Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
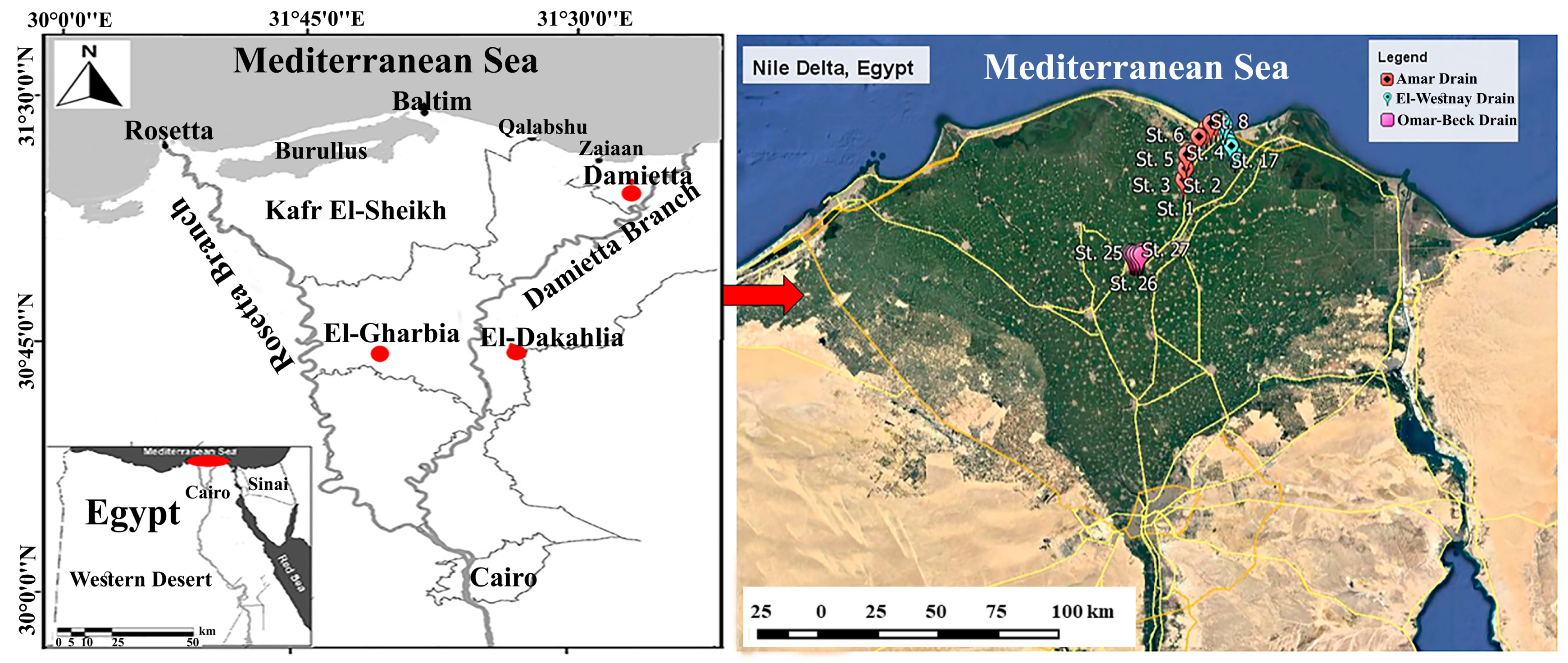
2.1. Study Area
2.2. Sampling Design and Processing
2.3. Sediment Analysis
2.4. Plant Analysis
2.5. Phytoremediation Potentials of the Selected Aquatic Macrophytes
2.6. Data Analysis
3. Results
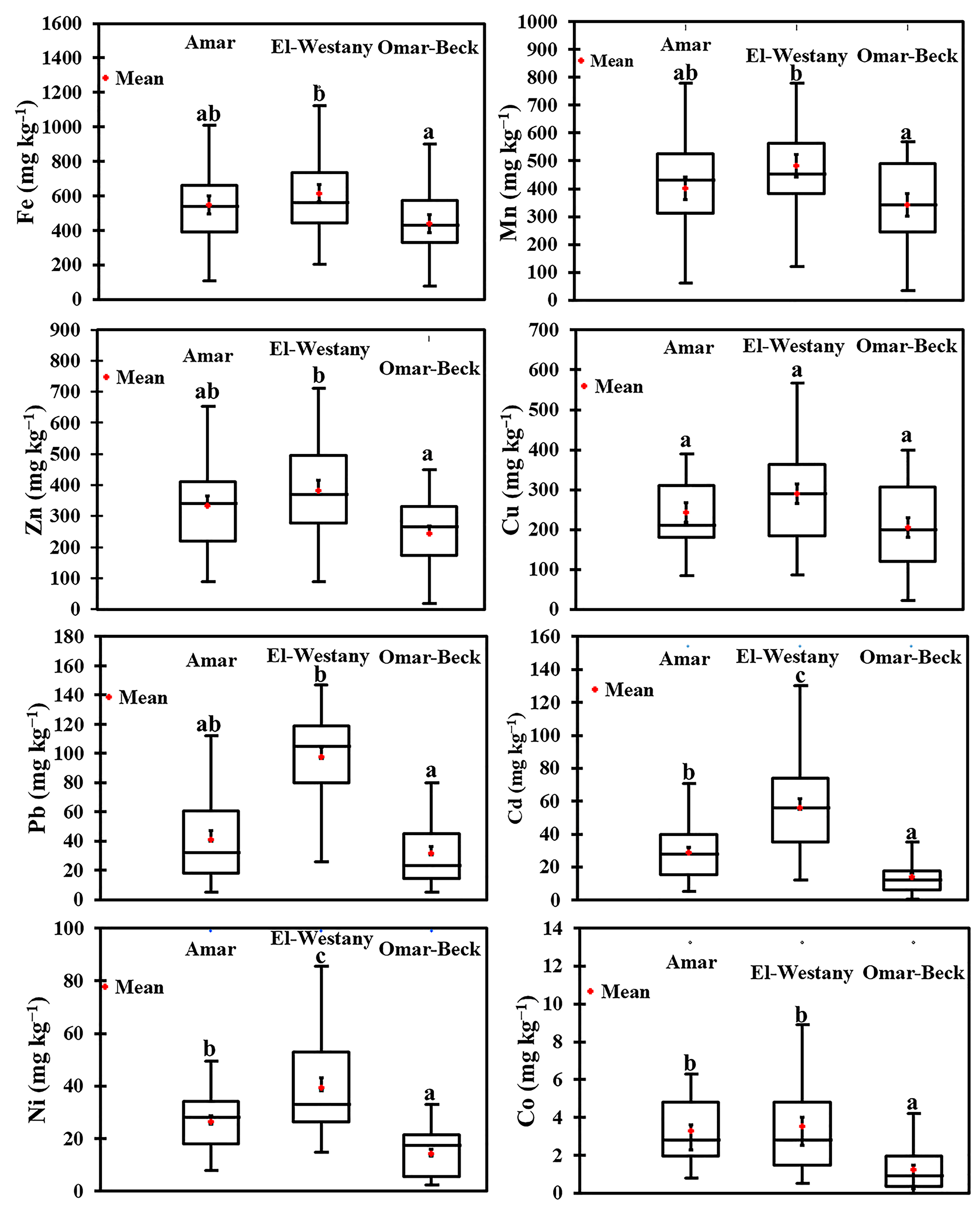
3.1. Sediment Heavy Metals in the Investigated Three Drains
3.2. Plant Heavy Metals
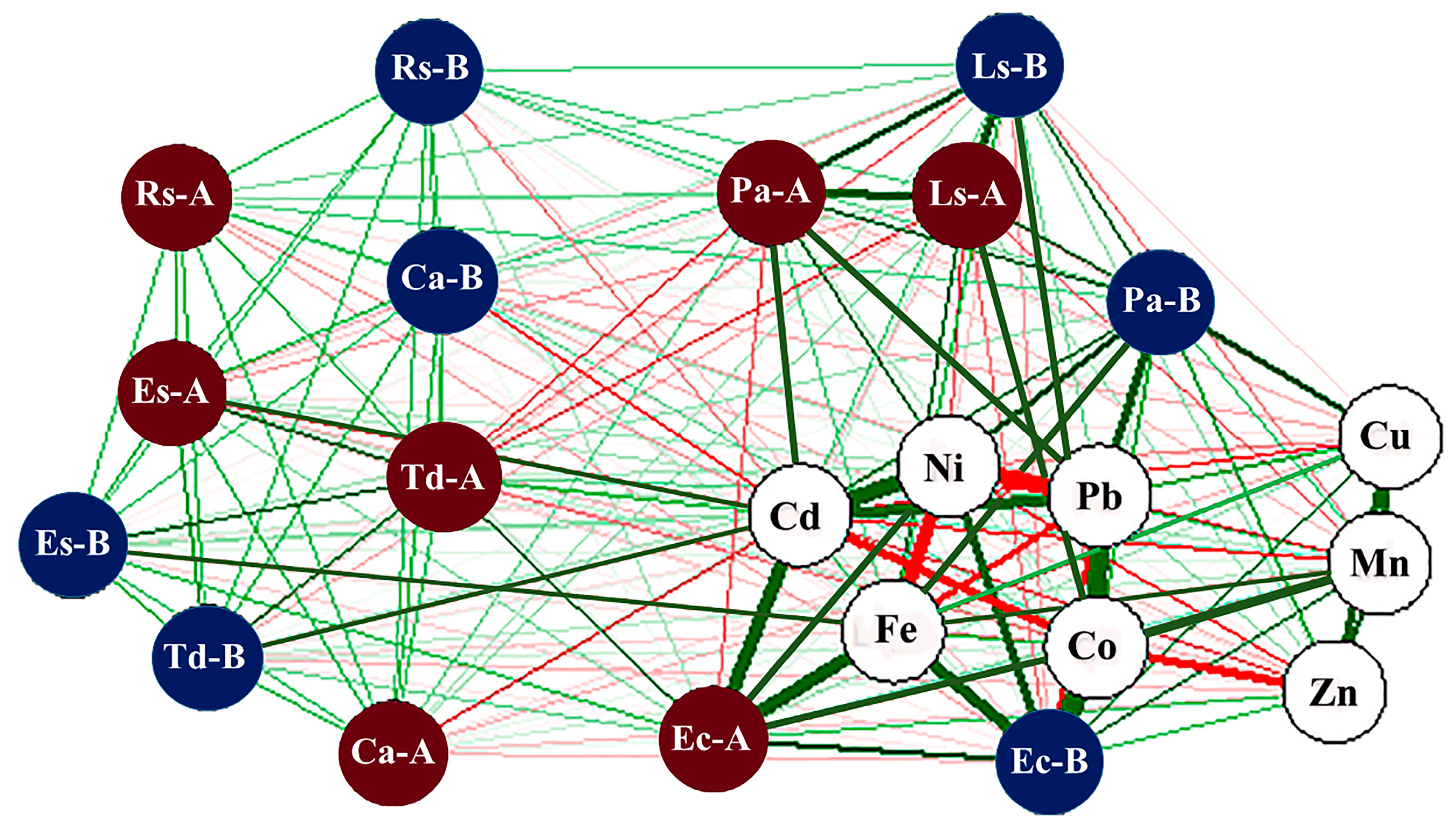
3.3. Interactive Effects of Drains, Plant Species, and Tissues on Sediment’s Heavy Metals
3.4. Heavy Metals Phytoremediation Assessment for Plant Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Metwalley, S.M.; Abbas, H.H. Irrigation: Water Resources, Types and Common Problems in Egypt. In Technological and Modern Irrigation Environment in Egypt; Springer: Berlin/Heidelberg, Germany, 2020; pp. 15–34. [Google Scholar]
- Authman, M.M.N.; Abbas, W.T.; Gaafar, A.Y. Metals Concentrations in Nile Tilapia (Oreochromis niloticus) from Illegal Fish Farm in Al-Minufiya Province, Egypt, and Their Effects on Some Tissues Structures. Ecotoxicol. Environ. Saf. 2012, 84, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Garbisu, C.; Alkorta, I. Basic Concepts on Heavy Metal Soil Bioremediation. Ejmp Ep Eur. J. Miner. Process. Environ. Prot. 2003, 3, 58–66. [Google Scholar]
- Eid, E.M.; Galal, T.M.; Shaltout, K.H.; El-Sheikh, M.A.; Asaeda, T.; Alatar, A.A.; Alfarhan, A.H.; Alharthi, A.; Alshehri, A.M.A.; Picó, Y. Biomonitoring Potential of the Native Aquatic Plant Typha domingensis by Predicting Trace Metals Accumulation in the Egyptian Lake Burullus. Sci. Total Environ. 2020, 714, 136603. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of Heavy Metals through Terrestrial Food Webs: A Review. Environ. Monit. Assess. 2015, 187, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant Growth Promoting Rhizobacteria and Endophytes Accelerate Phytoremediation of Metalliferous Soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of Siderophore-Producing Bacteria for Improving Heavy Metal Phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef]
