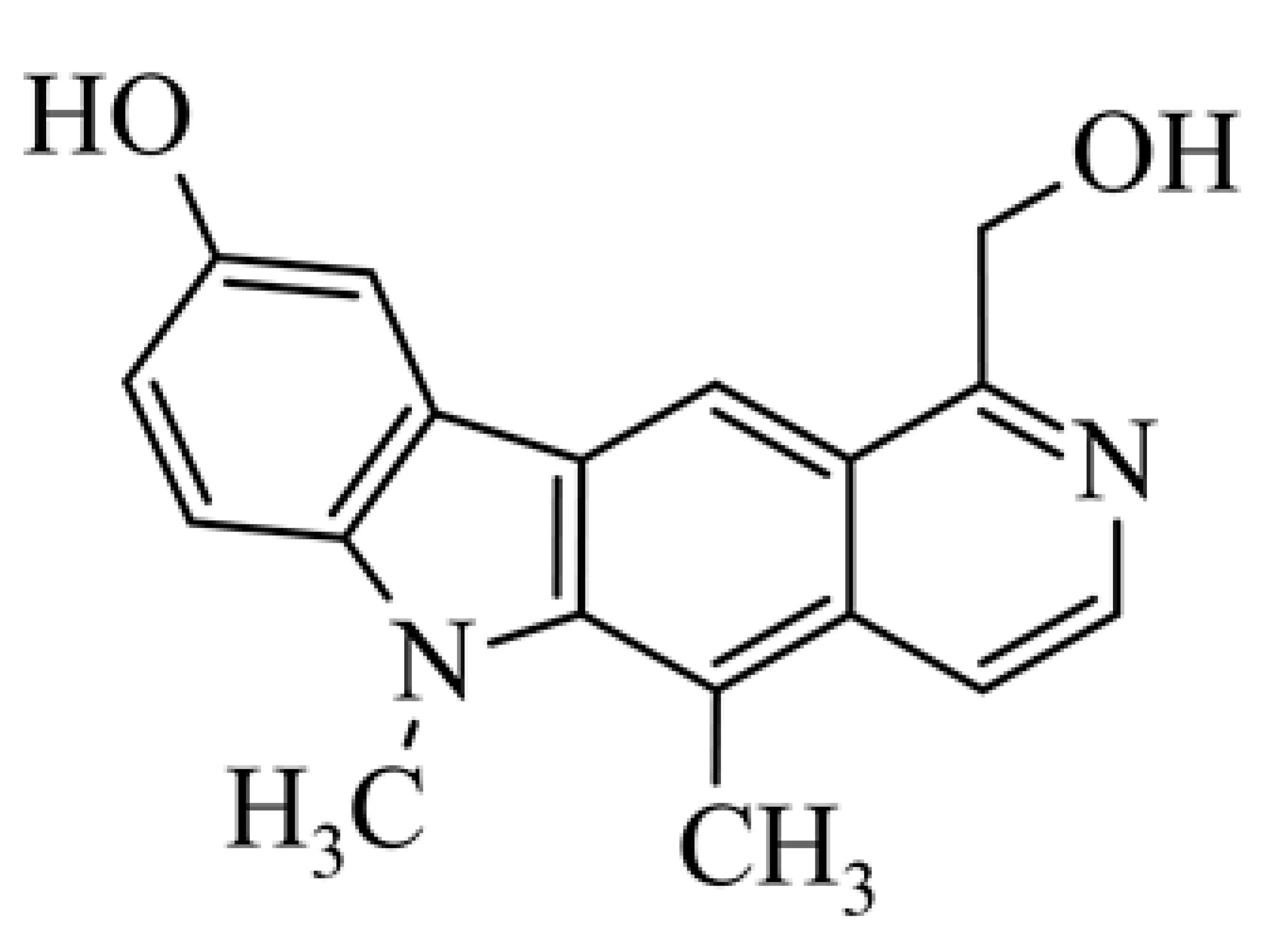 | 0.962 ± 0.52 | [43] | ||||
| 15 | 7-hydroxy-10,11-dimethyl-N-[2-(dimethylamino)ethyl]- -10H-pyrimido[4,5-b]carbazole-4-carboxamide |  | 0.010 | [44] | ||||
| 16 | 9-hydroxy-5,6-dimethyl-N-[2-(dimethylamino)ethyl]- 6H-pyrazino[2,3-b]carbazole-2-carboxamide | 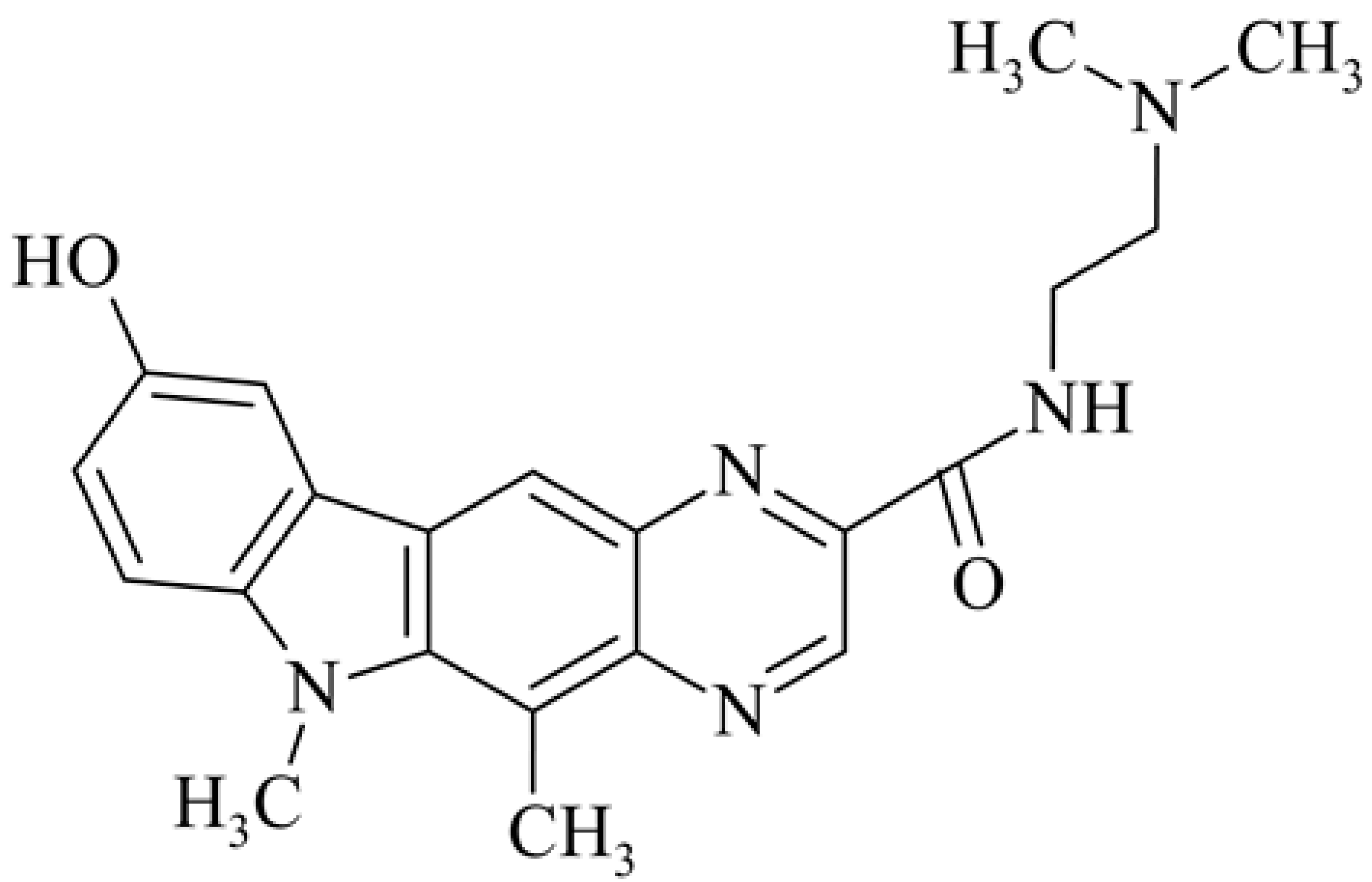 | 0.33 | [44] | ||||
| 17 | 1-(4-aminophenyl)-9-hydroxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazole 