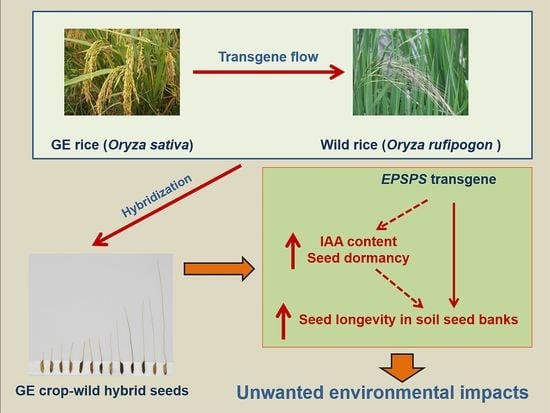
Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene
Abstract
Simple Summary
Abstract
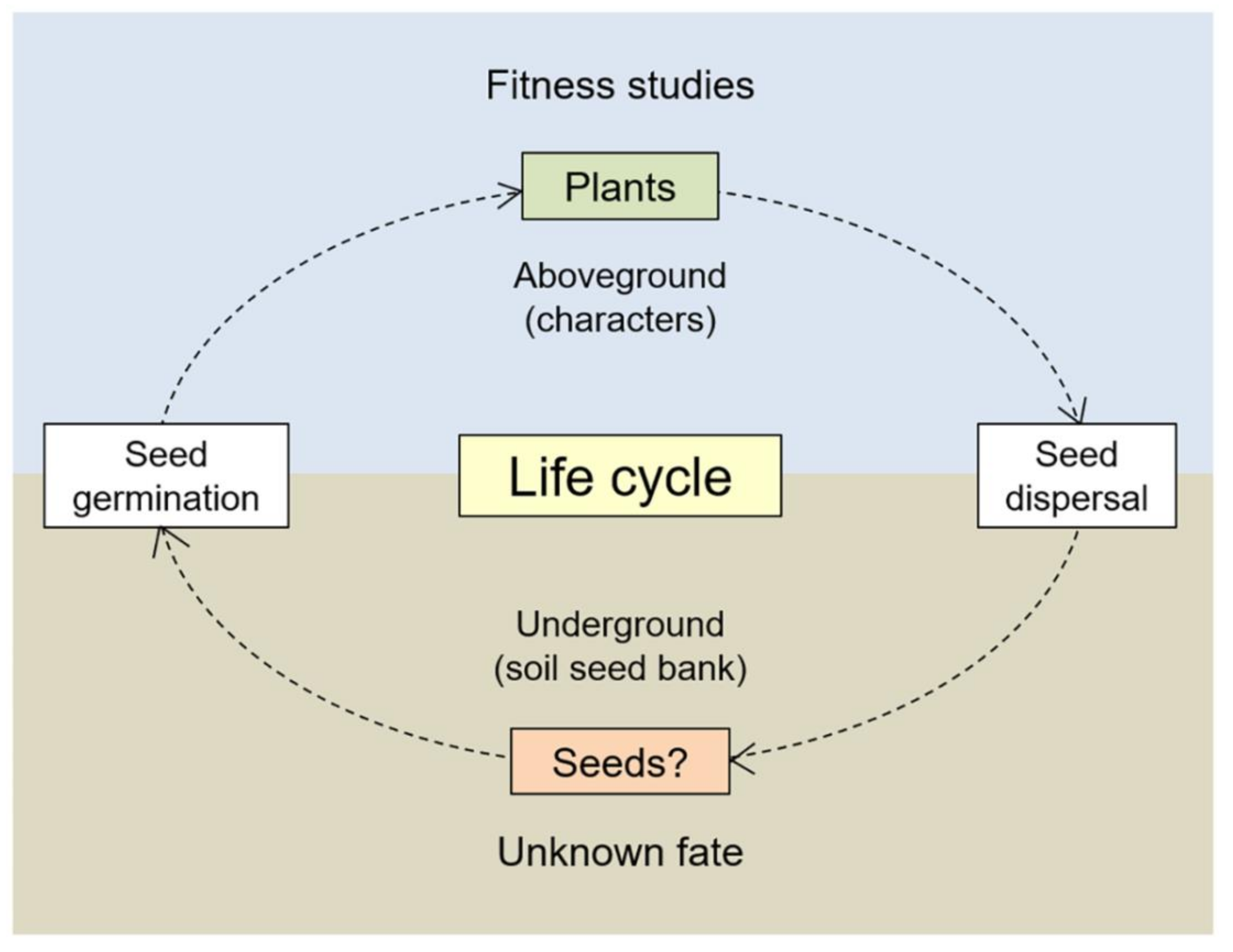
1. Introduction
2. Materials and Methods
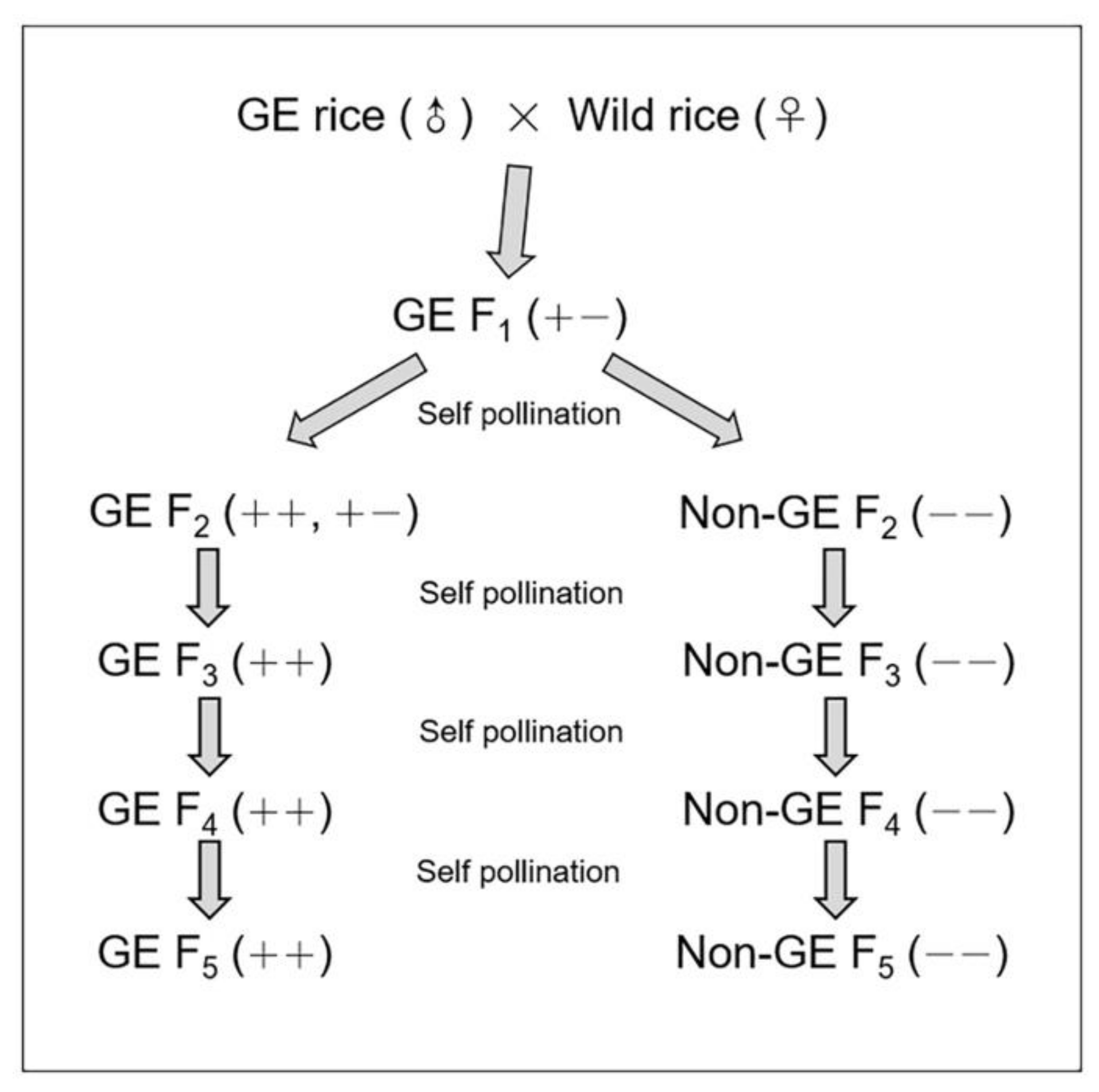
2.1. Plant Materials
2.2. Seed Dormancy Breaking
2.3. Soil Burial Treatment
2.4. Examination of Seed Germination and Dormancy
2.5. Examination of Plant Hormones in Seeds
2.6. Statistical Analysis
3. Results
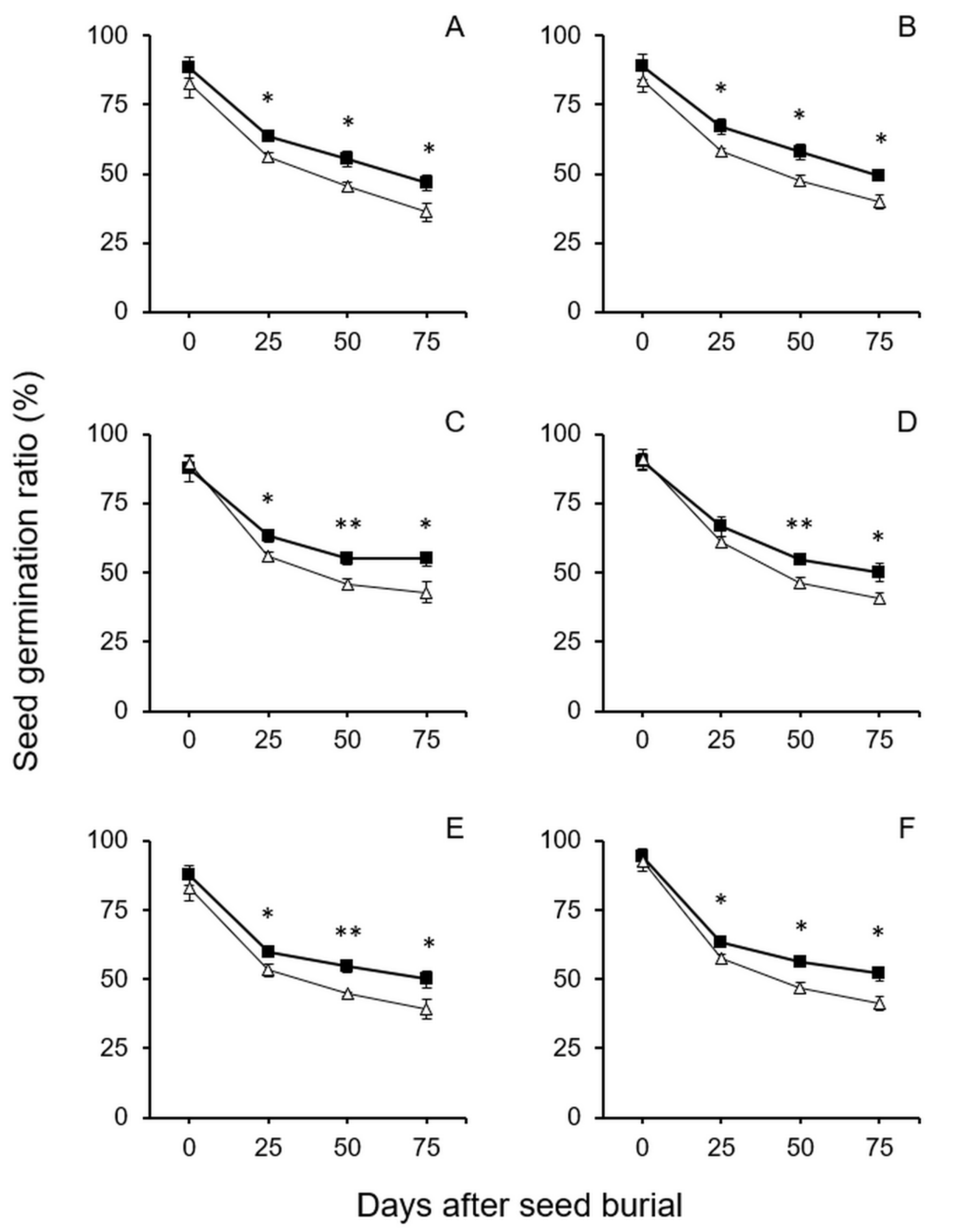
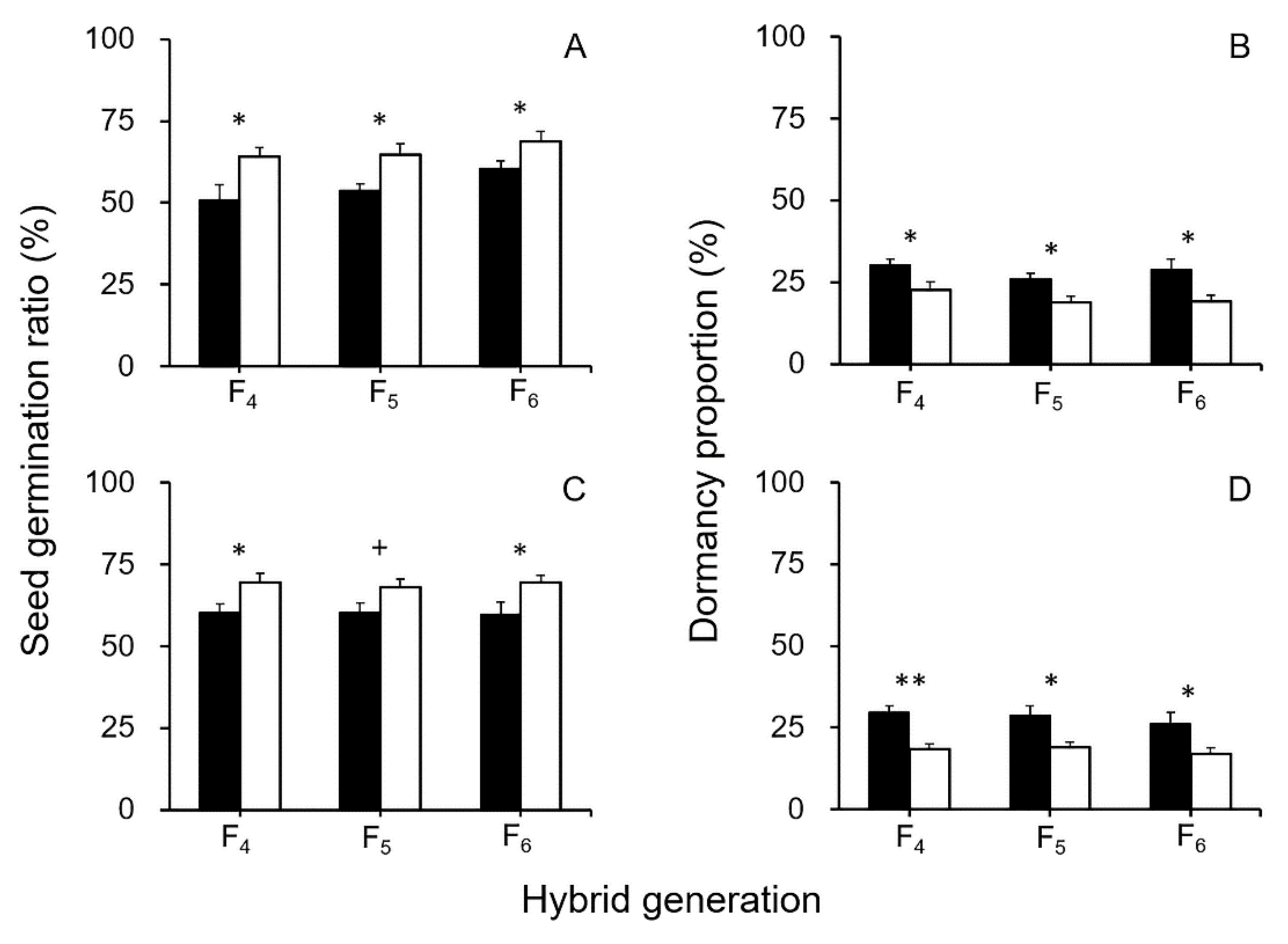
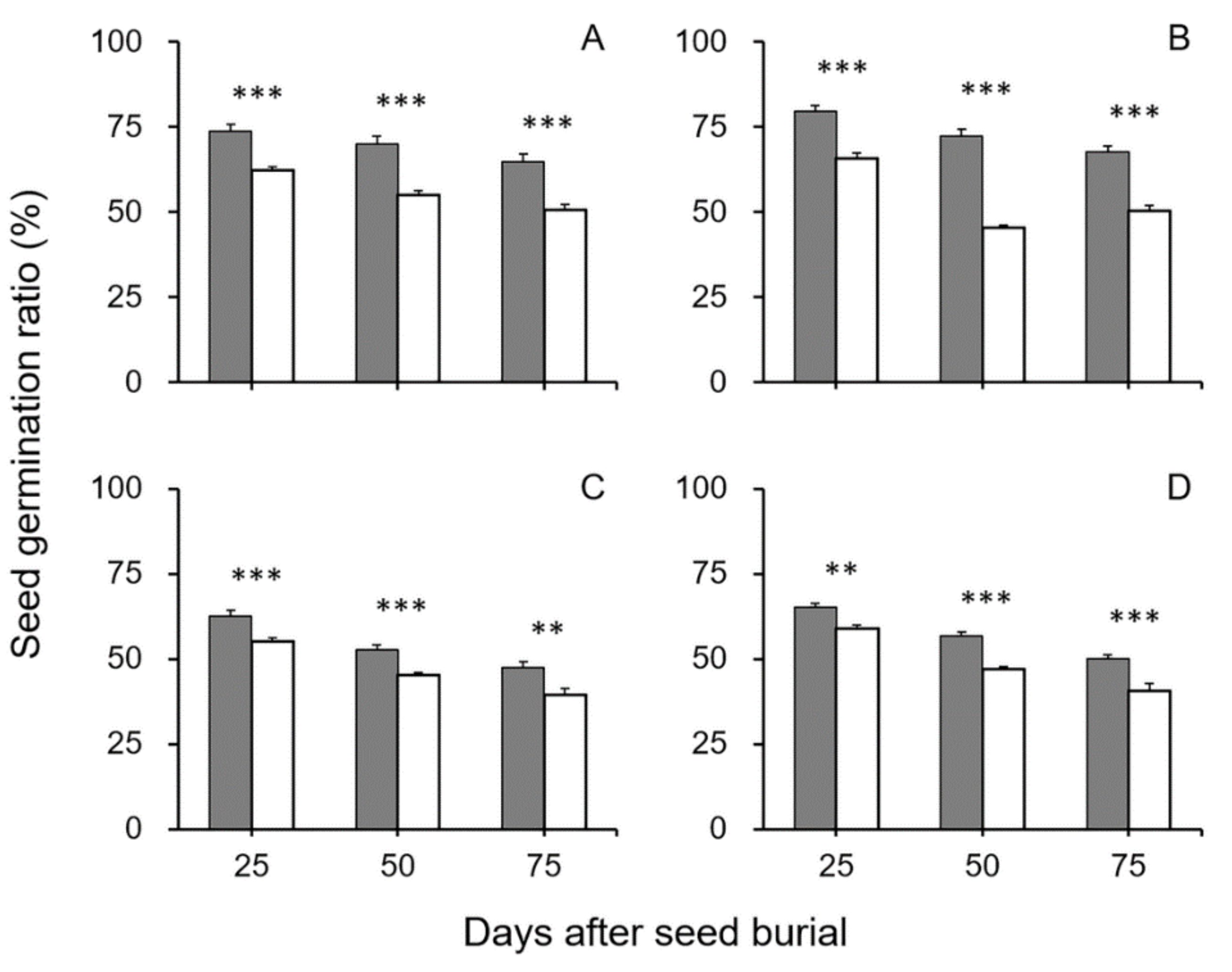
3.1. Survival and Longevity of GE and Non-GE Crop–Wild Hybrid Seeds After Soil Burial Treatments
3.2. Dormancy of GE and Non-GE Crop–Wild Hybrid Seeds
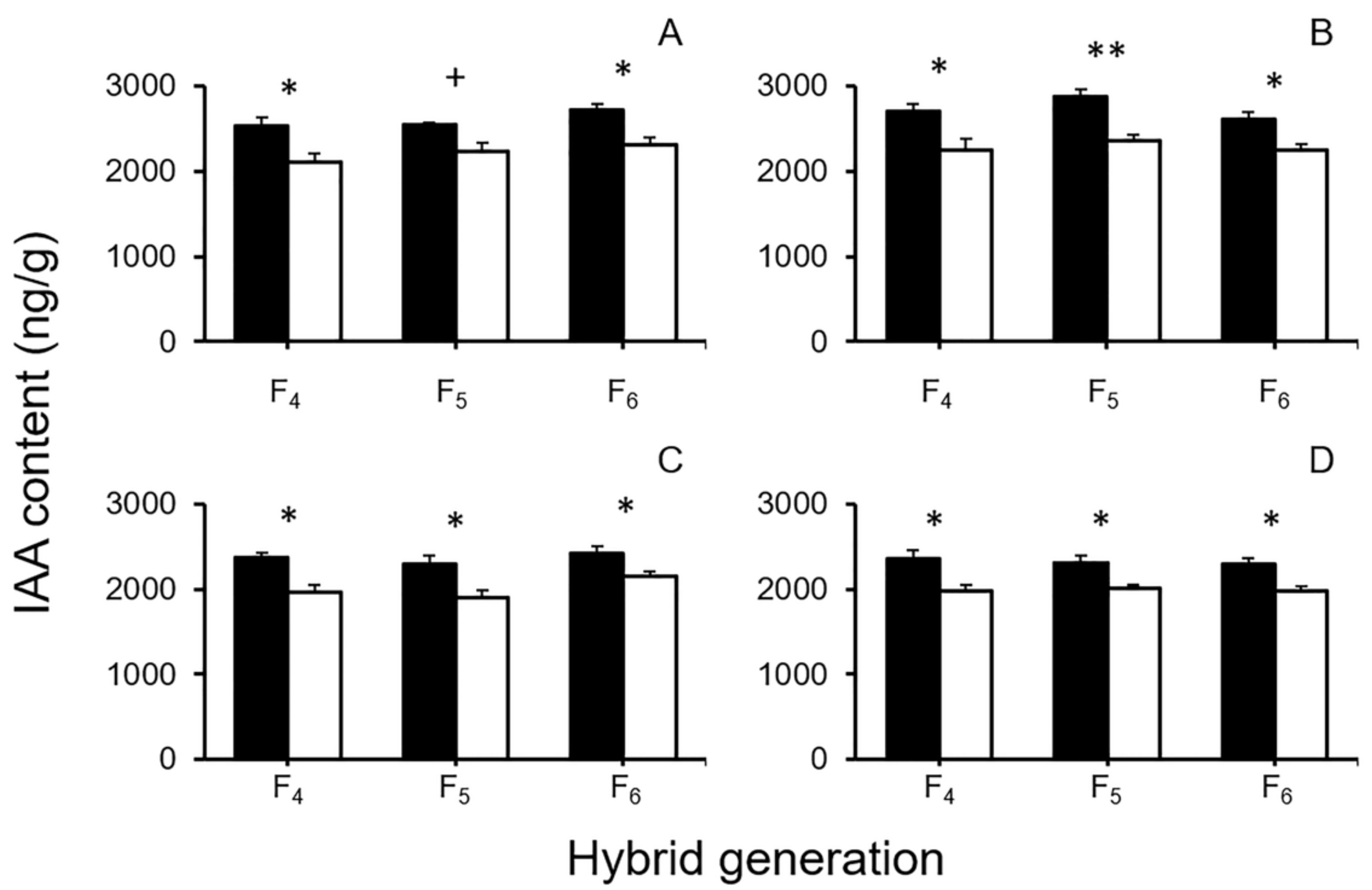
3.3. Changes of Plant Hormones in GE and Non-GE Crop–Wild Hybrid Seeds
4. Discussion
4.1. EPSPS Transgene Increases the Survival and Longevity of GE Crop–Wild Hybrid Seeds in Soil Seed Banks
4.2. Overexpressing EPSPS Transgene Increases Dormancy in GE Crop–Wild Hybrid Seeds
4.3. Overexpressing EPSPS Transgene Increases IAA Content in GE Crop–Wild Hybrid Seeds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISAAA. Global status of commercialized biotech/GM crops in 2019: Biotech crops drive socio-economic development and sustainable environment in the new frontier. In ISAAA Brief No. 55; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Linder, C.R.; Schmitt, J. Potential persistence of escaped transgenes: Performance of transgenic, oil-modified Brassica seeds and seedlings. Ecol. Appl. 1995, 5, 1056–1068. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Prentice, H.C.; Hancock, J.F. Gene flow and introgression from domesticated plants into their wild relatives. Annu. Rev. Ecol. Syst. 1999, 30, 539–563. [Google Scholar] [CrossRef]