- Eid, E.M.; Galal, T.M.; Sewelam, N.A.; Talha, N.I.; Abdallah, S.M. Phytoremediation of Heavy Metals by Four Aquatic Macrophytes and Their Potential Use as Contamination Indicators: A Comparative Assessment. Environ. Sci. Pollut. Res. 2020, 27, 12138–12151. [Google Scholar] [CrossRef]
- Tekin-Özan, S. Determination of Heavy Metal Levels in Water, Sediment and Tissues of Tench (Tinca tinca L. 1758) from Beyşehir Lake (Turkey). Environ. Monit. Assess. 2008, 145, 295–302. [Google Scholar] [CrossRef]
- Caselles-Osorio, A.; Vega, H.; Lancheros, J.C.; Casierra-Martínez, H.A.; Mosquera, J.E. Horizontal Subsurface-Flow Constructed Wetland Removal Efficiency Using Cyperus articulatus L. Ecol. Eng. 2017, 99, 479–485. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Reshi, Z.A.; Shah, M.A.; Rashid, I.; Ara, R.; Andrabi, S.M.A. Phytoremediation Potential of Phragmites australis in Hokersar Wetland-a Ramsar Site of Kashmir Himalaya. Int. J. Phytoremediat. 2014, 16, 1183–1191. [Google Scholar] [CrossRef]
- Bello, A.O.; Tawabini, B.S.; Khalil, A.B.; Boland, C.R.; Saleh, T.A. Phytoremediation of Cadmium-, Lead-and Nickel-Contaminated Water by Phragmites australis in Hydroponic Systems. Ecol. Eng. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Farahat, E.A.; Galal, T.M. Trace Metal Accumulation by Ranunculus sceleratus: Implications for Phytostabilization. Environ. Sci. Pollut. Res. 2018, 25, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A. Phytoremediation of Industrial Wastewater Potentiality by Typha domingensis. Int. J. Environ. Sci. Technol. 2011, 8, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Rezania, S.; Park, J.; Rupani, P.F.; Darajeh, N.; Xu, X.; Shahrokhishahraki, R. Phytoremediation Potential and Control of Phragmites australis as a Green Phytomass: An Overview. Environ. Sci. Pollut. Res. 2019, 26, 7428–7441. [Google Scholar] [CrossRef]