1-(4-aminophenyl)-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 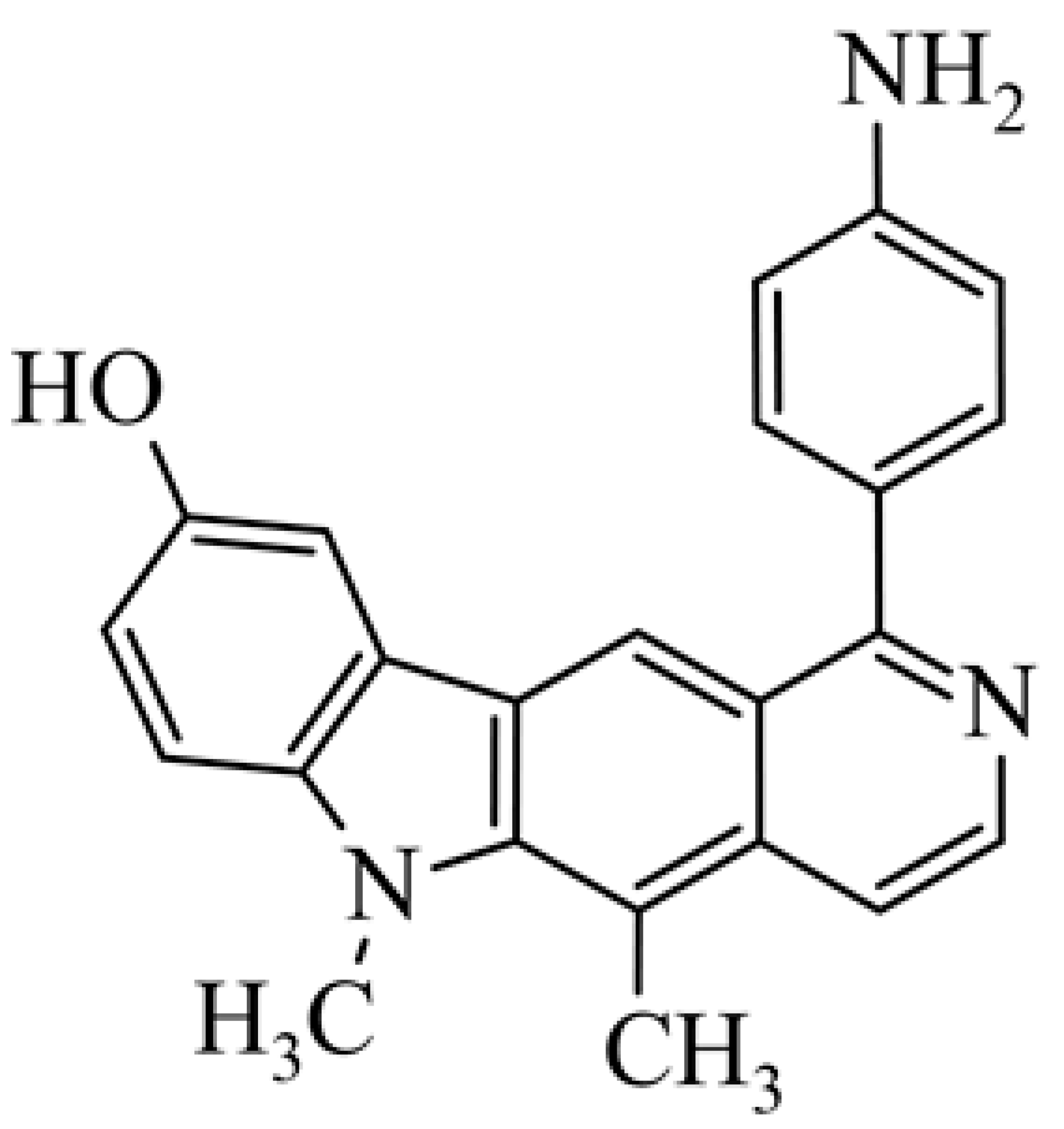 | 0.096 ± 0.031 | 0.51 ± 0.22 | [45] | |||
| 18 | 9-methoxy-5,6-dimethyl-1-{4-[N-[3-(dimethylamino)propyl]carbamoylphenyl}-6H-pyrido[4,3-b]carbazole N-[2-(dimethyla-mino)propyl]-4-(9-methoxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-1-yl)benzamide | 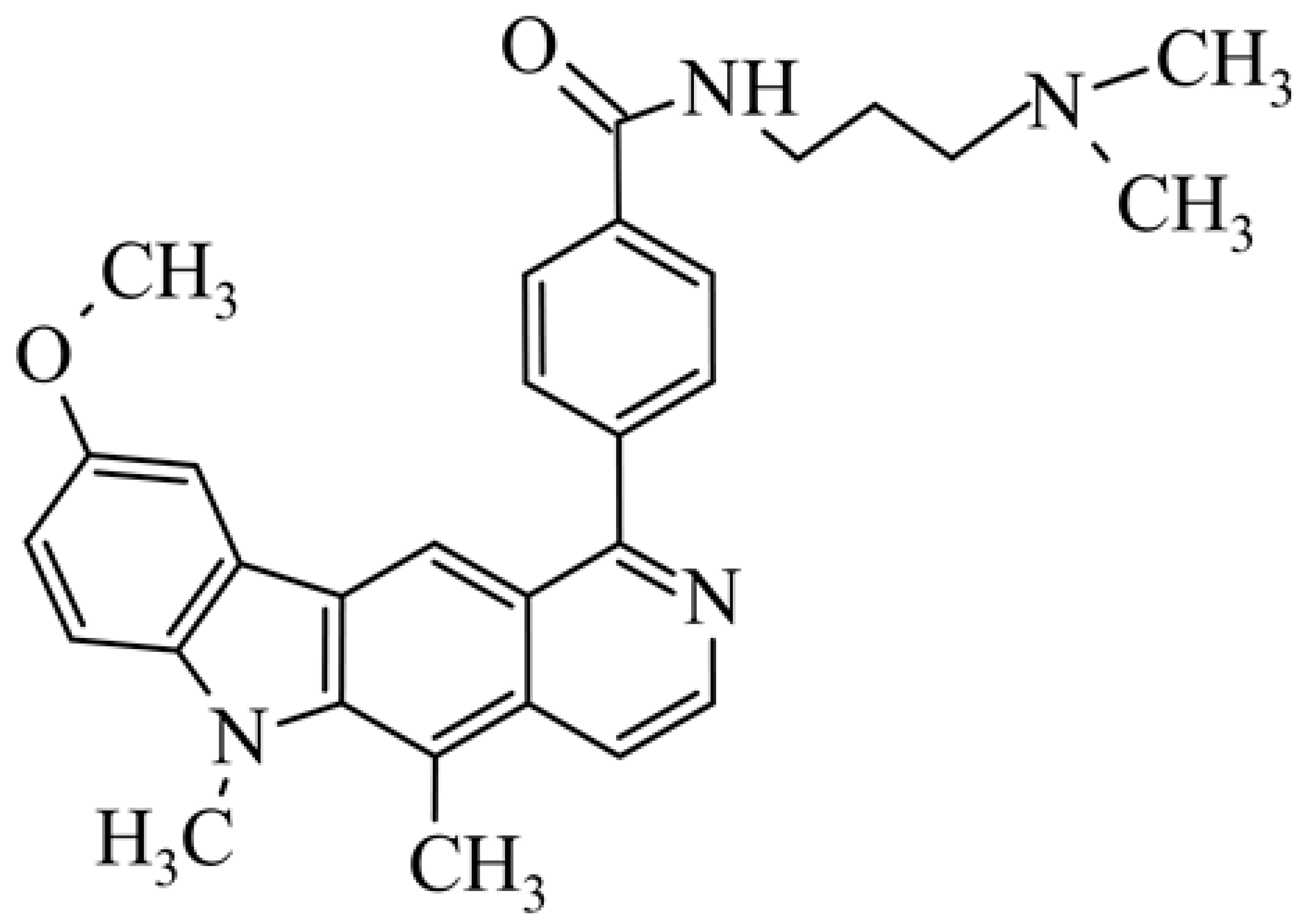 | 5.70 ± 0.33 | 4.30 ± 1.95 | [46] | |||