- Hall, L.; Topinka, K.; Huffman, J.; Davis, L.; Good, A. Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci. 2000, 48, 688–694. [Google Scholar] [CrossRef]
- Stewart, C.N.; Richards, H.A.; Halfhill, M.D. Transgenic plants and biosafety: Science, misconceptions and public perceptions. BioTechniques 2000, 29, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-R.; Snow, A.A. Gene flow from genetically modified rice and its environmental consequences. BioScience 2005, 55, 669–678. [Google Scholar] [CrossRef]
- Lu, B.-R. Transgene escape from GM crops and potential biosafety consequences: An environmental perspective. Collect Biosaf. Rev. 2008, 4, 66–141. [Google Scholar]
- Lu, B.-R.; Yang, X.; Ellstrand, N.C. Fitness correlates of crop transgene flow into weedy populations: A case study of weedy rice in China and other examples. Evol. Appl. 2016, 9, 857–870. [Google Scholar] [CrossRef]
- Doebley, J. Molecular evidence for gene flow among Zea species. BioScience 1990, 40, 443–448. [Google Scholar] [CrossRef]
- Song, Z.P.; Lu, B.-R.; Zhu, Y.G.; Chen, J.K. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. New Phytol. 2003, 157, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yuan, Q.-H.; Shi, L.; Qiang, Q.; Liu, W.-G.; Kuang, B.-G.; Zeng, D.-L.; Liao, Y.-L.; Cao, B.; Jia, S.-R. A large-scale field study of transgene flow from cultivated rice (Oryza sativa) to common wild rice (O. rufipogon) and barnyard grass (Echinochloa crusgalli). Plant Biotechnol. J. 2006, 4, 667–676. [Google Scholar] [CrossRef]
- Sun, G.; Dai, W.; Cui, R.; Qiang, S.; Song, X. Gene flow from glufosinate-resistant transgenic hybrid rice Xiang 125S/Bar68–1 to weedy rice and cultivated rice under different experimental designs. Euphytica 2015, 204, 211–227. [Google Scholar] [CrossRef]
