Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation
Abstract
Simple Summary
Abstract
1. Introduction
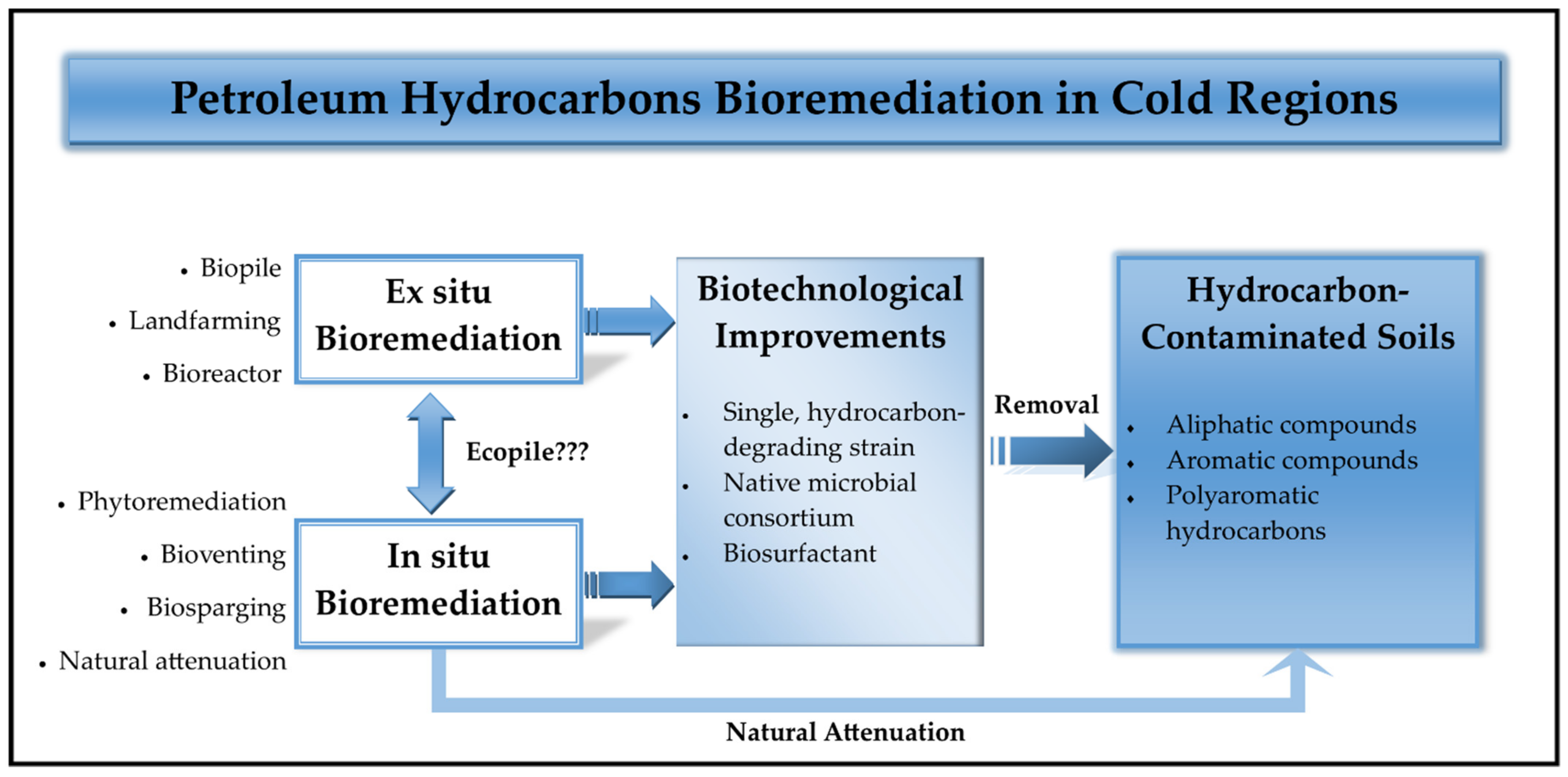
2. Petroleum Oil Bioremediation in Cold Regions
2.1. Hydrocarbons Bioremediation
2.1.1. Biostimulation
2.1.2. Bioaugmentation
2.2. Biodegradation Pathway and Its Metabolic Aspects
2.3. Bioremediation Research Trend in Cold Regions
3. Enhanced Bioremediation Studies in Cold Regions
3.1. In Situ Applications
3.1.1. Phytoremediation
3.1.2. Bioventing and Biosparging
3.2. Ex Situ Implementations
3.2.1. Biopile
3.2.2. Landfarming
3.2.3. Bioreactor
4. Other Potential Applications
4.1. Genetic Engineering
4.2. Enzyme Engineering
4.3. Immobilisation Tools
4.4. Microbial Biosurfactant
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keith, L.H. The source of US EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Core flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo-and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016, 220, 175–182. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, J. New insights into bioremediation strategies for oil-contaminated soil in cold environments. Int. Biodeterior. Biodegrad. 2019, 142, 58–72. [Google Scholar] [CrossRef]
- Cerro-Gálvez, E.; Casal, P.; Lundin, D.; Piña, B.; Pinhassi, J.; Dachs, J.; Vila-Costa, M. Microbial responses to anthropogenic dissolved organic carbon in the Arctic and Antarctic coastal seawaters. Environ. Microbiol. 2019, 21, 1466–1481. [Google Scholar] [CrossRef]
- Garneau, M.-È.; Michel, C.; Meisterhans, G.; Fortin, N.; King, T.L.; Greer, C.W.; Lee, K. Hydrocarbon biodegradation by Arctic sea-ice and sub-ice microbial communities during microcosm experiments, Northwest Passage (Nunavut, Canada). FEMS Microbiol. Ecol. 2016, 92, fiw130. [Google Scholar] [CrossRef]
- Gran-Scheuch, A.; Fuentes, E.; Bravo, D.M.; Jiménez, J.C.; Pérez-Donoso, J.M. Isolation and characterization of phenanthrene degrading bacteria from diesel fuel-contaminated Antarctic soils. Front. Microbiol. 2017, 8, 1634. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Malavenda, R.; Gerçe, B.; Papale, M.; Syldatk, C.; Hausmann, R.; Bruni, V.; Michaud, L.; Lo Giudice, A.; Amalfitano, S. Effects of a simulated acute oil spillage on bacterial communities from Arctic and Antarctic marine sediments. Microorganisms 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed]
- Filler, D.M.; Snape, I.; Barnes, D.L. Bioremediation of Petroleum Hydrocarbons in Cold Regions; Cambridge University Press: New York, NY, USA, 2008; ISBN 1-139-47004-3. [Google Scholar]
- Naseri, M.; Barabadi, A.; Barabady, J. Bioremediation treatment of hydrocarbon-contaminated Arctic soils: Influencing parameters. Environ. Sci. Pollut. Res. 2014, 21, 11250–11265. [Google Scholar] [CrossRef]
- Ferrarese, E.; Andreottola, G.; Oprea, I.A. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008, 152, 128–139. [Google Scholar] [CrossRef]
- Gomes, H.I.; Dias-Ferreira, C.; Ribeiro, A.B. Electrokinetic remediation of organochlorines in soil: Enhancement techniques and integration with other remediation technologies. Chemosphere 2012, 87, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumford, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Filler, D.M.; Van Stempvoort, D.R.; Leigh, M.B. Remediation of frozen ground contaminated with petroleum hydrocarbons: Feasibility and limits. In Permafrost Soils; Springer: Berlin, Germany, 2009; Volume 16, pp. 279–301. ISBN 978-3-540-69371-0. [Google Scholar]
- Soleimani, M.; Jaberi, N. Comparison of biological and thermal remediation methods in decontamination of oil polluted soils. J. Bioremed. Biodeg. 2014, 5, 1000e145. [Google Scholar] [CrossRef]
- Álvarez, L.M.; Ruberto, L.A.M.; Balbo, A.L.; Mac Cormack, W.P. Bioremediation of hydrocarbon-contaminated soils in cold regions: Development of a pre-optimized biostimulation biopile-scale field assay in Antarctica. Sci. Total Environ. 2017, 590, 194–203. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques–classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, B.; Chen, B.; Zhu, Z.; Lin, W.; Cao, T. Screening of biosurfactant producers from petroleum hydrocarbon contaminated sources in cold marine environments. Mar. Pollut. Bull. 2014, 86, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Simpanen, S.; Dahl, M.; Gerlach, M.; Mikkonen, A.; Malk, V.; Mikola, J.; Romantschuk, M. Biostimulation proved to be the most efficient method in the comparison of in situ soil remediation treatments after a simulated oil spill accident. Environ. Sci. Pollut. Res. 2016, 23, 25024–25038. [Google Scholar] [CrossRef] [PubMed]