- Lytle, J.S.; Lytle, T.F. Use of Plants for Toxicity Assessment of Estuarine Ecosystems. Environ. Toxicol. Chem. 2001, 20, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; El-Sheikh, M.A.; Asaeda, T. Seasonal Courses of Nutrients and Heavy Metals in Water, Sediment and above-and below-Ground Typha domingensis Biomass in Lake Burullus (Egypt): Perspectives for Phytoremediation. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 783–794. [Google Scholar] [CrossRef]
- Paz-Alberto, A.M.; Sigua, G.C. Phytoremediation: A Green Technology to Remove Environmental Pollutants. Am. J. Clim. Chang. 2013, 2, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, D. Phytoremediation of Heavy Metals from Industrial Effluent Using Constructed Wetland Technology. Appl. Ecol. Environ. Sci. 2013, 1, 92–97. [Google Scholar] [CrossRef]
- Saha, P.; Shinde, O.; Sarkar, S. Phytoremediation of Industrial Mines Wastewater Using Water Hyacinth. Int. J. Phytoremediat. 2017, 19, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Luo, C.; Lou, L.; Li, X.; Shen, Z. Bioaccumulation of Heavy Metals by the Aquatic Plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and Their Potential Use for Contamination Indicators and in Wastewater Treatment. Sci. Total Environ. 2008, 392, 22–29. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Badr, N.E.; El-Khatib, A.; Abo-El-Kassem, A. Heavy Metal Biomonitoring and Phytoremediation Potentialities of Aquatic Macrophytes in River Nile. Environ. Monit. Assess. 2012, 184, 1753–1771. [Google Scholar] [CrossRef]
- Kassaye, Y.A.; Skipperud, L.; Einset, J.; Salbu, B. Aquatic Macrophytes in Ethiopian Rift Valley Lakes; Their Trace Elements Concentration and Use as Pollution Indicators. Aquat. Bot. 2016, 134, 18–25. [Google Scholar] [CrossRef]
- Egyptian Environmental Affairs Agency (EEAA). Egypt State of the Environment Report; Ministry of Environment: Cairo, Egypt, 2017.