- Zhang, C.-J.; Yook, M.-J.; Park, H.-R.; Lim, S.-H.; Kim, J.-W.; Nah, G.J.; Song, H.-R.; Jo, B.H.; Roh, K.H.; Park, S.; et al. Assessment of potential environmental risks of transgene flow in smallholder farming systems in Asia: Brassica napus as a case study in Korea. Sci. Total Environ. 2018, 640–641, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X.; Wang, L.; Yan, H.; Su, J.; Wang, F.; Lu, B.-R. Limited ecological risk of insect-resistance transgene flow from cultivated rice to its wild ancestor based on life-cycle fitness assessment. Sci. Bull. 2016, 61, 1440–1450. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Jiang, X.; Wang, W.; Cai, X.; Su, J.; Wang, F.; Lu, B.-R. Genetically engineered rice endogenous 5-enolpyruvoylshikimate-3-phosphate synthase (epsps) transgene alters phenology and fitness of crop–wild hybrid offspring. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, C.E.; Presotto, A.; Carbonell, F.T.; Ureta, M.S. Transgene escape and persistence in an agroecosystem: The case of glyphosate-resistant Brassica rapa L. in central Argentina. Environ. Sci. Pollut. R. 2018, 25, 6251–6264. [Google Scholar] [CrossRef] [PubMed]
- Jenczewski, E.; Ronfort, J.; Chèvre, A.M. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environ. Biosaf. Res. 2002, 2, 9–24. [Google Scholar] [CrossRef]
- Londo, J.P.; Bautista, N.S.; Sagers, C.L.; Lee, E.H.; Watrud, L.S. Glyphosate drift promotes changes in fitness and transgene flow in canola (Brassica napus) and hybrids. Ann. Bot. 2010, 106, 957–965. [Google Scholar] [CrossRef]
- Yang, X.; Xia, H.; Wang, W.; Wang, F.; Su, J.; Snow, A.A.; Lu, B.-R. Transgenes for insect resistance reduce herbivory and enhance fecundity in advanced generations of crop–weed hybrids of rice. Evol. Appl. 2011, 4, 672–684. [Google Scholar] [CrossRef]
- Liu, Y.-B.; Darmency, H.; Stewart, N.C.; Tang, Z.-X.; Ma, K.-P. The effect of Bt-transgene introgression on plant growth and reproduction in wild Brassica juncea. Transgenic Res. 2014, 24, 537–547. [Google Scholar] [CrossRef]