- Stallwood, B.; Shears, J.; Williams, P.A.; Hughes, K.A. Low temperature bioremediation of oil-contaminated soil using biostimulation and bioaugmentation with a Pseudomonas sp. from maritime Antarctica. J. Appl. Microbiol. 2005, 99, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Huijer, K. Trends in oil spills from tanker ships 1995–2004. Int. Tanker Own. Pollut. Fed. (ITOPF) 2005, 30, 1–14. [Google Scholar]
- Knol, M.; Arbo, P. Oil spill response in the Arctic: Norwegian experiences and future perspectives. Mar. Policy 2014, 50, 171–177. [Google Scholar] [CrossRef]
- Poland, J.S.; Riddle, M.J.; Zeeb, B.A. Contaminants in the Arctic and the Antarctic: A comparison of sources, impacts, and remediation options. Polar Rec. 2003, 39, 369–383. [Google Scholar] [CrossRef]
- Miri, S.; Naghdi, M.; Rouissi, T.; Kaur Brar, S.; Martel, R. Recent biotechnological advances in petroleum hydrocarbons degradation under cold climate conditions: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 553–586. [Google Scholar] [CrossRef]
- Frolov, D. Peculiarities of weather and ground freezing conditions in Siberia and Russian Arctic in winter and spring period of 2019/2020. J. Phys. Conf. Ser. 2020, 1614, 012078. [Google Scholar] [CrossRef]
- Margesin, R.; Moertelmaier, C.; Mair, J. Low-temperature biodegradation of petroleum hydrocarbons (n-alkanes, phenol, anthracene, pyrene) by four actinobacterial strains. Int. Biodeterior. Biodegrad. 2013, 84, 185–191. [Google Scholar] [CrossRef]
- Fernández, P.M.; Martorell, M.M.; Blaser, M.G.; Ruberto, L.A.M.; de Figueroa, L.I.C.; Mac Cormack, W.P. Phenol degradation and heavy metal tolerance of Antarctic yeasts. Extremophiles 2017, 21, 445–457. [Google Scholar] [CrossRef]
- Subramaniam, K.; Shaharuddin, N.A.; Tengku-Mazuki, T.A.; Zulkharnain, A.; Khalil, K.A.; Convey, P.; Ahmad, S.A. Statistical optimisation for enhancement of phenol biodegradation by the Antarctic soil bacterium Arthrobacter sp. strain AQ5-15 using response surface methodology. J. Environ. Biol. 2020, 41, 1560–1569. [Google Scholar] [CrossRef]
- Tengku-Mazuki, T.A.; Subramaniam, K.; Zakaria, N.N.; Convey, P.; Khalil, K.A.; Lee, G.L.Y.; Zulkharnain, A.; Shaharuddin, N.A.; Ahmad, S.A. Optimization of phenol degradation by Antarctic bacterium Rhodococcus sp. Antarct. Sci. 2020, 32, 486–495. [Google Scholar] [CrossRef]
- Sepehr, S.; Shahnavaz, B.; Asoodeh, A.; Karrabi, M. Biodegradation of phenol by cold-tolerant bacteria isolated from alpine soils of Binaloud Mountains in Iran. J. Environ. Sci. Health A 2019, 54, 367–379. [Google Scholar] [CrossRef]
- McCarthy, K.; Walker, L.; Vigoren, L.; Bartel, J. Remediation of spilled petroleum hydrocarbons by in situ landfarming at an Arctic site. Cold Reg. Sci. Technol. 2004, 40, 31–39. [Google Scholar] [CrossRef]
- Hesham, A.E.-L.; Mawad, A.M.; Mostafa, Y.M.; Shoreit, A. Biodegradation ability and catabolic genes of petroleum-degrading Sphingomonas koreensis strain ASU-06 isolated from Egyptian oily soil. Biomed. Res. Int. 2014, 2014, 127674. [Google Scholar] [CrossRef] [PubMed]
- Gerginova, M.G.; Peneva, N.M.; Krumova, E.T.; Alexieva, Z.A. Biodegradation ability of fungal strains isolated from Antarctic towards PAH. In Proceedings of the 13th International Conference of Environmental Science and Technology, Athens, Greece, 5–7 September 2013; pp. 5–7. [Google Scholar]
- Ward, E.J.; Adkison, M.; Couture, J.; Dressel, S.C.; Litzow, M.A.; Moffitt, S.; Hoem Neher, T.; Trochta, J.; Brenner, R. Evaluating signals of oil spill impacts, climate, and species interactions in Pacific herring and Pacific salmon populations in Prince William Sound and Copper River, Alaska. PLoS ONE 2017, 12, e0172898. [Google Scholar] [CrossRef]
- Si-Zhong, Y.; Hui-Jun, J.; Zhi, W.; Rui-Xia, H.E.; Yan-Jun, J.I.; Xiu-Mei, L.I.; Shao-Peng, Y.U. Bioremediation of oil spills in cold environments: A Review. Pedosphere 2009, 19, 371–381. [Google Scholar]