- Eid, E.M.; Shaltout, K.H.; Moghanm, F.S.; Youssef, M.S.G.; El-Mohsnawy, E.; Haroun, S.A. Bioaccumulation and Translocation of Nine Heavy Metals by Eichhornia crassipes in Nile Delta, Egypt: Perspectives for Phytoremediation. Int. J. Phytoremediat. 2019, 21, 821–830. [Google Scholar] [CrossRef]
- Galal, T.M.; Al-Sodany, Y.M.; Al-Yasi, H.M. Phytostabilization as a Phytoremediation Strategy for Mitigating Water Pollutants by the Floating Macrophyte Ludwigia stolonifera (Guill. & Perr.) PH Raven. Int. J. Phytoremediat. 2020, 22, 373–382. [Google Scholar]
- Srour, D.; Abdelaal, M.; Mashaly, I.A. Floristic and Ecological Features of Three Drains in the North of Nile Delta of Egypt. Mansoura J. Biol. 2019, 40, 20–29. [Google Scholar]
- Boulos, L. Flora of Egypt Checklist, Revised Annotated Edition; Al Hadara Publishing: Cairo, Egypt, 2009. [Google Scholar]
- Otte, M.L.; Haarsma, M.S.; Broekman, R.A.; Rozema, J. Relation between Heavy Metal Concentrations in Salt Marsh Plants and Soil. Environ. Pollut. 1993, 82, 13–22. [Google Scholar] [CrossRef]
- Lu, R.K. Methods of Inorganic Pollutants Analysis. In Soil and Agro-chemical Analysis Methods; Agricultural Science and Technology Press: Beijing, China, 2000; pp. 205–266. [Google Scholar]
- Farahat, E.; Linderholm, H.W. The Effect of Long-Term Wastewater Irrigation on Accumulation and Transfer of Heavy Metals in Cupressus sempervirens Leaves and Adjacent Soils. Sci. Total Environ. 2015, 512, 1–7. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in Native Plants Growing on a Contaminated Florida Site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Gupta, S.; Nayek, S.; Saha, R.N.; Satpati, S. Assessment of Heavy Metal Accumulation in Macrophyte, Agricultural Soil, and Crop Plants Adjacent to Discharge Zone of Sponge Iron Factory. Environ. Geol. 2008, 55, 731–739. [Google Scholar] [CrossRef]
- Team R Development Core. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Team R Development Core: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 10 September 2020).