- Wang, W.; Xia, H.; Ting, X.; Si, H.J.; Cai, X.X.; Wang, F.; Su, J.; Snow, A.A.; Lu, B.-R. A novel 5-enolpyruvoylshikimate-3-phosphate (EPSP) synthase transgene for glyphosate resistance stimulates growth and fecundity in weedy rice (Oryza sativa) without herbicide. New Phytol. 2014, 202, 679–688. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Gundel, P.E.; Preston, C. Experimental methods for estimation of plant fitness costs associated with herbicide resistance genes. Weed Sci. 2015, 63, 203–216. [Google Scholar] [CrossRef]
- Yan, H.X.; Li, L.; Liu, P.; Jiang, X.Q.; Wang, L.; Fang, J.; Lin, Z.M.; Wang, F.; Su, J.; Lu, B.-R. Reduced weed seed shattering by silencing a cultivated rice gene: Strategic mitigation for escaped transgenes. Transgenic Res. 2017, 26, 465–475. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, H.; Wang, W.; Yang, X.; Wang, F.; Su, J.; Xia, H.; Xu, K.; Cai, X.; Lu, B.-R. Ambient insect pressure and recipient genotypes determine fecundity of transgenic crop-weed rice hybrid progeny: Implications for environmental biosafety assessment. Evol. Appl. 2016, 9, 847–856. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Zalamea, P.-C.; Dalling, J.W.; Sarmiento, C.; Arnold, A.E.; Delevich, C.; Berhow, M.A.; Ndobegang, A.; Gripenberg, S.; Davis, A.S. Dormancy-defense syndromes and tradeoffs between physical and chemical defenses in seeds of pioneer species. Ecology 2018, 99, 1988–1998. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Secondary seed dormancy prolongs persistence of volunteer canola in western Canada. Weed Sci. 2003, 51, 904–913. [Google Scholar] [CrossRef]
- Pipatpongpinyo, W.; Korkmaz, U.; Wu, H.; Kena, A.; Ye, H.; Feng, J.; Gu, X.-Y. Assembling seed dormancy genes into a system identified their effects on seedbank longevity in weedy rice. Heredity 2019, 124, 135–145. [Google Scholar] [CrossRef]
