1. Introduction
Human health is influenced by numerous factors, some under our control and some not. Global warming, aging of the population [
1], increased travel between the continents, poverty, the steady rise of multiple chronic diseases such as obesity [
2] and diabetes [
3] in the context of the recent epidemiological shift [
4] and the increasing usage of dexamethasone in the treatment of COVID-19 are all factors that determine the emergence of novel pathogens on the background of an altered host immune response.
Two examples of protozoa that have been viewed until recently as unable to infect humans are Colpoda steinii and Colpodella gonderi.
Colpoda steinii is a ciliate protozoan that was first discovered by Maupas in 1883 belonging to the family
Colpodidae, order
Colpodida, class
Colpodea, phylum
Ciliophora [
5]. Ciliates from the class
Colpodea are widespread around the globe and can be found in diverse habitats ranging from terrestrial/semiterrestrial (mosses, soil, bark of trees etc.) to aquatic environments (both fresh water—ponds, lakes, running waters and saltwater) [
6].
Colpoda steinii has a simple lifecycle between two morphologic forms: mobile trophozoites and cysts. The cysts provide resistance to the external environment for prolonged periods of time and have been found even in the Siberian permafrost [
7].
Colpoda steinii is considered to be non-pathogenic to humans but in spite of this fact several instances are cited in the literature that present cases of this parasite being present in urinary tract infections while no other microorganism was proven to be at the origin of the signs and symptoms exhibited by the patient [
8,
9,
10].
Colpodella gonderi (Foissner and Foissner, 1984) is a flagellated protozoan belonging to the order
Colpodellida (Cavalier-Smith, 1993) [
11]. Protozoa from the genus
Colpodella are free-living protozoa and the closest genetic relatives of Apicomplexans (phylum which includes important human pathogens such as
Plasmodium falciparum,
Cryptosporidium parvum,
Toxoplasma gondii).
Colpodella gonderi is considered a predatory flagellate and the literature cites multiple species of ciliates as being predated by this flagellate, including
Colpoda steinii [
12]. This protozoan is considered to be non-pathogenic to humans but two studies published in the last years cite different types of infection when no other proven pathogen was present except this parasite. The aforementioned studies describe an infection with similarities to
Babesia spp. infections and a tick-borne case in which the patient presented neurological symptoms [
13,
14].
To the best of our knowledge, no scientific accounts are present in the literature citing the presence of both Colpoda spp. and Colpodella spp. in any biological human samples.
2. Case Presentation
We report the case of an elderly female patient, aged 70, who was admitted to the pneumology hospital in Cluj-Napoca, Romania, with the main complaint of breathing difficulties, dyspnea with orthopnea that started a few hours before admission. Family history was not significant but the patient has a personal history of multiple chronic diseases including COPD Gold II, chronic respiratory insufficiency with oxygen therapy at home, chronic heart failure NYHA III, type 2 diabetes with insulin treatment and class 3 severe obesity (BMI = 44.1, Body mass index). The initial examinations in the ER (emergency room) revealed respiratory acidosis with hypoxemia and hypercapnia (pH 7.349, pCO2 56.2 mmHg, pO2 65.5 mmHg and SO2 82%), normal blood pressure and heart rate (Blood pressure = 120/80 mmHg, heart rate = 84 bpm). Multiple other examinations were performed including pulmonary X-ray that showed no significant radiologic abnormalities. The initial bloodwork showed only an abnormal level of blood glucose with values up to 450 mg/dL. The ER diagnostic was acute exacerbation of COPD with respiratory insufficiency and after stabilization the patient was transferred to the pneumology department.
The patient was hospitalized in the pneumology department for a total duration of 16 days, during which her condition steadily improved. Multiple routine tests were performed during her hospital stay, ranging from spirometry to bloodwork and urine tests.