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation Strategies for Soils Contaminated with Heavy Metals: Modifications and Future Perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The Influence of PH and Organic Matter Content in Paddy Soil on Heavy Metal Availability and Their Uptake by Rice Plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Weissmannová, H.D.; Pavlovský, J. Indices of Soil Contamination by Heavy Metals—Methodology of Calculation for Pollution Assessment (Minireview). Environ. Monit. Assess. 2017, 189, 1–25. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Bonanomi, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Ecological Risk Assessment of Heavy Metals along Three Main Drains in Nile Delta and Potential Phytoremediation by Macrophyte Plants. Plants 2020, 9, 910. [Google Scholar] [CrossRef]
- Farhat, H.I. Impact of Drain Effluent on Surficial Sediments in the Mediterranean Coastal Wetland: Sedimentological Characteristics and Metal Pollution Status at Lake Manzala, Egypt. J. Ocean Univ. China 2019, 18, 834–848. [Google Scholar] [CrossRef]
- Bradl, H.B. Sources and Origins of Heavy Metals. In Interface science and technology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 1–27. [Google Scholar]
- Khan, M.Z.H.; Hasan, M.R.; Khan, M.; Aktar, S.; Fatema, K. Distribution of Heavy Metals in Surface Sediments of the Bay of Bengal Coast. J. Toxicol. 2017, 2017, 9235764. [Google Scholar] [CrossRef]
- Segarra, M.J.; Szefer, P.; Wilson, M.J.; Bacon, J.; Bolalek, J. Chemical Forms and Distribution of Heavy Metals in Core Sediments from the Gdańsk Basin, Baltic Sea. Pol. J. Environ. Stud. 2007, 16, 505–515. [Google Scholar]
- Aitta, A.; El-Ramady, H.; Alshaal, T.; El-Henawy, A.; Shams, M.; Talha, N.; Elbehiry, F.; Brevik, E.C. Seasonal and Spatial Distribution of Soil Trace Elements around Kitchener Drain in the Northern Nile Delta, Egypt. Agriculture 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press/ Talyor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Galal, T.M.; Shedeed, Z.A.; Gharib, F.A.; Al-Yasi, H.M.; Mansour, K.H. The Role of Cyperus alopecuroides Rottb. Sedge in Monitoring Water Pollution in Contaminated Wetlands in Egypt: A Phytoremediation Approach. Environ. Sci. Pollut. Res. 2021, 28, 23005–23016. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Al-Sodany, Y.M.; Haroun, S.A.; Galal, T.M.; Ayed, H.; Khedher, K.M.; Jensen, K. Temporal Potential of Phragmites australis as a Phytoremediator to Remove Ni and Pb from Water and Sediment in Lake Burullus, Egypt. Bull. Environ. Contam. Toxicol. 2021, 106, 516–527. [Google Scholar] [PubMed]
- Bragato, C.; Brix, H.; Malagoli, M. Accumulation of Nutrients and Heavy Metals in Phragmites australis (Cav.) Trin. Ex Steudel and Bolboschoenus maritimus (L.) Palla in a Constructed Wetland of the Venice Lagoon Watershed. Environ. Pollut. 2006, 144, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Du Laing, G.; Van de Moortel, A.M.K.; Moors, W.; De Grauwe, P.; Meers, E.; Tack, F.M.G.; Verloo, M.G. Factors Affecting Metal Concentrations in Reed Plants (Phragmites australis) of Intertidal Marshes in the Scheldt Estuary. Ecol. Eng. 2009, 35, 310–318. [Google Scholar] [CrossRef]
- Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Performance of Selected Destruction Methods for the Determination of Heavy Metals in Reed Plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]