- Mall, U.; Singh, G.S. Soil seed bank dynamics: History and ecological significance in sustainability of different ecosystems. In Environment and Sustainable Development; Fulekar, H., Pathak, B., Kale, R.K., Eds.; Springer: New Delhi, India, 2014; pp. 31–46. [Google Scholar]
- Su, J.; Chen, G.M.; Tian, D.G.; Zhu, Z.; Wang, F. A gene encodes 5-enolpyruvylshikimate-3-phosphate mutagenized by errorprone PCR conferred rice with high glyphosate-tolerance. Mol. Plant Breed. 2008, 6, 830–836. (In Chinese) [Google Scholar]
- Fang, J.; Nan, P.; Gu, J.; Ge, X.; Feng, Y.-Q.; Lu, B.-R. Overexpressing exogenous 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) genes increases fecundity and auxin content of transgenic Arabidopsis plants. Front. Plant. Sci. 2018, 9, 233. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Sheri, V.; Manna, M.; Panditi, V.; Borphukan, B.; Ram, B.; Agarwal, A.; Fartyal, D.; Teotia, D. Overexpression of improved EPSPS gene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol. J. 2020, 18, 2504–2519. [Google Scholar] [CrossRef]
- Ma, T.; Yuan, Y.; Lin, K.; Nan, P.; Lu, B. Overexpression EPSPS transgene enhances chlorophyll synthesis in hybrid progeny between cultivated rice and weedy rice. J. Fudan Univ. Nat. Sci. 2020, 59, 185–193. (In Chinese) [Google Scholar]
- Wu, J.; Fang, J.; Cai, X.; Lu, B. Overexpression 5-enolpyruvylshikimate-3-phosphate synthase gene increase lignin content of transgenic progeny derived from hybrids of EPSPS transgenic rice with weedy and wild rice. J. Fudan Univ. Nat. Sci. 2020, 59, 666–676. (In Chinese) [Google Scholar]
- Burke, J.M.; Rieseberg, L.H. Fitness effects of transgenic disease resistance in sunflowers. Science 2003, 300, 1250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snow, A.A.; Plison, D.; Rieseberg, L.H.; Paulsen, M.J.; Pleskac, N.; Reagon, M.R.; Wolf, D.E.; Selbo, M. A Bt transgene reduces herbivory and enhances fecundity in wild sunflowers. Ecol. Appl. 2003, 13, 279–286. [Google Scholar] [CrossRef]