- Bragg, J.R.; Prince, R.C.; Harner, E.J.; Atlas, R.M. Effectiveness of bioremediation for the Exxon Valdez oil spill. Nature 1994, 368, 413–418. [Google Scholar] [CrossRef]
- Anjum, R.; Rahman, M.; Masood, F.; Malik, A. Bioremediation of pesticides from soil and wastewater. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 295–328. ISBN 978-94-007-1591-2. [Google Scholar]
- Du, W.; Wan, Y.; Zhong, N.; Fei, J.; Zhang, Z.; Chen, L.; Hao, J. Status quo of soil petroleum contamination and evolution of bioremediation. Pet. Sci. 2011, 8, 502–514. [Google Scholar] [CrossRef]
- Aislabie, J.; Saul, D.J.; Foght, J.M. Bioremediation of hydrocarbon-contaminated polar soils. Extremophiles 2006, 10, 171–179. [Google Scholar] [CrossRef]
- Giblin, A.E.; Laundre, J.A.; Nadelhoffer, K.J.; Shaver, G.R. Measuring nutrient availability in Arctic soils using ion exchange resins: A field test. Soil Sci. Soc. Am. J. 1994, 58, 1154–1162. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmention: A review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar]
- Kim, J.; Lee, A.H.; Chang, W. Enhanced bioremediation of nutrient-amended, petroleum hydrocarbon-contaminated soils over a cold-climate winter: The rate and extent of hydrocarbon biodegradation and microbial response in a pilot-scale biopile subjected to natural seasonal freeze-thaw temperatures. Sci. Total Environ. 2018, 612, 903–913. [Google Scholar]
- Jeong, S.-W.; Jeong, J.; Kim, J. Simple surface foam application enhances bioremediation of oil-contaminated soil in cold conditions. J. Hazard. Mater. 2015, 286, 164–170. [Google Scholar] [CrossRef]
- Marchand, C.; Mench, M.; Jani, Y.; Kaczala, F.; Notini, P.; Hijri, M.; Hogland, W. Pilot scale aided-phytoremediation of a co-contaminated soil. Sci. Total Environ. 2018, 618, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Sartaj, M. Optimization of field scale biopiles for bioremediation of petroleum hydrocarbon contaminated soil at low temperature conditions by response surface methodology (RSM). Int. Biodeterior. Biodegrad. 2014, 89, 103–109. [Google Scholar] [CrossRef]
- Chang, W.; Dyen, M.; Spagnuolo, L.; Simon, P.; Whyte, L.; Ghoshal, S. Biodegradation of semi-and non-volatile petroleum hydrocarbons in aged, contaminated soils from a sub-Arctic site: Laboratory pilot-scale experiments at site temperatures. Chemosphere 2010, 80, 319–326. [Google Scholar] [CrossRef]
- Rayner, J.L.; Snape, I.; Walworth, J.L.; Harvey, P.M.; Ferguson, S.H. Petroleum–hydrocarbon contamination and remediation by microbioventing at sub-Antarctic Macquarie Island. Cold Reg. Sci. Technol. 2007, 48, 139–153. [Google Scholar] [CrossRef]
- Paudyn, K.; Rutter, A.; Rowe, R.K.; Poland, J.S. Remediation of hydrocarbon contaminated soils in the Canadian Arctic by landfarming. Cold Reg. Sci. Technol. 2008, 53, 102–114. [Google Scholar] [CrossRef]
- Whelan, M.J.; Coulon, F.; Hince, G.; Rayner, J.; McWatters, R.; Spedding, T.; Snape, I. Fate and transport of petroleum hydrocarbons in engineered biopiles in polar regions. Chemosphere 2015, 131, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.M.; Ruberto, L.A.M.; Gurevich, J.M.; Mac Cormack, W.P. Environmental factors affecting reproducibility of bioremediation field assays in Antarctica. Cold Reg. Sci. Technol. 2020, 169, 102915. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Biological decontamination of oil spills in cold environments. J. Chem. Technol. Biotechnol. 1999, 74, 381–389. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zakaria, N.N.; Roslee, A.F.A.; Shukor, M.Y.; Zulkharnain, A.; Napis, S.; Convey, P.; Alias, S.A.; Gonzalez-Rocha, G.; Ahmad, S.A. Biodegradation of diesel oil by cold-adapted bacterial strains of Arthrobacter spp. from Antarctica. Antarct. Sci. 2020, 32, 341–353. [Google Scholar] [CrossRef]
- VanderZwaag, D.; Huebert, R.; Ferrara, S. The Artic environmental protection strategy, Artic council and multilateral environmental initiatives: Tinkering while the Artic marine environmental totter’s. Denv. J. Int’l L. Pol’y 2001, 30, 131. [Google Scholar]