- Batool, R.; Hameed, M.; Ashraf, M.; Fatima, S.; Nawaz, T.; Ahmad, M.S.A. Structural and Functional Response to Metal Toxicity in Aquatic Cyperus alopecuroides Rottb. Limnologica 2014, 48, 46–56. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative Performance of Trace Element Bioaccumulation and Biomonitoring in the Plant Species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H. Monthly Variations of Trace Elements Accumulation and Distribution in Above-and below-Ground Biomass of Phragmites australis (Cav.) Trin. Ex Steudel in Lake Burullus (Egypt): A Biomonitoring Application. Ecol. Eng. 2014, 73, 17–25. [Google Scholar] [CrossRef]
- Agunbiade, F.O.; Olu-Owolabi, B.I.; Adebowale, K.O. Phytoremediation Potential of Eichhornia crassipes in Metal-Contaminated Coastal Water. Bioresour. Technol. 2009, 100, 4521–4526. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Santos, J.A.G. Three New Arsenic Hyperaccumulating Ferns. Sci. Total Environ. 2006, 364, 24–31. [Google Scholar] [CrossRef]
- Bonanno, G.; Borg, J.A.; Di Martino, V. Levels of Heavy Metals in Wetland and Marine Vascular Plants and Their Biomonitoring Potential: A Comparative Assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef]
- Weis, J.S.; Weis, P. Metal Uptake, Transport and Release by Wetland Plants: Implications for Phytoremediation and Restoration. Environ. Int. 2004, 30, 685–700. [Google Scholar] [CrossRef]
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A. Heavy Metals in Aquatic Plants and Sediments from Water Systems in Macedonia, Greece. Ecotoxicol. Environ. Saf. 1995, 32, 73–80. [Google Scholar] [CrossRef]
- Carrión, C.; Ponce-de León, C.; Cram, S.; Sommer, I.; Hernández, M.; Vanegas, C. Potential Use of Water Hyacinth (Eichhornia crassipes) in Xochimilco for Metal Phytoremediation. Agrociencia 2012, 46, 609–620. [Google Scholar]
- Baldantoni, D.; Ligrone, R.; Alfani, A. Macro-and Trace-Element Concentrations in Leaves and Roots of Phragmites australis in a Volcanic Lake in Southern Italy. J. Geochemical Explor. 2009, 101, 166–174. [Google Scholar] [CrossRef]
- Phusantisampan, T.; Meeinkuirt, W.; Saengwilai, P.; Pichtel, J.; Chaiyarat, R. Phytostabilization Potential of Two Ecotypes of Vetiveria zizanioides in Cadmium-Contaminated Soils: Greenhouse and Field Experiments. Environ. Sci. Pollut. Res. 2016, 23, 20027–20038. [Google Scholar] [CrossRef]
- Kamari, A.; Yusof, N.; Abdullah, H.; Haraguchi, A.; Abas, M.F. Assessment of Heavy Metals in Water, Sediment, Anabas testudineus and Eichhornia crassipes in a Former Mining Pond in Perak, Malaysia. Chem. Ecol. 2017, 33, 637–651. [Google Scholar] [CrossRef]
- Olivares-Rieumont, S.; Lima, L.; De la Rosa, D.; Graham, D.W.; Columbie, I.; Santana, J.L.; Sánchez, M.J. Water Hyacinths (Eichhornia crassipes) as Indicators of Heavy Metal Impact of a Large Landfill on the Almendares River near Havana, Cuba. Bull. Environ. Contam. Toxicol. 2007, 79, 583–587. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Almuqrin, A.H.; Aloraini, D.A.; Khedher, K.M.; Taher, M.A.; Alfarhan, A.H.; Picó, Y.; Barcelo, D. Uptake Prediction of Nine Heavy Metals by Eichhornia crassipes Grown in Irrigation Canals: A Biomonitoring Approach. Sci. Total Environ. 2021, 782, 146887. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C. Phytoremediation Efficiency of Eichhornia crassipes in Fly Ash Pond. Int. J. Phytoremediation 2016, 18, 450–452. [Google Scholar] [CrossRef]