- Lakon, G. The topographical tetrazolium method for determining the germinating capacity of seeds. Plant Physiol. 1949, 24, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Beres, Z.T.; Jin, L.; Parrish, J.T.; Zhao, W.; David, M.; Snow, A.A.; Wu, K. Effects of over-expressing a native gene encoding 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) on glyphosate resistance in Arabidopsis thaliana. PLoS ONE 2017, 12, e0175820. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Zhu, X.-Y.; Lu, B.-R. Soil burial induced dormancy in weedy rice seeds through hormone level changes: Implications in adaptive evolution and weed control. J. Syst. Evol. 2021, 1–13. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Bewey, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Dormancy and the control of germination. In Seeds Physiology of Development, Germination and Dormancy; Bewley, J.D., Bradford, K.J., Hilhorst, H.W.M., Nonogaki, H., Eds.; Springer: New York, NY, USA, 2013; pp. 247–297. [Google Scholar]
- Liu, Y.; Koornneef, M.; Soppe, W.J.J. The absence of histone H2B monoubiquitination in the Arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 2007, 19, 433–444. [Google Scholar] [CrossRef]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Hanada, A.; Yaeno, T.; Shirasu, K.; Yao, H.; et al. The main auxin biosynthesis pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [PubMed]
- Di, D.-W.; Zhang, C.; Luo, P.; An, C.-W.; Guo, G.-Q. The biosynthesis of auxin: How many paths truly lead to IAA? Plant Growth Regul. 2016, 78, 275–285. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhao, Y.; Feng, Z.; Li, Q.; Yang, H.Q.; Luan, S.; Li, J.; He, Z.H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef]