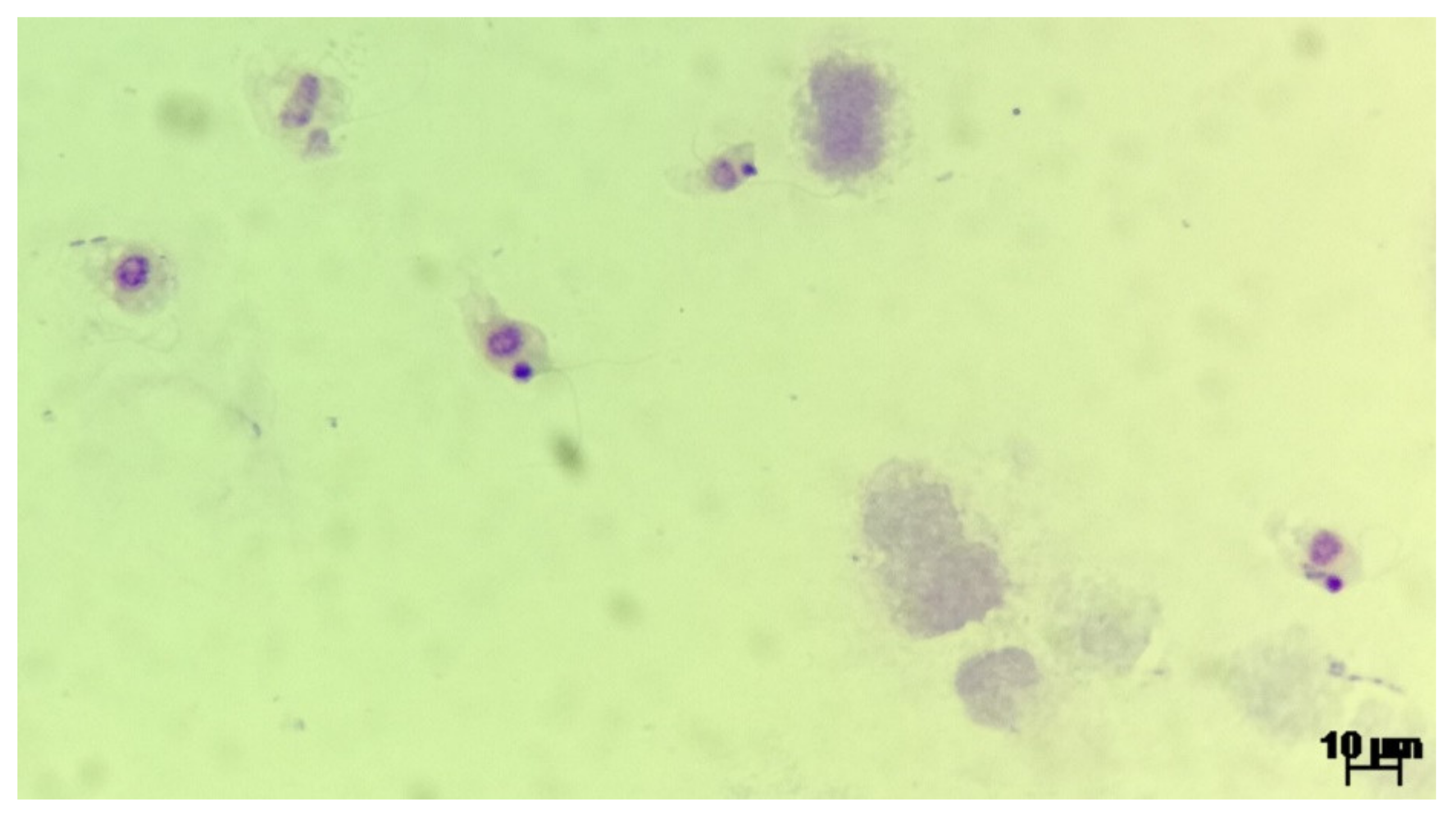
Microscopic analysis of the urine sediment revealed the following results: 25–30 leukocytes/microscopic field, 8–10 squamous epithelial cells/field, rare bacterial flora and mobile parasitic trophozoites. The urine culture was negative.
Upon urine examination we managed to ascertain the presence of two distinct parasite genera, based on morphology and movement type. We identified trophozoites from the
Colpoda genus, most probably
Colpoda steinii according to the specific ciliate type mobility and following morphological characteristics: size ~40 × 20 μm (length and width), kidney shaped, covered with short cilia, cystotome cleft at about one-third of the distance from the anterior end, contractile vacuole at the posterior extremity, ovoid macronucleus and multiple food vacuoles. The morphology of one observed
Colpoda spp. trophozoites is presented in
Figure 1. The second identified parasite was a protozoan of the
Colpodella genus, most probably
Colpodella gonderi, with specific flagellate like movement patterns and the following morphological characteristics: size ~15 × 10 μm (length and width), egg shaped with an anterior pointed end, spherical nucleus localized relatively in the middle of the body, spherical inclusion at the end of the body, two flagella of an approximate length of 1.5–2 the size of the body. The morphology of multiple
Colpodella spp. trophozoites is presented in
Figure 2 and the movement patterns are presented in
Supplementary Video S1. We measured the intensity of the infection by counting the number of observed trophozoites in multiple microscopic fields, using 40× objective. In the case of
Colpodella spp. we observed ~20 trophozoites/field, as seen in
Supplementary Video S1.