| 19 | 9-hydroxy-5,6-dimethyl-1-{4-[N-[2-(dimethylamino)ethyl]carbamoyl]phenyl}-6H-pyrido[4,3-b]carbazole N-[2-(dimethyla-mino)ethyl]-4-(9-hydroxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-1-yl)benzamide | 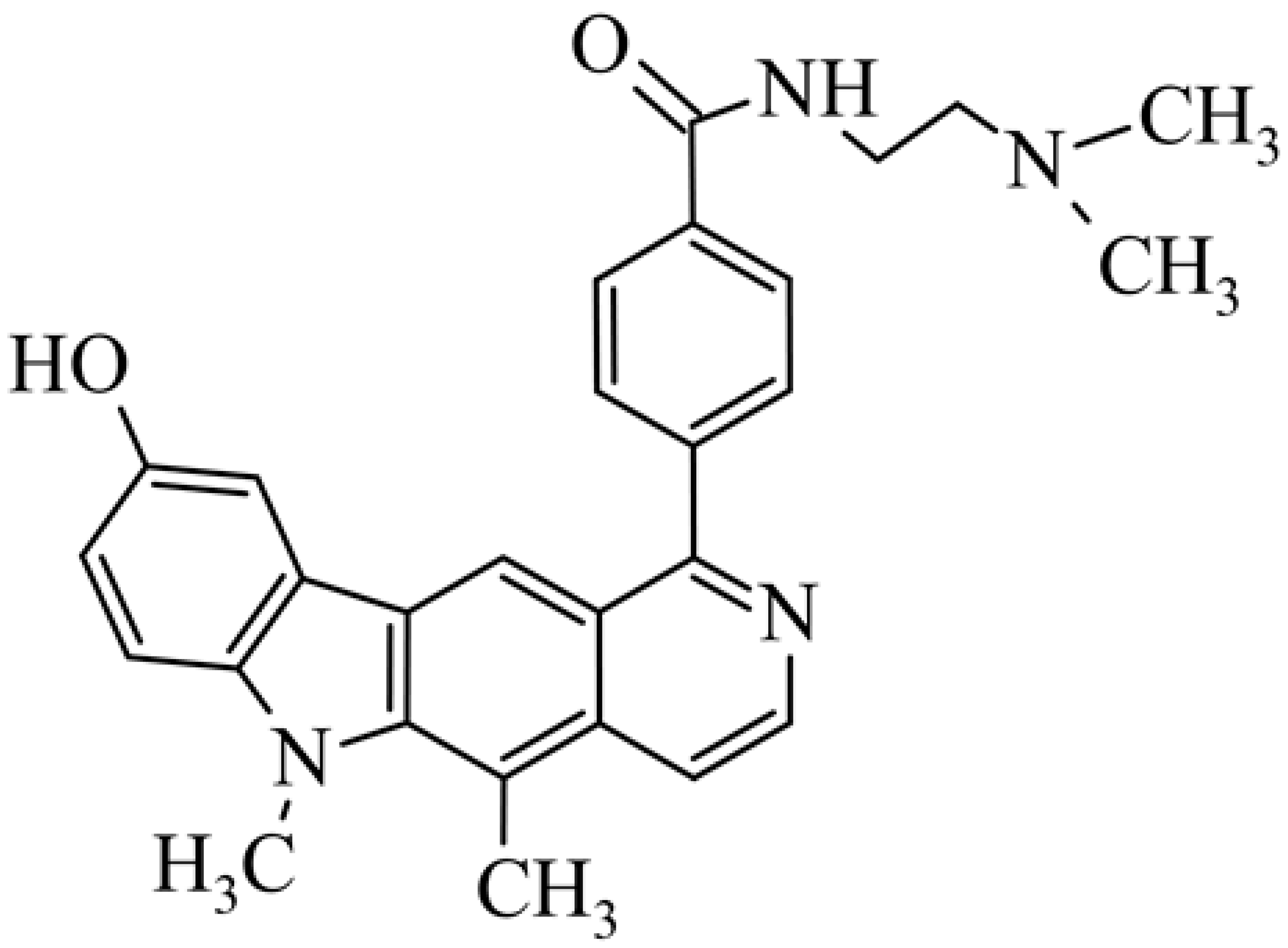 | 7.15 ± 2.76 | 8.19 ± 1.41 | [46] | |||
| 20 | 9-hydroxy-5,6-dimethyl-1-{3-[N-[2-(dimethylamino)ethyl]carbamoyl]phenyl}-6H-pyrido[4,3-b]carbazole N-[2-(dimethyla-mino)ethyl]-3-(9-hydroxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-1-yl)benzamide | 