- Ramaih, S.; Guedira, M.; Paulsen, G.M. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Func. Plant Biol. 2003, 30, 939–945. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Liu, Y.; Zhao, J.; Fu, J.; Ren, X.; Wang, G.; Wang, J. Exogenous auxin regulates multi-metabolic network and embryo development, controlling seed secondary dormancy and germination in Nicotiana tabacum L. BMC Plant Biol. 2016, 16, 41. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed]

| Hybrid Seed | F4 | F5 | F6 | |||
|---|---|---|---|---|---|---|
| GE | Non-GE | GE | Non-GE | GE | Non-GE | |
| EP3–w1 | 27.9 ± 2.2 1 | 17.9 ± 1.2 ** 2 | 25.4 ± 1.4 | 18.3 ± 2.2 * | 27.5 ± 2.4 | 19.2 ± 1.8 * |
| EP3–w2 | 25.4 ± 2.1 | 16.7 ± 1.2 ** | 23.8 ± 1.1 | 18.3 ± 2.0 * | 28.3 ± 0.5 | 18.8 ± 1.9 ** |
| Hybrid Lineage | Hormone | F4 | F5 | F6 | |||
|---|---|---|---|---|---|---|---|
| GE | Non-GE | GE | Non-GE | GE | Non-GE | ||
| EP3–w1 | ABA | 90.3 ± 4.7 1 | 65.5 ± 7.2 * 2 | 76.4 ± 5.8 | 65.3 ± 1.4 NS | 84.0 ± 5.9 | 82.3 ± 9.0 NS |
| cis-OPDA | 3.7 ± 1.4 | 3.1 ± 1.3 NS | 4.1 ± 0.8 | 3.0 ± 0.3 NS | 4.0 ± 0.2 | 4.5 ± 0.4 NS | |
| GA3 | 1.2 ± 0.1 | 1.1 ± 0.2 NS | 1.1 ± 0.2 | 1.1 ± 0.2 NS | 1.2 ± 0.2 | 1.1 ± 0.2 NS | |
| JA | 4.6 ± 0.4 | 7.4 ± 0.4 ** | 7.4 ± 0.4 | 6.7 ± 0.9 NS | 7.0 ± 1.1 | 6.9 ± 0.6 NS | |
| SA | 921.0 ± 102.1 | 816.8 ± 98.6 NS | 817.5 ± 55.4 | 822.2 ± 42.7 NS | 910.8 ± 73.9 | 957.0 ± 12.1 NS | |
| EP3–w2 | ABA | 75.2 ± 5.7 | 62.8 ± 5.3 NS | 88.9 ± 3.2 | 63.3 ± 7.9 * | 75.7 ± 7.2 | 64.0 ± 5.8 NS |
| cis-OPDA | 3.5 ± 0.3 | 3.5 ± 0.5 NS | 4.0 ± 0.1 | 4.0 ± 0.0 NS | 4.0 ± 0.0 | 4.6 ± 0.4 NS | |
| GA3 | 1.4 ± 0.4 | 1.1 ± 0.3 NS | 1.0 ± 0.3 | 1.2 ± 0.2 NS | 1.0 ± 0.1 | 1.1 ± 0.1 NS | |
| JA | 7.5 ± 0.5 | 6.6 ± 1.0 NS | 5.7 ± 1.4 | 4.8 ± 0.7 NS | 6.2 ± 0.1 | 6.7 ± 0.4 NS | |
| SA | 847.4 ± 26.9 | 856.2 ± 19.3 NS | 876.5 ± 18.0 | 959.0 ± 46.3 NS | 905.8 ± 73.9 | 887.5 ± 48.2 NS | |