Colpoda spp. trophozoites were observed in much smaller numbers.
Noteworthy is the fact that our patient did not have any other urinary pathology, nor did she exhibit any urinary symptoms before or during the hospital stay.
We managed to obtain multiple urine samples during the hospitalization of our patient and observed the following chronological evolution of urinary parasitic contamination. In the first sample we managed to ascertain the existence of 2 distinct parasite trophozoites, namely Colpoda spp. and Colpodella spp., based on the specific morphology and movement patterns. In the next samples collected in the following days, only one protozoal species could be found in the urine—Colpodella spp. Upon discharge from the hospital, we managed to obtain one final urine sample in which no parasite trophozoites could be found. We did not observe parasitic cysts in any of the analyzed samples.
The treatment of our patient focused mainly on the management of her chronic diseases and included both a pharmacological approach and non-invasive oxygen-therapy. Our patient was treated with 2 antibiotics during her stay, ceftriaxone and metronidazole, with different treatment durations. Ceftriaxone was administered for the duration of hospitalization, in the dosage 1 g/24 h, for the prophylaxis of potential bacterial infections. In addition, metronidazole was administered for 5 days, 250 mg every 12 h. The treatment with metronidazole was started as soon as the first urine analysis was interpreted and the parasites observed. The subsequent urine sediment examinations showed the disappearance of Colpoda spp. firstly, and upon discharge the disappearance of Colpodella spp.
3. Discussion
Several limitations were encountered in regards to the diagnostic workflow. Firstly, we were unable to perform a PCR assay for the identification of the observed protozoa. Lacking a nucleic acid identification assay, we had to rely solely on the morphological identification on wet mounts and Giemsa-stained smears. Secondly, we did not screen our patient for the intestinal or genital presence of this protozoa in order to exclude a contamination from this body sites. Thirdly, we did not screen other patients proactively for the presence of these parasites, but in the normal workflow of biological samples no other cases having these parasites were identified before, during or after the timeframe of hospitalization of our patient.
Before admitting that our patient had a rare urinary parasitic infection, we tried to rule out all other possibilities, the main one being the accidental contamination of the sample with the parasites. During this timeframe, urine sediment samples from other patients hospitalized in our clinic were analyzed according to the same protocol and no other sample tested positive for the presence of parasites. Urine samples were collected according to aseptic standards, in single use sterile plastic containers. We did not manage to screen our patient for the intestinal or genital presence of these parasites. The presence of parasite trophozoites in our patient’s urine was proven on multiple days, in different urine samples, with a decreasing number as expected in a patient treated with metronidazole. The last urine sample that was analyzed before discharge from the hospital tested negative for parasite trophozoites, presence of leukocytes or bacteria. During the course of this case, no concomitant bacterial urinary infection was observed in our patient, in addition to the protozoa.
Compared to the work of Costache et al. [
10], we were unable to witness the process of encystation or excystation, nor did we see any formed
Colpoda spp. cysts. The most plausible explanation for this fact could be related to the mistiming of the examinations with the life cycle of the parasite. Even though unlikely, two other explanations exist as well. Firstly, the combined treatment with ceftriaxone and metronidazole might have a better effect on
Colpoda spp. compared to
Colpodella spp., and thus, explaining the fact that we only managed to observe the ciliate in the first examination and not in the following days after starting the treatment. Secondly, although highly unlikely, a predation of
Colpoda spp. by
Colpodella spp. might have occurred, thus rendering the observation of ciliates impossible on further examinations. An argument for this hypothesis comes from the discrepancy in the numbers of the two parasites: we observed only a few
Colpoda spp. trophozoites as compared to increased numbers of flagellates.
The novelty of our case comes from the observation of an associated urinary infection with two different protozoa, a ciliate and a flagellate and from the fact that there are no other cited cases in the literature in which
Colpodella spp. was found in the urinary tract [
13,
14].
The source of contamination in the case of our patient can only be speculated upon. These parasites are often found in the environment and can easily contaminate any water body though dust. It is not inconceivable that our patient was contaminated by using rain water instead of tap water for bathing or simply by bathing with water contaminated by dust though an open window. While we managed to get thorough information about our patient’s medical history, relevant details about her living conditions and habits were left out.