- Hoover, R.B.; Pikuta, E.V. Psychrophilic and psychrotolerant microbial extremophiles in polar environments. In Polar Microbiology: The Ecology, Biodiversity and Bioremediation Potential of Microorganisms in Extremely Cold Environments; CRC Press: Boca Raton, FL, USA, 2009; pp. 115–156. ISBN 9780367384593. [Google Scholar]
- Roslee, A.F.A.; Zakaria, N.N.; Convey, P.; Zulkharnain, A.; Lee, G.L.Y.; Gomez-Fuentes, C.; Ahmad, S.A. Statistical optimisation of growth conditions and diesel degradation by the Antarctic bacterium, Rhodococcus sp. strain AQ5‒07. Extremophiles 2020, 24, 277–291. [Google Scholar] [CrossRef]
- Lee, G.L.Y.; Ahmad, S.A.; Yasid, N.A.; Zulkharnain, A.; Convey, P.; Johari, W.L.W.; Alias, S.A.; Gonzalez-Rocha, G.; Shukor, M.Y. Biodegradation of phenol by cold-adapted bacteria from Antarctic soils. Polar Biol. 2018, 41, 553–562. [Google Scholar] [CrossRef]
- Lin, X.; Yang, B.; Shen, J.; Du, N. Biodegradation of crude oil by an Arctic psychrotrophic bacterium Pseudoalteromomas sp. P29. Curr. Microbiol. 2009, 59, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Akbari, A.; David, C.A.; Ghoshal, S. Selective biostimulation of cold-and salt-tolerant hydrocarbon-degrading Dietzia maris in petroleum-contaminated sub-Arctic soils with high salinity. J. Chem. Technol. Biotechnol. 2018, 93, 294–304. [Google Scholar] [CrossRef]
- Crisafi, F.; Giuliano, L.; Yakimov, M.M.; Azzaro, M.; Denaro, R. Isolation and degradation potential of a cold-adapted oil/PAH-degrading marine bacterial consortium from Kongsfjorden (Arctic region). Rend Lincei 2016, 27, 261–270. [Google Scholar] [CrossRef]
- Bell, T.H.; Yergeau, E.; Maynard, C.; Juck, D.; Whyte, L.G.; Greer, C.W. Predictable bacterial composition and hydrocarbon degradation in Arctic soils following diesel and nutrient disturbance. ISME J. 2013, 7, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Brakstad, O.G.; Lofthus, S.; Ribicic, D.; Netzer, R. Biodegradation of petroleum oil in cold marine environments. In Psychrophiles: From Biodiversity to Biotechnology; Springer: Berlin, Germany, 2017; pp. 613–644. ISBN 978-3-540-74335-4. [Google Scholar]
- Li, H.; Li, X.; Yu, T.; Wang, F.; Qu, C. Study on extreme microbial degradation of petroleum hydrocarbons. IOP Conf. Ser. Mater. Sci. Eng. 2019, 484, 012040. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Binupriya, A.R.; Baik, S.-H.; Yun, S.-E. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. CLEAN–Soil Air Water 2008, 36, 92–96. [Google Scholar] [CrossRef]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef]
- Ruberto, L.; Vazquez, S.C.; Dias, R.L.; Hernández, E.A.; Coria, S.H.; Levin, G.; Balbo, A.L.; Mac Cormack, W.P. Small-scale studies towards a rational use of bioaugmentation in an Antarctic hydrocarbon-contaminated soil. Antarct. Sci. 2010, 22, 463. [Google Scholar] [CrossRef]
- Abbasian, F.; Lockington, R.; Mallavarapu, M.; Naidu, R. A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl. BioChem. Biotechnol. 2015, 176, 670–699. [Google Scholar] [CrossRef]
- Aislabie, J.; Foght, J. Hydrocarbon-Degrading Bacteria in Contaminated Soils; Cambridge University Press: Cambridge, UK, 2008; pp. 69–83. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Liao, H.; Tang, M.; Luo, L.; Li, C.; Chiclana, F.; Zeng, X.-J. A bibliometric analysis and visualization of medical big data research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Bazm, S.; Kalantar, S.M.; Mirzaei, M. Bibliometric mapping and clustering analysis of Iranian papers on reproductive medicine in Scopus database (2010-2014). Int. J. Reprod. Biomed. 2016, 14, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Lee, H.; Chen, Y.-H.; Chae, Y. Bibliometric analysis of research assessing the use of acupuncture for pain treatment over the past 20 years. J. Pain Res. 2020, 13, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; An, H.; Wang, Y.; Huang, J.; Gao, X. Evolutionary features of academic articles co-keyword network and keywords co-occurrence network: Based on two-mode affiliation network. Physica A Stat. Mech. Its Appl. 2016, 450, 657–669. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual. Universiteit Leiden, CWTS Meaningful Metrics. Available online: https://www.vosviewer.com/getting-started (accessed on 8 January 2021).