| Hybrid Lineage | Hormone | F4 | F5 | F6 | |||
|---|---|---|---|---|---|---|---|
| GE | Non-GE | GE | Non-GE | GE | Non-GE | ||
| EP3–w1 | ABA | 53.8 ± 5.0 1 | 43.0 ± 3.9 NS 2 | 42.5 ± 8.3 | 35.4 ± 3.7 NS | 60.0 ± 2.7 | 46.4 ± 9.0 NS |
| cis-OPDA | 1.3 ± 0.1 | 1.4 ± 0.1 NS | 1.3 ± 0.3 | 1.6 ± 0.7 NS | 1.8 ± 0.5 | 2.7 ± 0.2 NS | |
| GA3 | 4.4 ± 0.6 | 3.4 ± 0.0 NS | 3.2 ± 0.1 | 3.3 ± 0.3 NS | 4.5 ± 1.1 | 4.0 ± 0.3 NS | |
| JA | 2.5 ± 0.7 | 1.8 ± 0.1 NS | 1.4 ± 0.2 | 1.5 ± 0.1 NS | 2.3 ± 0.2 | 1.7 ± 0.3 NS | |
| SA | 660.6 ± 127.5 | 493.4 ± 82.4 NS | 669.5 ± 50.6 | 732.8 ± 7.5 NS | 566.6 ± 77.4 | 717.8 ± 35.9 NS | |
| EP3–w2 | ABA | 55.3 ± 6.6 | 42.9 ± 2.5 NS | 29.4 ± 2.3 | 32.6 ± 4.6 NS | 46.2 ± 7.8 | 37.1 ± 2.8 NS |
| cis-OPDA | 1.2 ± 0.1 | 1.3 ± 0.4 NS | 1.6 ± 0.3 | 1.0 ± 0.3 NS | 2.0 ± 0.2 | 2.3 ± 0.4 NS | |
| GA3 | 3.7 ± 0.1 | 3.8 ± 0.4 NS | 4.8 ± 3.8 | 3.8 ± 0.4 NS | 4.5 ± 0.9 | 5.4 ± 0.1 NS | |
| JA | 2.7 ± 1.0 | 1.5 ± 0.4 NS | 1.2 ± 0.1 | 1.0 ± 0.1 NS | 2.0 ± 0.6 | 1.3 ± 0.1 NS | |
| SA | 722.7 ± 35.1 | 636.5 ± 33.6 NS | 680.6 ± 35.9 | 581.2 ± 124.9 NS | 605.2 ± 51.9 | 697.5 ± 64.6 NS | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.-Q.; Yang, X.; Lu, B.-R. Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene. Biology 2021, 10, 562. https://doi.org/10.3390/biology10060562
Jiang X-Q, Yang X, Lu B-R. Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene. Biology. 2021; 10(6):562. https://doi.org/10.3390/biology10060562
Chicago/Turabian StyleJiang, Xiao-Qi, Xiao Yang, and Bao-Rong Lu. 2021. "Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene" Biology 10, no. 6: 562. https://doi.org/10.3390/biology10060562
APA StyleJiang, X.-Q., Yang, X., & Lu, B.-R. (2021). Increased Longevity and Dormancy of Soil-Buried Seeds from Advanced Crop–Wild Rice Hybrids Overexpressing the EPSPS Transgene. Biology, 10(6), 562. https://doi.org/10.3390/biology10060562