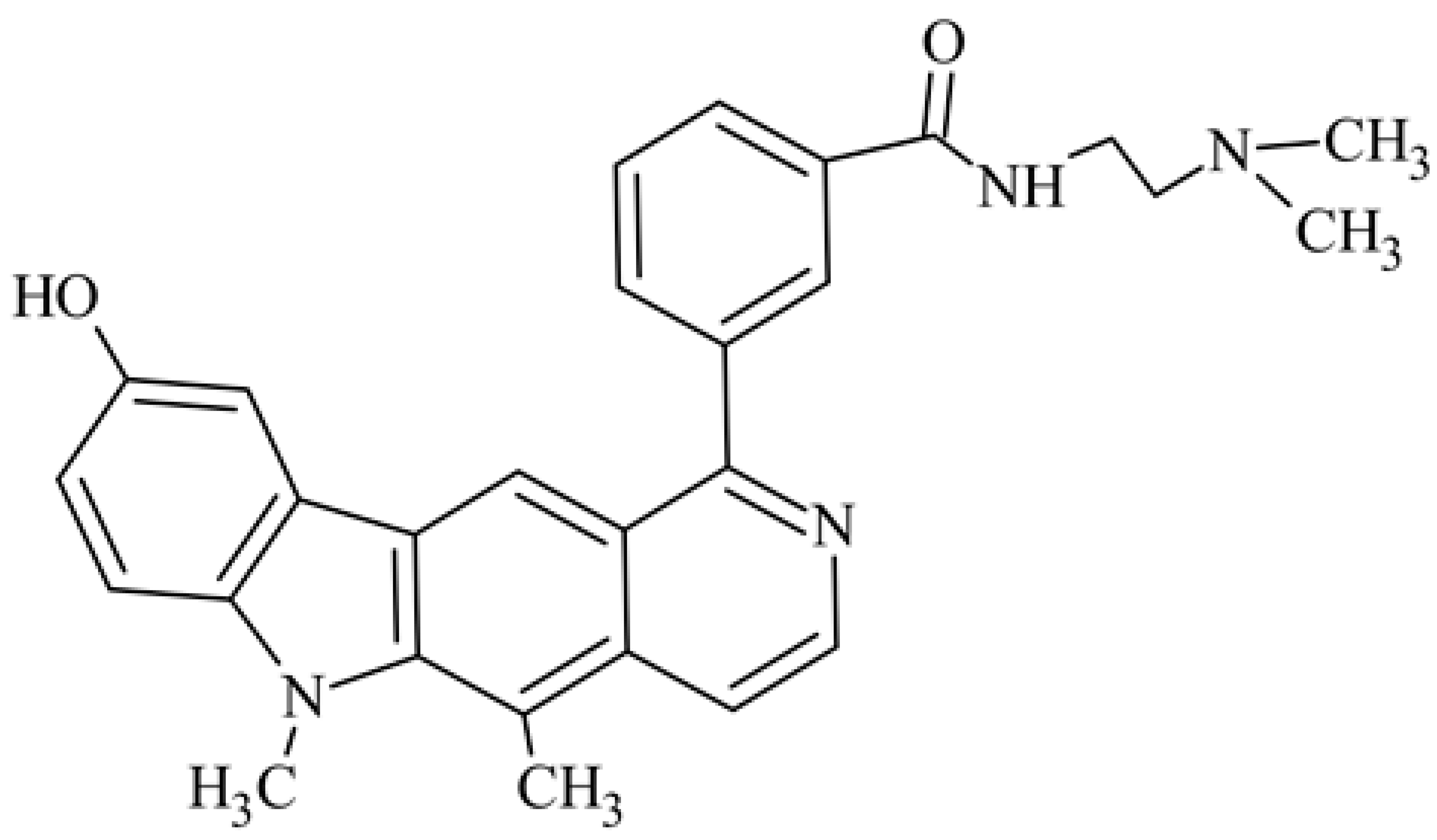 | 6.08 ± 3.96 | 8.25 ± 2.14 | [46] | |||
| 21 | 5,6-dimethyl-1-(4-nitro-phenyl)-6H-pyrido[4,3-b]carbazol-9-ol | 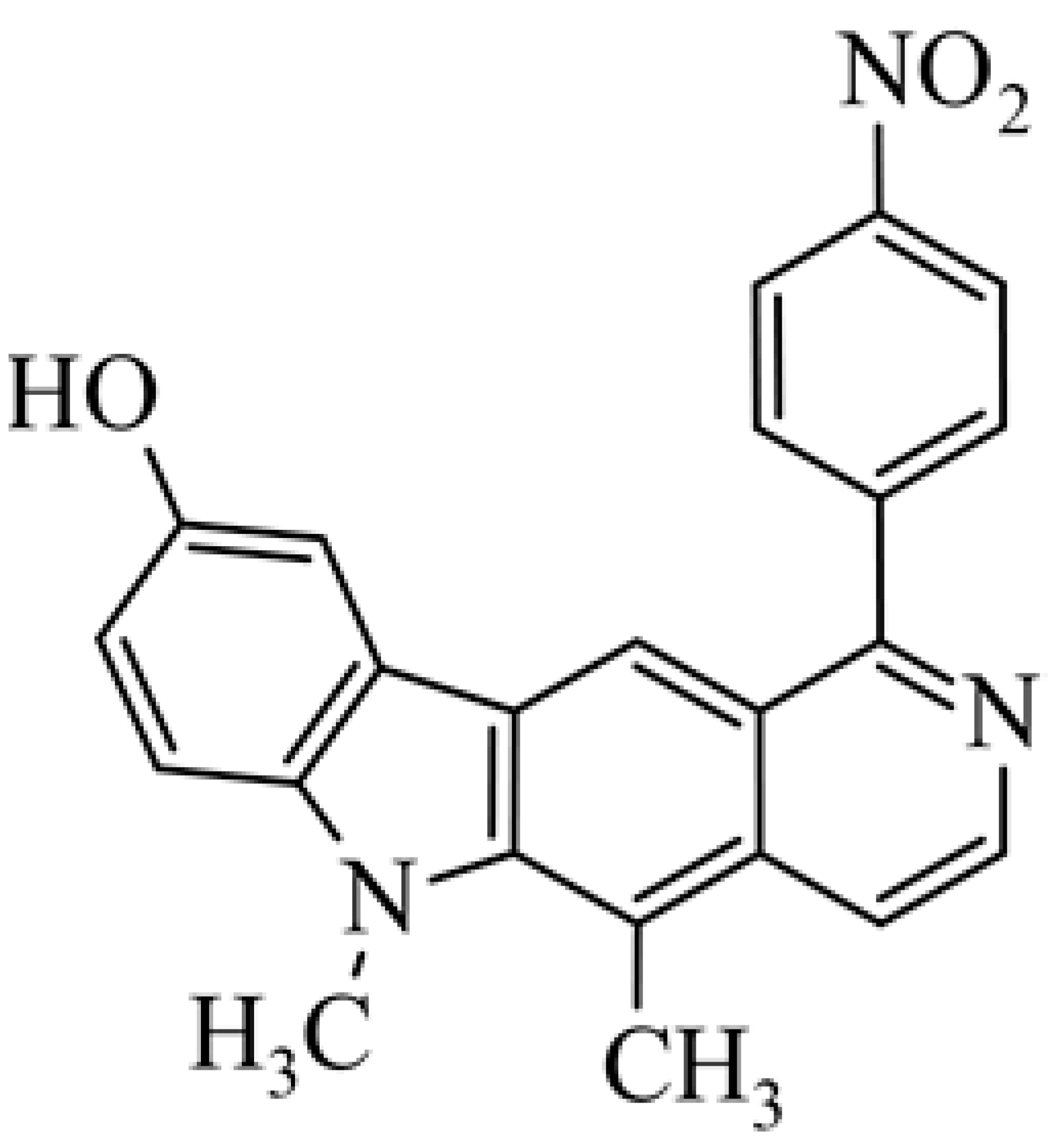 | 2.37 ± 3.41 | [47] | ||||
| 22 | 1-(4-hydroxy-phenyl)-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | 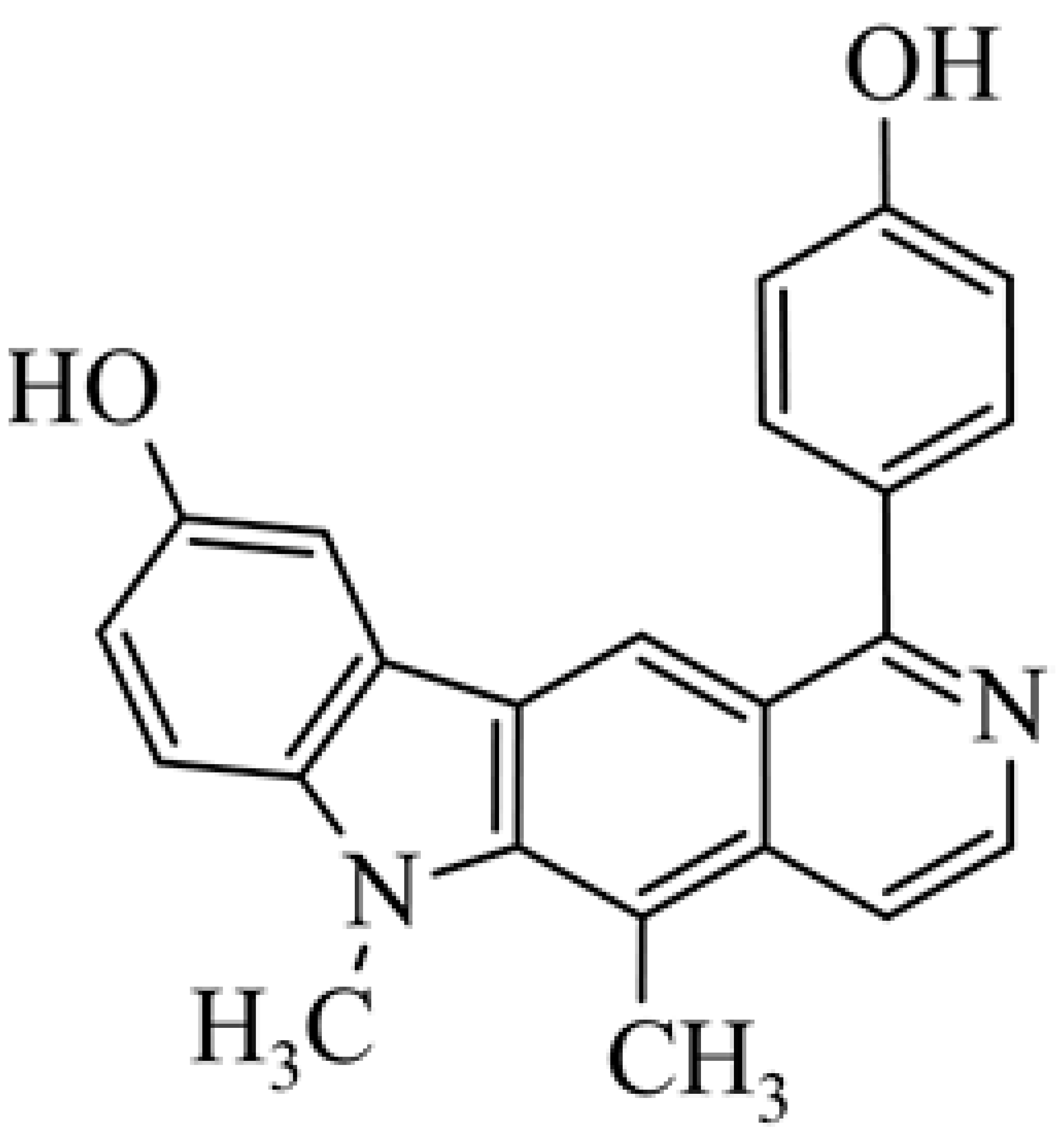 | 1.76 ± 0.05 | [47] | ||||
| 23 | 6-(9-hydroxy-5,6-dimethyl-6H-pyrido[4,3-b]carbazol-1-yl)pyridine-2-carboxylic acid [2-(dimethylamino)ethyl]amide | 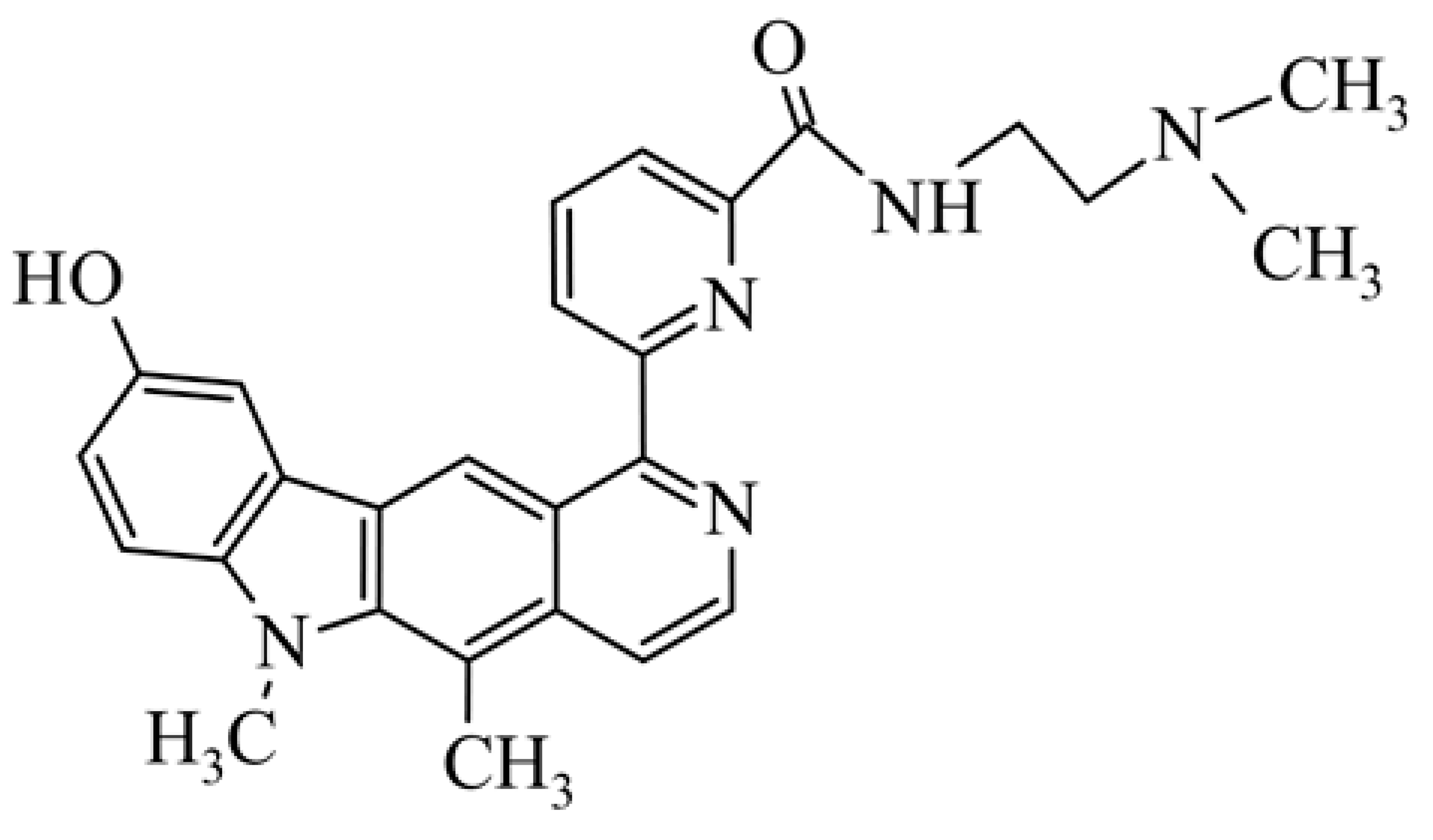 | 0.050 ± 0.011 | 0.095 ± 0.040 | 0.23 ± 0.020 | 40 (P388) | 207 (T/C) | [41] |
| 24 | 5,6-dimethyl-1-(6-methyl-pyridin-2-yl)-6H-pyrido[4,3-b]carbazol-9-ol |  | 0.9 | 5.03 | [42] | |||
| 25 | 5-methyl-6-(2-dimethylamino-ethyl)-1-(6-methyl-pyridin-2-yl)-6H-pyrido[4,3-b]carbazol-9-ol | 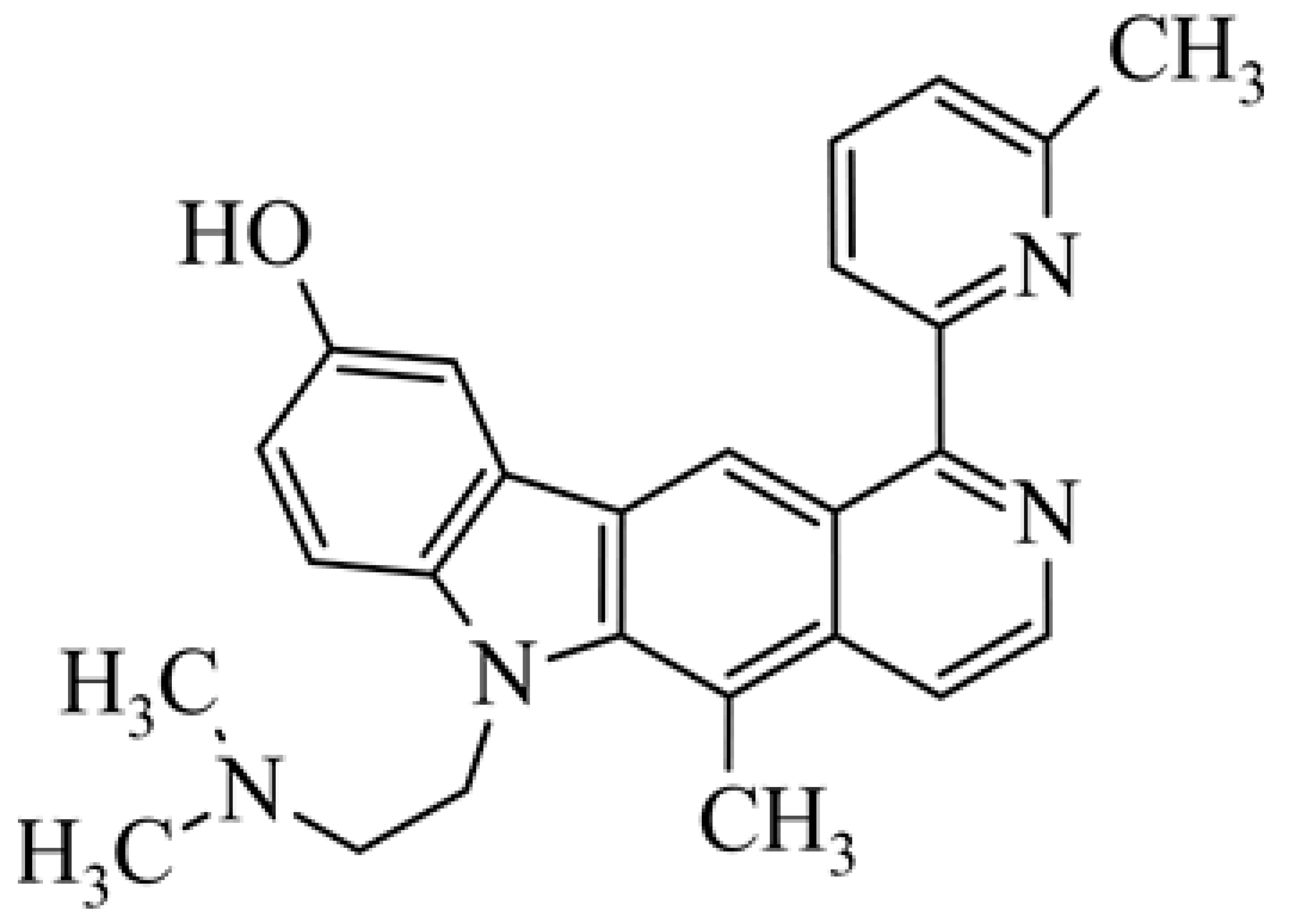 | 1.50 | 2.12 | [48] | |||