- Gu, D.; Li, J.; Li, X.; Liang, C. Visualizing the knowledge structure and evolution of big data research in healthcare informatics. Int. J. Med. Inform. 2017, 98, 22–32. [Google Scholar] [CrossRef]
- Leewis, M.-C.; Reynolds, C.M.; Leigh, M.B. Long-term effects of nutrient addition and phytoremediation on diesel and crude oil contaminated soils in subarctic Alaska. Cold Reg. Sci. Technol. 2013, 96, 129–137. [Google Scholar] [CrossRef]
- Palmroth, M.R.; Pichtel, J.; Puhakka, J.A. Phytoremediation of subarctic soil contaminated with diesel fuel. Bioresour. Technol. 2002, 84, 221–228. [Google Scholar] [CrossRef]
- King, M.M.; Kinner, N.E.; Deming, D.P.; Simonton, J.A.; Belden, L.M. Bioventing of no. 2 fuel oil: Effects of air flowrate, temperature, nutrient amendment, and acclimation. Bioremediat. J. 2014, 24, 47–60. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, S.; Hu, L.; Wang, Y.; Yao, L.; Liu, Y.; Yuan, Z. Removal efficiency and the mineralization mechanism during enhanced bioventing remediation of oil-contaminated soils. Pol. J. Environ. Stud. 2016, 25, 1955–1963. [Google Scholar] [CrossRef]
- Germaine, K.J.; Byrne, J.; Liu, X.; Keohane, J.; Culhane, J.; Lally, R.D.; Kiwanuka, S.; Ryan, D.; Dowling, D.N. Ecopiling: A combined phytoremediation and passive biopiling system for remediating hydrocarbon impacted soils at field scale. Front. Plant. Sci. 2015, 5, 756. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; Spada, V.; Sciarrillo, R. Assessment of three approaches of bioremediation (natural attenuation, landfarming and bioaugmentation–assisted landfarming) for a petroleum hydrocarbons contaminated soil. Chemosphere 2017, 170, 10–16. [Google Scholar] [CrossRef]
- Robles-González, I.V.; Fava, F.; Poggi-Varaldo, H.M. A review on slurry bioreactors for bioremediation of soils and sediments. Microb. Cell Fact. 2008, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Zangi-Kotler, M.; Ben-Dov, E.; Tiehm, A.; Kushmaro, A. Microbial community structure and dynamics in a membrane bioreactor supplemented with the flame retardant dibromoneopentyl glycol. Environ. Sci. Pollut. Res. 2015, 22, 17615–17624. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Approaches for remediation of sites contaminated with total petroleum hydrocarbons. In Total Petroleum Hydrocarbons; Springer International Publishing: Cham, Switzerland, 2020; pp. 167–205. [Google Scholar]
- Tekere, M. Microbial Bioremediation and Different Bioreactors Designs Applied. In Biotechnology and Bioengineering; Jacob-Lopes, E., Zepka, L.Q., Eds.; IntechOpen: Rijeka, Croatia, 2019; pp. 1–19. ISBN 978-1-83962-661-6. [Google Scholar]
- Kumar, R.; Yadav, P. Novel and cost-effective technologies for hydrocarbon bioremediation. In Microbial Action on Hydrocarbons; Springer: Singapore, 2018; pp. 543–565. ISBN 978-981-13-1840-5. [Google Scholar]
- Bramley-Alves, J.; Wasley, J.; King, C.K.; Powell, S.; Robinson, S.A. Phytoremediation of hydrocarbon contaminants in subantarctic soils: An effective management option. J. Environ. Manag. 2014, 142, 60–69. [Google Scholar] [CrossRef]
- Lim, M.W.; Von Lau, E.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil—present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess. Eng 2013, 18, 431–439. [Google Scholar] [CrossRef]
- Kumar, V.; Shahi, S.K.; Singh, S. Bioremediation: An eco-sustainable approach for restoration of contaminated sites. In Microbial Bioprospecting for Sustainable Development; Springer: Singapore, 2018; pp. 115–136. ISBN 978-981-13-0053-0. [Google Scholar]
- Dubchak, S.; Bondar, O. Bioremediation and phytoremediation: Best approach for rehabilitation of soils for future use. In Remediation Measures for Radioactively Contaminated Areas; Springer International Publishing: Cham, Switzerland, 2019; pp. 201–221. ISBN 978-3-319-73398-2. [Google Scholar]
- Ferrera-Rodríguez, O.; Greer, C.W.; Juck, D.; Consaul, L.L.; Martínez-Romero, E.; Whyte, L.G. Hydrocarbon-degrading potential of microbial communities from Arctic plants. J. Appl. Microbiol. 2013, 114, 71–83. [Google Scholar] [CrossRef]
- Mair, J.; Schinner, F.; Margesin, R. A Feasibility Study on the Bioremediation of hydrocarbon-contaminated soil from an alpine former military site: Effects of temperature and biostimulation. Cold Reg. Sci. Technol. 2013, 96, 122–128. [Google Scholar] [CrossRef]
- Camenzuli, D.; Freidman, B.L. On-site and in situ remediation technologies applicable to petroleum hydrocarbon contaminated sites in the Antarctic and Arctic. Polar Res. 2015, 34, 24492. [Google Scholar] [CrossRef]
- Philp, J.C.; Atlas, R.M. Bioremediation of contaminated soils and aquifers. In Bioremediation; American Society of Microbiology: Washington, DC, USA, 2005; pp. 139–236. ISBN 9781555817596. [Google Scholar]
- da Silva, S.; Gonçalves, I.; Gomes de Almeida, F.C.; Padilha da Rocha e Silva, N.M.; Casazza, A.A.; Converti, A.; Asfora Sarubbo, L. Soil bioremediation: Overview of technologies and trends. Energies 2020, 13, 4664. [Google Scholar] [CrossRef]
- Shah, J.K.; Sayles, G.D.; Suidan, M.T.; Mihopoulos, P.; Kaskassian, S. Anaerobic bioventing of unsaturated zone contaminated with DDT and DNT. Water Sci. Technol. 2001, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Johnson, R.L.; Bruce, C.L.; Leeson, A. Advances in in situ air sparging/biosparging. Bioremediat. J. 2001, 5, 251–266. [Google Scholar] [CrossRef]