- Saleh, H.M.; Aglan, R.F.; Mahmoud, H.H. Ludwigia stolonifera for Remediation of Toxic Metals from Simulated Wastewater. Chem. Ecol. 2019, 35, 164–178. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.; Urey, E.; Koca, F.D. Temporal Variation of Heavy Metal Accumulation and Translocation Characteristics of Narrow-Leaved Cattail (Typha angustifolia L.). Environ. Sci. Pollut. Res. 2015, 22, 17886–17896. [Google Scholar] [CrossRef] [PubMed]
- Klink, A. A Comparison of Trace Metal Bioaccumulation and Distribution in Typha latifolia and Phragmites australis: Implication for Phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef]
- Marchand, L.; Nsanganwimana, F.; Cook, B.J.; Vystavna, Y.; Huneau, F.; Le Coustumer, P.; Lamy, J.B.; Oustrière, N.; Mench, M. Trace Element Transfer from Soil to Leaves of Macrophytes along the Jalle d’Eysines River, France and Their Potential Use as Contamination Biomonitors. Ecol. Indic. 2014, 46, 425–437. [Google Scholar] [CrossRef]
- Deng, H.; Ye, Z.H.; Wong, M.H. Accumulation of Lead, Zinc, Copper and Cadmium by 12 Wetland Plant Species Thriving in Metal-Contaminated Sites in China. Environ. Pollut. 2004, 132, 29–40. [Google Scholar] [CrossRef]
- Teuchies, J.; Jacobs, S.; Oosterlee, L.; Bervoets, L.; Meire, P. Role of Plants in Metal Cycling in a Tidal Wetland: Implications for Phytoremidiation. Sci. Total Environ. 2013, 445, 146–154. [Google Scholar] [CrossRef]
- Qian, J.; Zayed, A.; Zhu, Y.; Yu, M.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: III. Uptake and Accumulation of Ten Trace Elements by Twelve Plant Species. J. Environ. Qual. 1999, 28, 1448–1455. [Google Scholar] [CrossRef]
| Heavy Metal | Plant Species | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.a. | E.s. | P.a. | R.s. | T.d. | E.c. | L.s. | ||||||||
| AG | BG | AG | BG | AG | BG | AG | BG | AG | BG | AG | BG | AG | BG | |
| Fe | 118.27 ab | 133.53 a | 1031.67 ab | 1298.23 ab | 1465.60 b | 2043.83 b | 71.96 a | 169.28 ab | 529.87 ab | 813.50 ab | 794.30 ab | 1826.83 ab | 120.49 a | 140.18 a |
| Cu | 6.34 a | 18.40 a | 36.90 ab | 66.67 ab | 24.18 ab | 83.00 ab | 23.69 ab | 67.73 ab | 35.90 ab | 59.50 ab | 78.47 b | 147.70 b | 12.55 ab | 36.16 ab |
| Zn | 29.73 a | 51.11 a | 189.33 ab | 237.40 ab | 86.40 ab | 178.77 ab | 18.30 a | 119.07 ab | 79.33 ab | 95.37 ab | 319.23 b | 553.27 b | 61.44 ab | 138.80 ab |
| Mn | 16.07 a | 30.04 a | 782.13 ab | 933.43 ab | 1126.67 ab | 1630.00 b | 18.00 ab | 50.58 ab | 591.07 ab | 930.68 ab | 1163.93 b | 1783.20 b | 94.48 ab | 153.03 ab |
| Co | 3.07 a | 6.50 a | 6.63 ab | 10.27 ab | 10.09 ab | 57.69 b | 5.71 ab | 10.79 ab | 13.00 ab | 34.50 ab | 21.17 b | 30.33 ab | 10.07 ab | 12.75 ab |
| Cd | 5.31 a | 9.57 ab | 13.27 ab | 24.93 ab | 36.20 ab | 137.30 b | 4.66 a | 5.23 a | 21.83 ab | 43.40 ab | 31.17 ab | 39.67 ab | 76.20 b | 47.97 ab |
| Ni | 4.54 a | 9.80 a | 26.35 ab | 85.00 ab | 28.50 ab | 149.40 b | 16.46 ab | 64.72 ab | 48.20 ab | 107.53 ab | 91.40 b | 113.50 ab | 69.00 ab | 94.13 ab |
| Pb | 28.50 ab | 36.87 a | 50.33 ab | 146.80 ab | 44.46 ab | 162.67 ab | 19.50 a | 35.45 a | 38.83 ab | 64.17 ab | 217.33 b | 344.00 b | 207.30 b | 209.87 ab |