| 26 | 5,6-dimethyl-1-(2-methyl-pyridin-4-yl)-6H-pyrido[4,3-b]carbazol-9-ol | 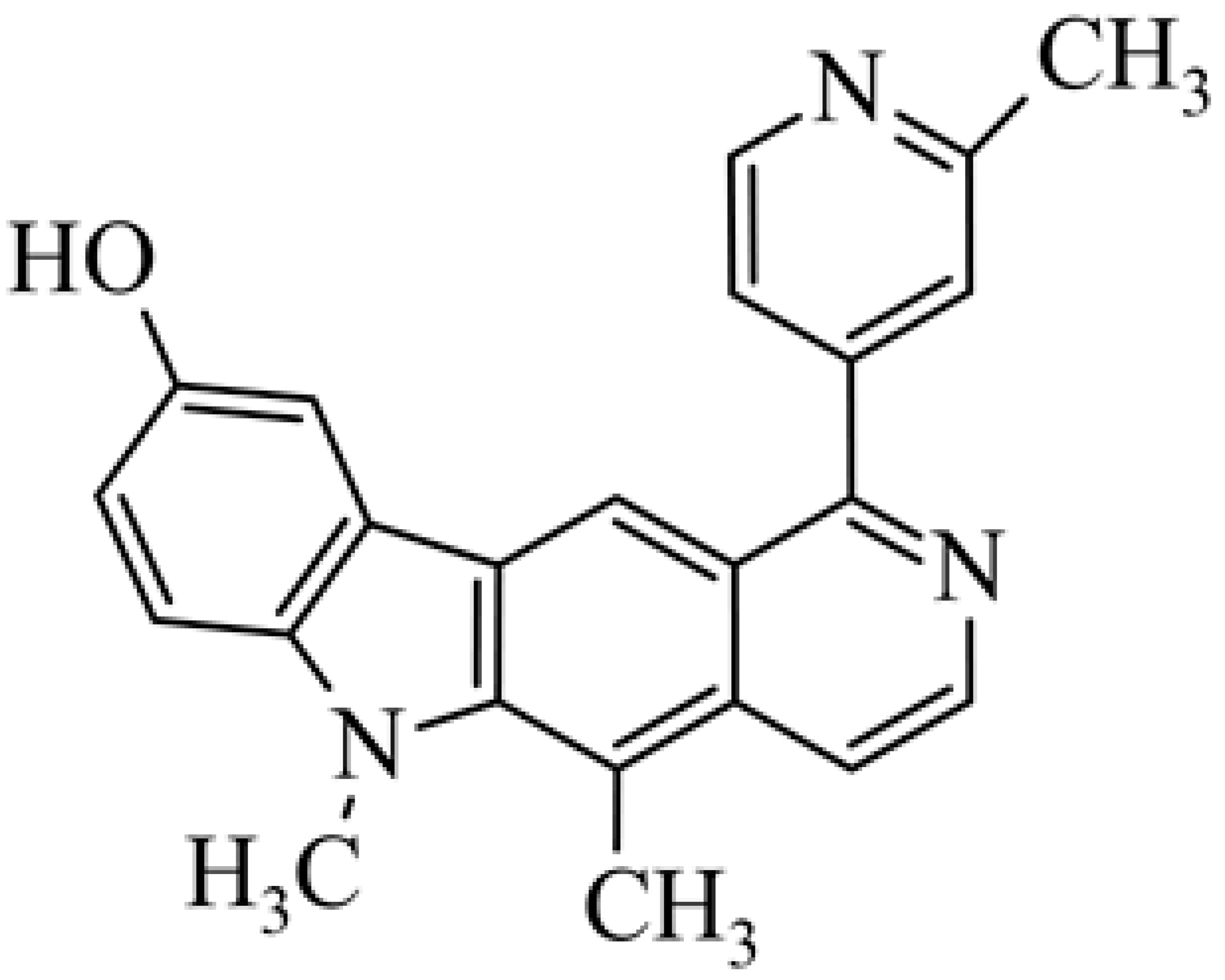 | 0.8 | [49] | ||||
| 27 | 5,6-dimethyl-1-(6-methyl-pyridin-3-yl)-6H-pyrido[4,3-b]carbazol-9-ol | 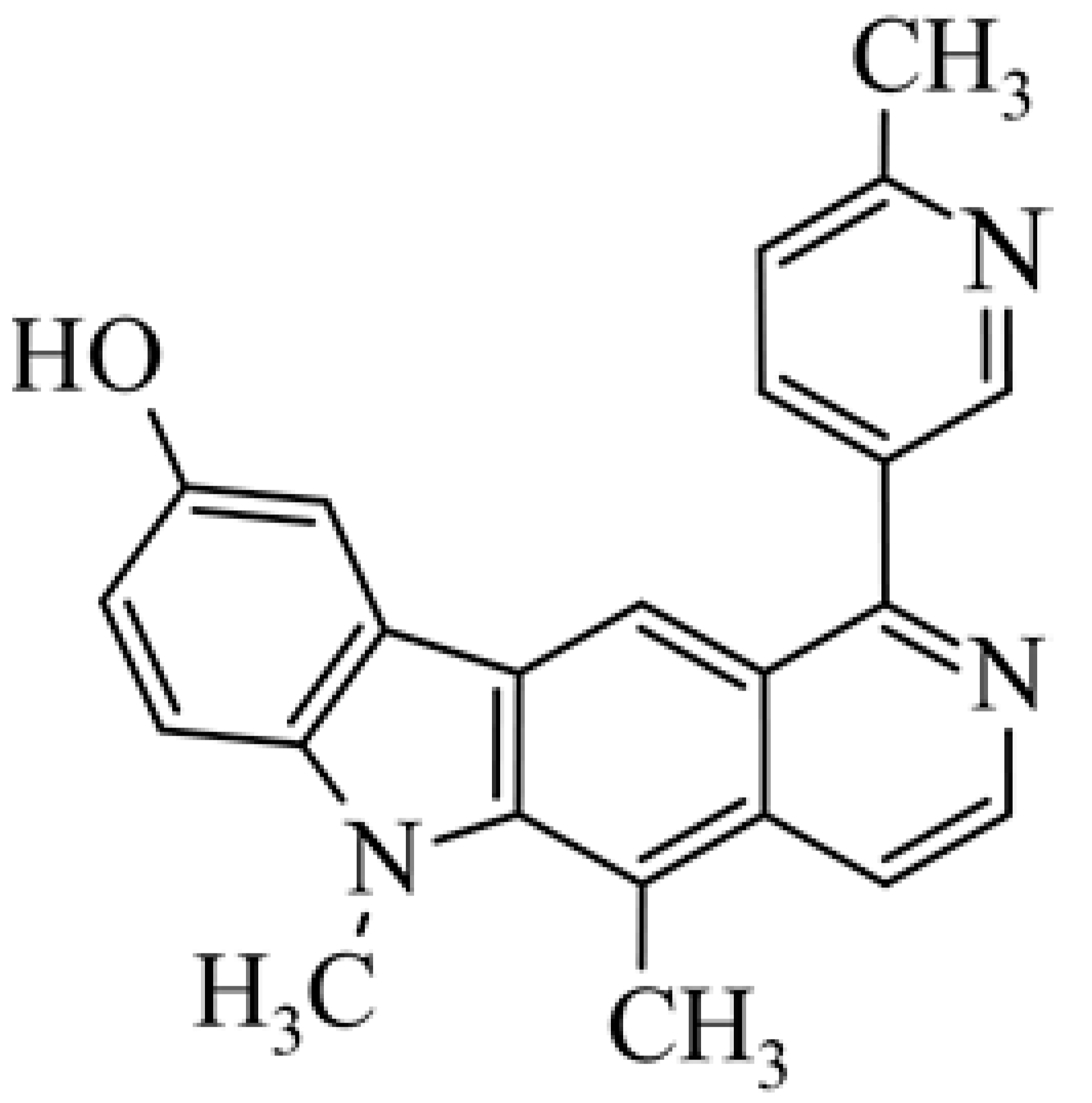 | 0.85 | [50] | ||||
| 28 | 9-methoxy-5,6-dimethyl-1-(1-methyl-4-nitro-3H-imidazol-5-yl)-6H-pyrido[4,3-b]carbazole | 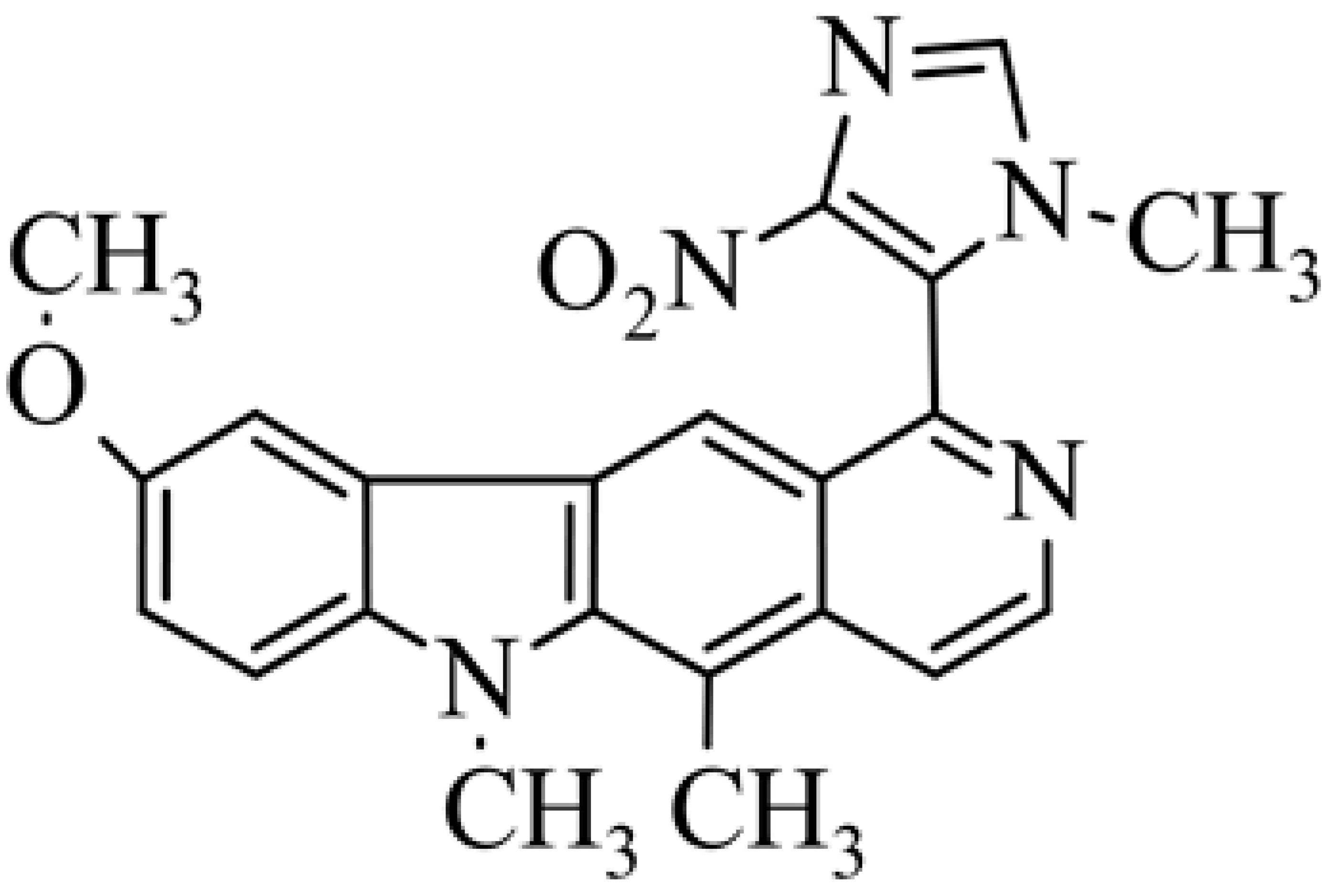 | 4.74± 1.30 (hypoxic 0.57 ± 0.033) | 5.96 ± 1.10 (hypoxic 0.69 ± 0.053) | [51] | |||