The main differential diagnosis that we performed was to exclude the following pathogens:
Trichomonas vaginalis (one of the most frequently recovered flagellates from urine samples) [
15] and
Balantidium coli (considered to be the only ciliate pathogenic to humans). No other parasites were found in the urine. Based on morphological characteristics and examination of the movement pattern, we excluded all the aforementioned pathogens.
Regarding the treatment, the evidence seems to suggest the concomitant treatment regimen with ceftriaxone and metronidazole to be effective against urinary contamination with Colpoda spp. and Colpodella spp.
We are aware of the coincidence of presenting a similarly rare case of urinary infection as previous work from Costache et al. [
10] in Cluj-Napoca, Romania. This occurrence might suggest a common local link that requires further investigation in upcoming studies.
Given the rarity of cited cases in the literature, the presence of the second case in the same geographic location may sound weird. However, a possible explanation of this situation might be the increased reliance on microscopical examinations for urine and stool samples in Romania, as opposed to other European countries. Wide spread access to automated diagnostic systems may come with the downside of missing rare microorganisms present in different samples. Furthermore, the lack of accessible molecular diagnosis in some countries can only add to the potential confusion. Therefore, an increased probability of misdiagnosis or lack of identification of rare pathogens can be seen across the literature.
Further studies are required to assess the frequency of this kind of infections, to assess the risk factors, to determine the route of infection, to clarify the physiopathology and to propose standardized treatment regimens for them.
4. Review of Literature
We performed a review of the medical literature regarding the isolation of
Colpoda spp. and
Colpodella spp. from humans, searching on PubMed and Cochrane library electronic database, up to 15 March 2021. The results are summarized in
Table 1. We considered the following terms included in the studies title or abstract: “colpoda”, “colpodella” combined with the Boolean operator” AND” along with” human”,” urine”,” blood” and ”diagnostic”. We excluded studies written in languages other than English and French. We found a selection of five studies, four case reports and one original article that we included in our review.
Regarding
Colpodella spp., we found two case reports both from China and both patients were females in their fifties. Yuan C et al. reported a case of
Colpodella spp. recovered from the blood of a patient with hemolytic anemia and immunosuppression due to solitary natural killer cell deficiency who responded well to the treatment with atovaquone and azithromycin for 8 weeks [
13]. Jiang J et al. reported a case where the parasite was found in the cerebrospinal fluid of a patient with neurologic symptoms who was treated with doxycycline [
14].
Since there are just two case reports of this parasite isolated from humans it is difficult to draw a conclusion in terms of pathological involvement and treatment. However, regarding the diagnostic, in both cases researchers used 18S rRNA PCR for molecular diagnosis. Unfortunately, molecular diagnostic techniques are still not available at large scale so we want to emphasize the importance of morphologic diagnosis of
Colpodella spp. The most important morphologic features are presented in
Table 2.
Regarding
Colpoda spp., we found 3 articles, two case reports and one research article. The case reported by Costache C et al. from Romania [
10] and the case reported by Guy Y et al. from Algeria [
8] are describing the parasite
Colpoda spp. isolated from urine and identified based on morphologic characteristics. On the other hand, Bouchoucha I et al. [
16] performed an analysis of several contact lens solutions, recovered from patients with the clinical diagnosis of keratitis and corneal ulcer and discovered the presence of the parasite
Colpoda steinii in one specimen of contact lens solution. In that study, the diagnostic of the ciliated protozoan was performed using 18S rRNA PCR. However, this study shows a second source of potential interaction between humans and the rare ciliate,
Colpoda spp.
Even though Colpoda spp. is not considered a human pathogen, in all the case reports from the literature that we included in the review, this ciliate parasite was isolated from the urine of the patients. Further research is required to establish if this parasite is able to survive in the urinary bladder of healthy individuals or just in that of immunosuppressed ones.
There is an established link in the literature concerning immunosuppression and the increased incidence of bacterial or protozoan infections. Including the case presented by us, in four out of the six case reports included in our review the patients presented a form of immunosuppression. The presented evidence points to the existence of a plausible link between immunosuppression and infections caused by unusual pathogens.
In the case of rare parasites, even if molecular diagnosis represents the most accurate tool, optical microscopy diagnosis based on morphologic criteria should not be neglected.