- Wu, Y.W.; Huang, G.H.; Chakma, A.; Zeng, G.M. Separation of petroleum hydrocarbons from soil and groundwater through enhanced bioremediation. Energy Sources 2005, 27, 221–232. [Google Scholar] [CrossRef]
- Kao, C.M.; Chen, C.Y.; Chen, S.C.; Chien, H.Y.; Chen, Y.L. Application of in situ biosparging to remediate a petroleum-hydrocarbon spill site: Field and microbial evaluation. Chemosphere 2008, 70, 1492–1499. [Google Scholar] [CrossRef]
- Brown, L.D.; Ulrich, A.C. Bioremediation of oil spills on land. In Handbook of Oil Spill Science and Technology; Fingas, M., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 395–406. ISBN 978-1-118-98998-2. [Google Scholar]
- Sanscartier, D.; Zeeb, B.; Koch, I.; Reimer, K. Bioremediation of diesel-contaminated soil by heated and humidified biopile system in cold climates. Cold Reg. Sci. Technol. 2009, 55, 167–173. [Google Scholar] [CrossRef]
- Nikolopoulou, M.; Pasadakis, N.; Norf, H.; Kalogerakis, N. Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Mar. Pollut. Bull. 2013, 77, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chikere, C.B.; Okoye, A.U.; Okpokwasili, G.C. Microbial community profiling of active oleophilic bacteria involved in bioreactor-based crude-oil polluted sediment treatment. J. Appl. Environ. Microbiol. 2016, 4, 1–20. [Google Scholar]
- Lu, M.; Zhang, Z.; Sun, S.; Wang, Q.; Zhong, W. Enhanced degradation of bioremediation residues in petroleum-contaminated soil using a two-liquid-phase bioslurry reactor. Chemosphere 2009, 77, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Palanisami, T.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. In-situ remediation approaches for the management of contaminated sites: A comprehensive overview. In Reviews of Environmental Contamination and Toxicology; Springer: Berlin, Germany, 2016; Volume 236, pp. 1–115. ISBN 978-3-319-20013-2. [Google Scholar]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; Santana, F.; Gonzalez-Lopez, J.; Mack, L.; Gonzalez-Martinez, A. Polar Arctic Circle Biomass enhances performance and stability of aerobic granular sludge systems operated under different temperatures. Bioresour. Technol. 2020, 300, 122650. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Muñoz-Palazon, B.; Hurtado-Martinez, M.; Mikola, A.; Gonzalez-Lopez, J.; Vahala, R.; Gonzalez-Martinez, A. Analysis of microbial communities involved in organic matter and nitrogen removal in a full-scale moving bed biofilm reactor located near the Polar Arctic Circle. Int. Biodeterior. Biodegrad. 2020, 146, 104830. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Roslee, A.F.A.; Abdul Khalil, K.; Napis, S.; Convey, P.; Gomez-Fuentes, C.; Ahmad, S.A. Response surface methodology optimization and kinetics of diesel degradation by a cold-adapted antarctic bacterium, Arthrobacter sp. strain AQ5-05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Kumar, S.; Dagar, V.K.; Khasa, Y.P.; Kuhad, R.C. Genetically modified microorganisms (GMOs) for bioremediation. In Biotechnology for Environmental Management and Resource Recovery; Springer: New Delhi, India, 2013; pp. 191–218. ISBN 978-81-322-0876-1. [Google Scholar]
- Luz, A.P.; Pellizari, V.H.; Whyte, L.G.; Greer, C.W. A survey of indigenous microbial hydrocarbon degradation genes in soils from Antarctica and Brazil. Can. J. Microbiol. 2004, 50, 323–333. [Google Scholar] [CrossRef]
- Master, E.R.; Mohn, W.W. Induction of bphA, encoding biphenyl dioxygenase, in two polychlorinated biphenyl-degrading bacteria, psychrotolerant Pseudomonas strain Cam-1 and mesophilic Burkholderia strain LB400. Appl. Environ. Microbiol. 2001, 67, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, R.J.; Inniss, W.E.; Glick, B.R.; Robinson, C.; Mayfield, C.I. Transfer and expression of mesophilic plasmid-mediated degradative capacity in a psychrotrophic bacterium. Appl. Environ. Microbiol. 1988, 54, 638–641. [Google Scholar] [CrossRef]
- Parrilli, E.; Papa, R.; Tutino, M.L.; Sannia, G. Engineering of a psychrophilic bacterium for the bioremediation of aromatic compounds. Bioeng. Bugs 2010, 1, 213–216. [Google Scholar] [CrossRef]
- Monti, M.R.; Smania, A.M.; Fabro, G.; Alvarez, M.E.; Argarana, C.E. Engineering Pseudomonas fluorescens for biodegradation of 2, 4-dinitrotoluene. Appl. Environ. Microbiol. 2005, 71, 8864–8872. [Google Scholar] [CrossRef]
- Kayıhan, D.S.; Kayıhan, C.; Özden Çiftçi, Y. Transgenic tobacco plants overexpressing a cold-adaptive nitroreductase gene exhibited enhanced 2, 4-dinitrotoluene detoxification rate at low temperature. Int. J. Phytoremediat. 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Li, Q.-S.; Ogawa, J.; Schmid, R.D.; Shimizu, S. Engineering cytochrome P450 BM-3 for oxidation of polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 2001, 67, 5735–5739. [Google Scholar] [CrossRef]
- Sideri, A.; Goyal, A.; Di Nardo, G.; Tsotsou, G.E.; Gilardi, G. Hydroxylation of non-substituted polycyclic aromatic hydrocarbons by cytochrome P450 BM3 engineered by directed evolution. J. Inorg. Biochem 2013, 120, 1–7. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M.G. Use of nanotechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Murugesan, K.; Chang, Y.-Y.; Kim, E.-J.; Chang, Y.-S. Degradation of polybrominated diphenyl ethers by a sequential treatment with nanoscale zero valent iron and aerobic biodegradation. J. Chem. Technol. Biotechnol. 2012, 87, 216–224. [Google Scholar] [CrossRef]