| 29 | 9-methoxy-5,6-dimethyl-1-(1-methyl-4-nitro-2H-pyrazol-5-yl)-6H-pyrido[4,3-b]carbazole | 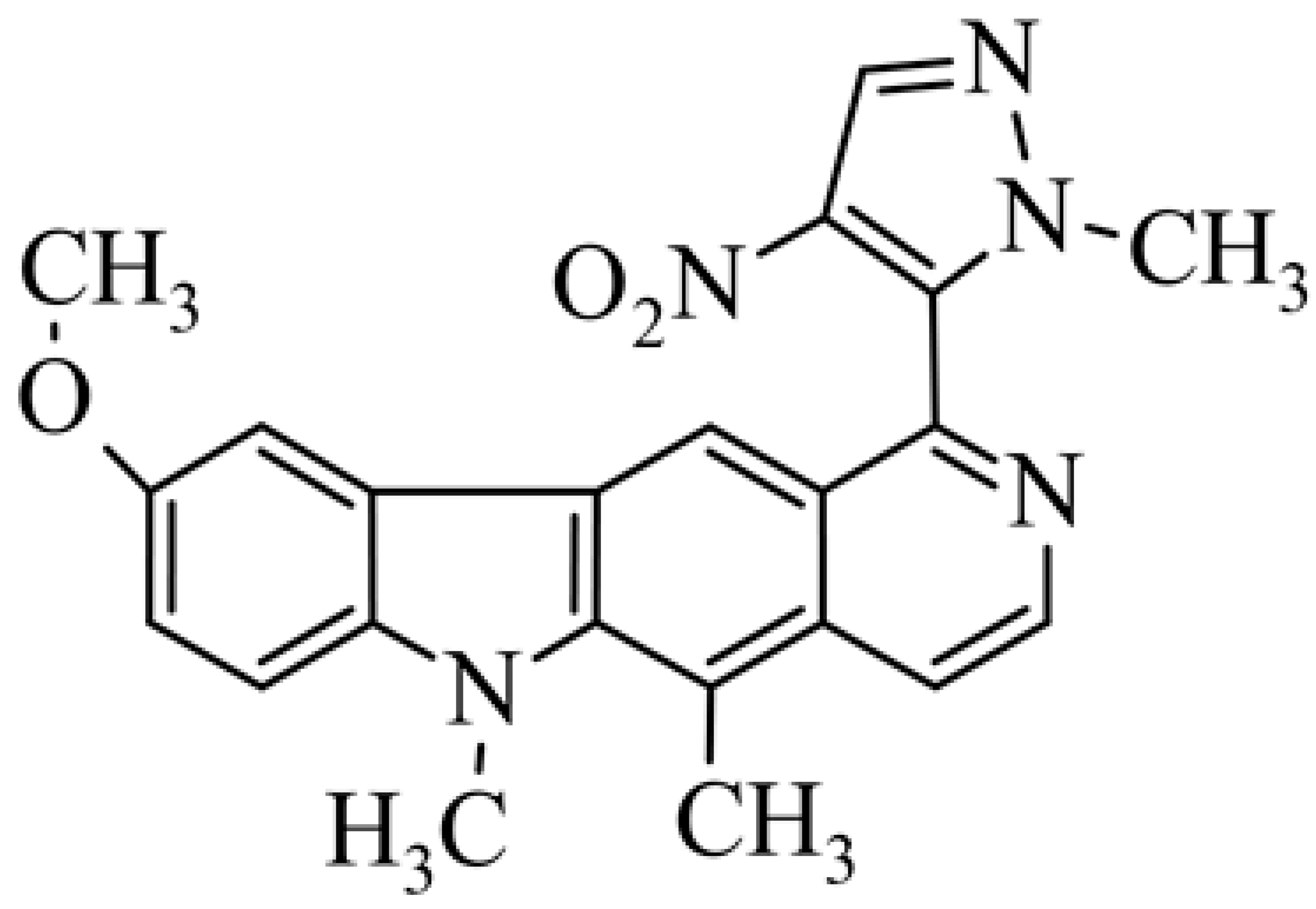 | 30.50 ± 5.05 (hypoxic 0.65 ± 0.019) | 11.25 ± 3.42 (hypoxic 0.81 ± 0.045) | [51] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tylińska, B.; Wiatrak, B. Bioactive Olivacine Derivatives—Potential Application in Cancer Therapy. Biology 2021, 10, 564. https://doi.org/10.3390/biology10060564
Tylińska B, Wiatrak B. Bioactive Olivacine Derivatives—Potential Application in Cancer Therapy. Biology. 2021; 10(6):564. https://doi.org/10.3390/biology10060564
Chicago/Turabian StyleTylińska, Beata, and Benita Wiatrak. 2021. "Bioactive Olivacine Derivatives—Potential Application in Cancer Therapy" Biology 10, no. 6: 564. https://doi.org/10.3390/biology10060564
APA StyleTylińska, B., & Wiatrak, B. (2021). Bioactive Olivacine Derivatives—Potential Application in Cancer Therapy. Biology, 10(6), 564. https://doi.org/10.3390/biology10060564