| Variable | Heavy Metal | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Cu | Zn | Mn | Co | Cd | Ni | Pb | |
| Drain (D) | 239.25 *** | 1.65 ns | 3.29 ** | 8.63 ** | 0.03 ns | 7.46 ** | 1.20 ns | 112.20 *** |
| Species (S) | 20.18 ** | 30.15 ** | 45.60 ** | 12.10 *** | 5.90 ** | 22.50 *** | 7.50 *** | 8.23 ** |
| Tissue (T) | 1.50 ns | 85.10 *** | 10.90 *** | 5.80 *** | 1.45 ns | 8.45 *** | 23.10 ns | 2.80 *** |
| D × S | 5.40 *** | 3.50 *** | 5.30 *** | 84.12 ** | 5.20 *** | 22.40 *** | 10.15 *** | 11.00 ** |
| D × T | 7.50 ns | 0.85 ns | 10.50 *** | 5.40 *** | 1.50 ns | 3.20 ** | 0.75 ns | 9.12 ** |
| S × T | 5.30 *** | 3.12 ** | 10.90 *** | 2.50 *** | 10.09 ** | 5.50 *** | 7.30 *** | 4.10 ** |
| D × S × T | 15.30 *** | 3.50 ** | 4.20 ** | 8.10 *** | 5.20 *** | 3.80 ** | 9.10 ** | 5.50 *** |
| Heavy Metal | Plant Species | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.a. | E.s. | P.a. | R.s. | T.d. | E.c. | L.s. | ||||||||
| BF | TF | BF | TF | BF | TF | BF | TF | BF | TF | BF | TF | BF | TF | |
| Fe | 0.25 a | 0.89 a | 2.43 ab | 0.79 a | 3.83 b | 0.72 a | 0.32 a | 0.43 a | 1.52 ab | 0.65 a | 3.42 ab | 0.43 a | 0.26 a | 0.85 a |
| Cu | 0.07 a | 0.34 ab | 0.27 ab | 0.55 ab | 0.34 ab | 0.29 a | 0.28 ab | 0.35 ab | 0.24 ab | 0.60 b | 0.60 b | 0.53 ab | 0.15 ab | 0.35 ab |
| Zn | 0.16 a | 0.58 ab | 0.74 ab | 0.80 b | 0.56 ab | 0.48 ab | 0.37 ab | 0.15 a | 0.30 ab | 0.83 b | 1.72 b | 0.58 ab | 0.43 ab | 0.44 ab |
| Mn | 0.07 a | 0.53 ab | 2.29 ab | 0.84 b | 3.99 ab | 0.69 ab | 0.12 ab | 0.36 a | 2.28 ab | 0.64 ab | 4.37 b | 0.65 ab | 0.38 ab | 0.61 ab |
| Co | 0.62 a | 0.47 ab | 0.98 ab | 0.65 ab | 5.49 b | 0.17 a | 1.02 ab | 0.53 ab | 3.29 ab | 0.38 ab | 2.89 ab | 0.70 ab | 1.21 ab | 0.79 b |
| Cd | 0.40 ab | 0.55 ab | 1.05 ab | 0.53 ab | 5.78 b | 0.26 a | 0.22 a | 0.89 ab | 1.83 ab | 0.50 ab | 1.67 ab | 0.79 ab | 2.02 ab | 1.59 b |
| Ni | 0.37 a | 0.46 ab | 3.18 ab | 0.31 ab | 5.59 b | 0.19 a | 2.42 ab | 0.25 ab | 4.02 ab | 0.45 ab | 4.25 ab | 0.81 b | 3.52 ab | 0.73 ab |
| Pb | 0.65 a | 0.77 ab | 2.59 ab | 0.34 ab | 2.87 ab | 0.27 a | 0.62 a | 0.55 ab | 1.13 ab | 0.60 ab | 6.06 b | 0.63 ab | 3.69 ab | 0.99 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, M.; Mashaly, I.A.; Srour, D.S.; Dakhil, M.A.; El-Liethy, M.A.; El-Keblawy, A.; El-Barougy, R.F.; Halmy, M.W.A.; El-Sherbeny, G.A. Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt. Biology 2021, 10, 560. https://doi.org/10.3390/biology10060560
Abdelaal M, Mashaly IA, Srour DS, Dakhil MA, El-Liethy MA, El-Keblawy A, El-Barougy RF, Halmy MWA, El-Sherbeny GA. Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt. Biology. 2021; 10(6):560. https://doi.org/10.3390/biology10060560
Chicago/Turabian StyleAbdelaal, Mohamed, Ibrahim A. Mashaly, Dina S. Srour, Mohammed A. Dakhil, Mohamed Azab El-Liethy, Ali El-Keblawy, Reham F. El-Barougy, Marwa Waseem A. Halmy, and Ghada A. El-Sherbeny. 2021. "Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt" Biology 10, no. 6: 560. https://doi.org/10.3390/biology10060560
APA StyleAbdelaal, M., Mashaly, I. A., Srour, D. S., Dakhil, M. A., El-Liethy, M. A., El-Keblawy, A., El-Barougy, R. F., Halmy, M. W. A., & El-Sherbeny, G. A. (2021). Phytoremediation Perspectives of Seven Aquatic Macrophytes for Removal of Heavy Metals from Polluted Drains in the Nile Delta of Egypt. Biology, 10(6), 560. https://doi.org/10.3390/biology10060560