- Le, T.T.; Yoon, H.; Son, M.-H.; Kang, Y.-G.; Chang, Y.-S. Treatability of hexabromocyclododecane using pd/fe nanoparticles in the soil-plant system: Effects of humic acids. Sci. Total Environ. 2019, 689, 444–450. [Google Scholar] [CrossRef]
- Ghorbannezhad, H.; Moghimi, H.; Taheri, R.A. Enhanced biodegradation of phenol by magnetically immobilized Trichosporon cutaneum. Ann. Microbiol. 2018, 68, 485–491. [Google Scholar] [CrossRef]
- Farber, R.; Rosenberg, A.; Rozenfeld, S.; Banet, G.; Cahan, R. Bioremediation of artificial diesel-contaminated soil using bacterial consortium immobilized to plasma-pretreated wood waste. Microorganisms 2019, 7, 497. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jang, S.-H.; Chung, H.-S. Improving the stability of cold-adapted enzymes by immobilization. Catalysts 2017, 7, 112. [Google Scholar] [CrossRef]
- Fareed, A.; Riaz, S.; Nawaz, I.; Iqbal, M.; Ahmed, R.; Hussain, J.; Hussain, A.; Rashid, A.; Naqvi, T.A. Immobilized cells of a novel bacterium increased the degradation of n-methylated carbamates under low temperature conditions. Heliyon 2019, 5, e02740. [Google Scholar] [CrossRef] [PubMed]
- Alessandrello, M.J.; Tomás, M.S.J.; Raimondo, E.E.; Vullo, D.L.; Ferrero, M.A. Petroleum oil removal by immobilized bacterial cells on polyurethane foam under different temperature conditions. Mar. Pollut. Bull. 2017, 122, 156–160. [Google Scholar] [CrossRef]
- De Ory, I.; Cabrera, G.; Ramirez, M.; Blandino, A. Immobilization of cells on polyurethane foam. In Immobilization of Enzymes and Cells; Humana Press: Totowa, NJ, USA, 2006; Volume 22, pp. 357–365. ISBN 978-1-59745-053-9. [Google Scholar]
- Ye, J.; Huang, L.; Zhang, Y.; Chen, Y.; Chen, G. Acclimation, screening and immobilization of a high-efficient and low-temperature plant oil-degrading strain. J. Saf. Environ. 2012, 2, 1–8. [Google Scholar]
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565. [Google Scholar] [CrossRef]
- Ibrahim, S.; Abdul Khalil, K.; Zahri, K.N.M.; Gomez-Fuentes, C.; Convey, P.; Zulkarnain, A.; Sabri, S.; Alias, S.A.; González-Rocha, G.; Ahmad, S.A. Biosurfactant production and growth kinetics studies of the waste canola oil-degrading bacterium Rhodococcus erythropolis AQ5-07 from Antarctica. Molecules 2020, 25, 3878. [Google Scholar] [CrossRef]
- Luong, T.M.; Ponamoreva, O.N.; Nechaeva, I.A.; Petrikov, K.V.; Delegan, Y.A.; Surin, A.K.; Linklater, D.; Filonov, A.E. Characterization of biosurfactants produced by the oil-degrading bacterium Rhodococcus erythropolis S67 at low temperature. World J. Microbiol. Biotechnol. 2018, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Janek, T.; Łukaszewicz, M.; Krasowska, A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiol. 2012, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Malavenda, R.; Rizzo, C.; Michaud, L.; Gerçe, B.; Bruni, V.; Syldatk, C.; Hausmann, R.; Giudice, A.L. Biosurfactant production by Arctic and Antarctic bacteria growing on hydrocarbons. Polar Biol. 2015, 38, 1565–1574. [Google Scholar] [CrossRef]
- Xia, M.; Fu, D.; Chakraborty, R.; Singh, R.P.; Terry, N. Enhanced crude oil depletion by constructed bacterial consortium comprising bioemulsifier producer and petroleum hydrocarbon degraders. Bioresour. Technol. 2019, 282, 456–463. [Google Scholar] [CrossRef] [PubMed]


| Pollutants | Examples | Description | References |
|---|---|---|---|
| Petroleum | Diesel and crude oil | Common oil types found in cold regions that produce toxic effects to the cold ecosystem. | [20,21,22,23,24] |
| Aliphatic hydrocarbon | n-alkanes (C6–C22) | Major constituents of petroleum oils with linear chain arrangement that can be easily degraded by most of the hydrocarbon-degrading bacteria. | [25,26] |
| Aromatic hydrocarbon | Benzene, toluene, ethylbenzene, xylene (BTEX) and phenol | Highly volatile, single-ring compounds released by diesel- or petroleum-based products. BTEX compounds are highly unstable and will be readily converted into stable phenolic compounds. | [27,28,29,30] |
| Polycyclic aromatic hydrocarbon (PAH) | Naphthalene, anthracene, phenanthrene and pyrene | Most recalcitrant pollutants derived from petroleum oils. These compounds contain multiple ring structures that make them highly stable and difficult to be biodegraded. | [31,32] |
| Microorganisms | Origins | Petroleum-Based Contaminants | Removal Efficiency | References |
|---|---|---|---|---|
| Arthrobacter sp. strain AQ5–15 A | King George Island, Antarctica | Phenol | 99.4% | [27] |
| Rhodococcus sp. strain AQ5–14 A | King George Island, Antarctica | Phenol | 99.1% | [28] |
| Pseudomonas sp. A, Stenotrophomonas spp. A and Shinella spp. A | Alpine Binaloud Mountains, Iran | Phenol | 99% | [29] |
| Sphingomonas koreensis strain ASU–06 A | Oil-contaminated soil, Egypt | PAHs (Nap, Phe, Ant and pyrene) | 98.6% | [31] |
| Rhodococcus sp. strain AQ5–07 A | King George Island, Antarctica | Diesel oil | 90.3% | [54,55] |
| Pseudoalteromonas sp. strain P29 A | Arctic marine sediment | Mixed and vacuum crude oil | 80–90% | [56] |
| Arthrobacter spp. strains AQ5-05 A and AQ5-06 A | King George Island, Antarctica | Diesel oil | 47.5% (AQ5-06) and 41% (AQ5-05) | [51] |
| Dietzia maris strain NWWC4 A | Subarctic Canada | Arctic diesel | 37% ± 6% | [57] |
| Ceratobasidum stevensii strain B6 C and Fusarium solani C | Livingston Island, Antarctica | PAHs (Ant and Phe) | 40–89.5% | [32] |
| Pseudoalteromonas spp. A, Marinobacter spp. A, Oleispira sp. A, Alcanivorax sp. A, Sphingopyxis sp. A, Rhodobacter sp. A and Hyphomonas sp. A | Svalbard, Arctic | Arabian crude oil | 17.2–81.9% | [58] |
| Rhodococcus erythropolis strain BZ4 A, Rhodococcus cercidiphyllus strain BZ22 A, Arthrobacter sulfureus strain BZ73 A and Pimelobacter simplex strain BZ91 A | South Tyrol, Italy | Linear, aromatic and polyaromatic hydrocarbons (n-alkanes of C12–C22, phenol, Ant and pyrene) | 11–100% | [25] |
| Cryptococcus spp. B, Candida spp. B, Rhodotorula spp. B, Mrakia spp.B, Candida spp. B, Cistobasidium spp. B and Pichia spp. B | King George Island, Antarctica | Linear and aromatic hydrocarbons (Phenol, methanol and n-hexadecane) | 13–78% | [26] |
| Technique | Description | Pros | Cons | References |
|---|---|---|---|---|
| Phytoremediation | Useful plants are selected and planted at the polluted site |
|
| [50,75,76] |
| Bioventing/biosparging | Air injection to the soil surface or into deeper soil |
|
| [53,77,78] |
| Biopile | Polluted soil is excavated and piled up aboveground with the exploitation of fertilizer, temperature, and irrigation. |
|
| [15,30,44,49,79] |
| Landfarming | Contaminated soil is excavated and spread on a treatment bed supplied with tilling system |
|
| [42,45,47,80] |
| Bioreactor | Polluted soil is excavated into incubation tank supplied with water, oxygen and other requirements. |
|
| [16,81,82,83,84] |
| In Situ Bioremediation | Location | Enhancements | Treatment Period | Removal Efficiency | References |
|---|---|---|---|---|---|
| Phytoremediation | Subarctic Alaska (PPS) | BST by agricultural fertiliser (20 N: 20 P: 10 K) | Re-examined after 15 years | TPH reduction by 80–95% | [75] |
| Sweden (PPS) | BST by 10% w/w organic municipal compost | 5 months | Removal of 38% (MMW hydrocarbon), 40% (HMW hydrocarbon) | [43] | |
| Sub-Arctic (DPS) | BST by fertiliser (16.6% N, 4% P and 25.3% K) | 330 days | Diesel removal of 97% | [76] | |
| Bioventing | New England (PPS) | BST by fertiliser (100 C: 10 N: 1.5 P) + aeration rate at 275 cm3/min | 12 months | TPH removal of 82.5% | [77] |
| Subarctic Macquarie Island (DPS) | BST by N fertiliser (125 mg kg−1) + 9 optimised micro-injection (6 mm) | 12 months | Removal rate of 1020 mg kg−1 per day | [46] |
| Ex Situ Bioremediation | Location | Enhancements | Treatment Period | Removal Efficiency | References |
|---|---|---|---|---|---|
| Biopile | Antarctica (PPS) | BST by NH4NO3 and MSP | 50 days | Removal of isoprenoid hydrocarbons by 75.8% | [15] |
| Antarctica (PPS) | BST by NH4NO3 and MSP + sunlight (157 h exposure) | 2 months | TPH reduction by 75% | [42] | |
| Canada (PPS) | BST by mature municipal compost and BAT by bacterial consortium | 94 days | TPH removal of 74–82% | [36] | |
| Republic of Ireland (PPS) | BST by fertiliser (25 N: 4 P) and BAT by microbial consortium + phytoremediators | 24 months | Below the detectable level with initial TPH concentration of 1613 mg kg−1 | [92] | |
| Landfarming | Sub-Arctic (PPS) | BST by fertiliser (2 MSP: 1 urea) + aggressive tilling | 56 days | BTEX and gasoline compounds below the detectable level | [40] |
| Italy (PPS) | BST by MPP, MSP, NH4Cl and NaCl + periodic tilling and BAT by bacterial consortium | 3 months | 86% TPH removal | [93] | |
| Canada (PPS) | BST by fertiliser (100 C: 9 N: 1 P) + 2000 mg kg−1 CaCO3 + periodic tilling | 2 months | 75% TPH removal | [37] | |
| Canadian Arctic (DPS) | BST by urea and (NH4)2HPO4 + optimised rototilling | 3 months | 80% TPH removal | [39] | |
| Korea (PPS) | BST by fertiliser (100 C: 10 N: 1 P) and BAT by oil-degrading microbes | 33 days | 73.7% TPH removal | [34] |
| Targeted Genes | Recombinant Strain | Temperature | Hydrocarbons Nature | References |
|---|---|---|---|---|
| Genetically Modified Bacteria | ||||
| TOL | Pseudomonas putida Q5T | 0 °C | Toluene | [109] |
| ToMO | Pseudoalteromonas haloplanktis TAC125 | 15 °C | Derivatives of benzene, phenol, xylene and compounds of toluene, naphthalene | [110] |
| DntAaAbAcAd dntB and dntD | Pseudomonas fluorescens RE | 10 °C | 2,4-dinitrotoluene (DNT) | [111] |
| BphA and bphE | Pseudomonas sp. Cam-10 | 7 °C | Polychlorinated biphenyls | [108] |
| Transgenic Plant | [112] | |||
| Ntr | Nicotiana tabacum | 4 °C | 2,4- DNT | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, H.S.; Zakaria, N.N.; Zulkharnain, A.; Sabri, S.; Gomez-Fuentes, C.; Ahmad, S.A. Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation. Biology 2021, 10, 354. https://doi.org/10.3390/biology10050354
Yap HS, Zakaria NN, Zulkharnain A, Sabri S, Gomez-Fuentes C, Ahmad SA. Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation. Biology. 2021; 10(5):354. https://doi.org/10.3390/biology10050354
Chicago/Turabian StyleYap, How Swen, Nur Nadhirah Zakaria, Azham Zulkharnain, Suriana Sabri, Claudio Gomez-Fuentes, and Siti Aqlima Ahmad. 2021. "Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation" Biology 10, no. 5: 354. https://doi.org/10.3390/biology10050354
APA StyleYap, H. S., Zakaria, N. N., Zulkharnain, A., Sabri, S., Gomez-Fuentes, C., & Ahmad, S. A. (2021). Bibliometric Analysis of Hydrocarbon Bioremediation in Cold Regions and a Review on Enhanced Soil Bioremediation. Biology, 10(5), 354. https://doi.org/10.3390/biology10050354











