Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established?
Abstract
Simple Summary
Abstract
1. Introduction
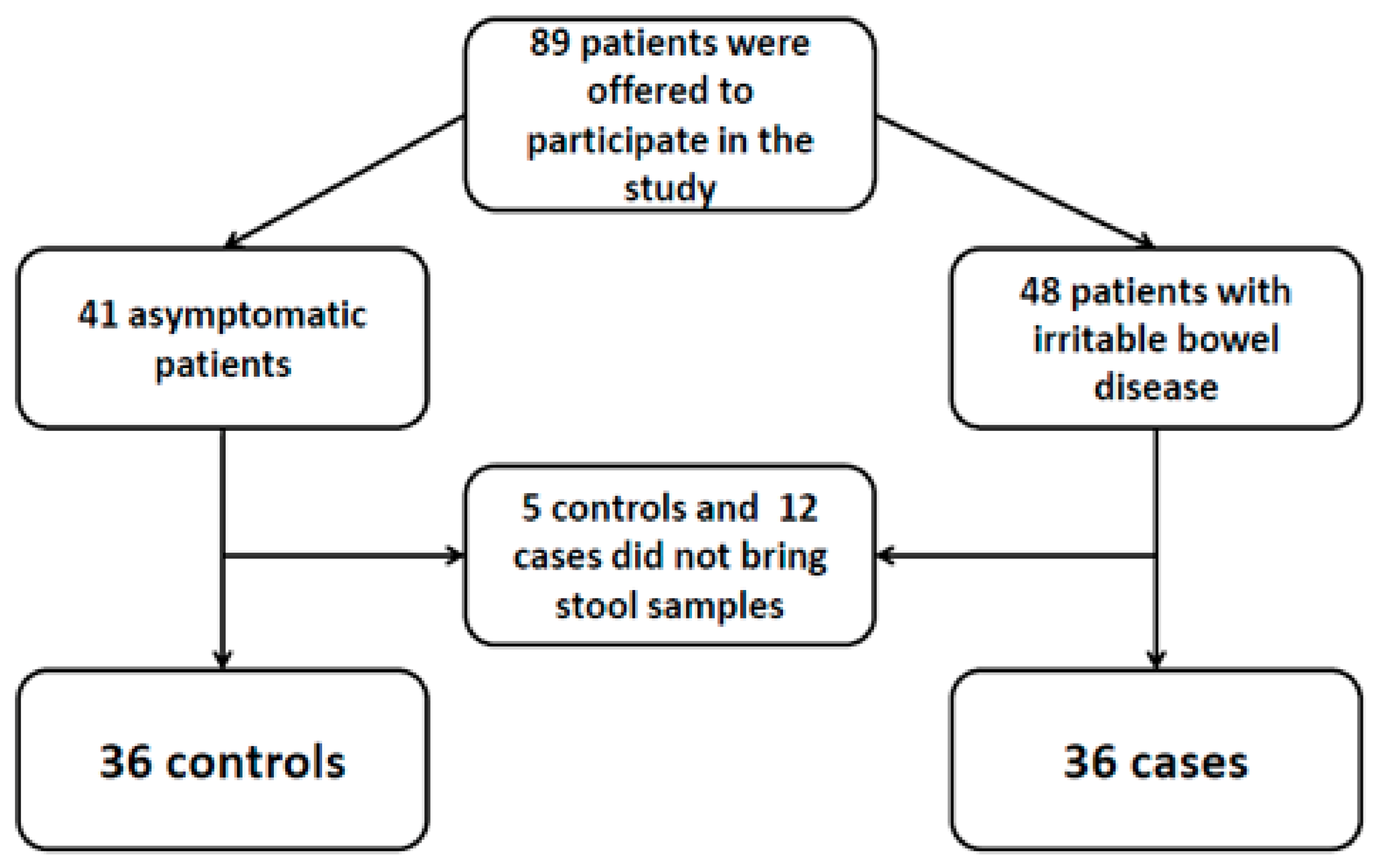
2. Methods
2.1. Statistical and Ethical Issues
2.2. Microbiological Techniques
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Appendix A
References
- Fletcher, S.M.; Stark, D.; Harkness, J.; Ellis, J.T. Enteric protozoa in the developed world: A public health perspective. Clin. Microbiol. Rev. 2012, 25, 420–449. [Google Scholar] [CrossRef]
- Tan, K.S.W. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Clark, C.G. Pre-empting Pandora’s Box: Blastocystis Subtypes Revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef]
- Maloney, J.G.; Lombard, J.E.; Urie, N.J.; Shivley, C.B.; Santin, M. Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol. Res. 2019, 118, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Update on the pathogenic potential and treatment options for Blastocystis sp. Gut Pathog. 2014, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Alonso, C.; Santos, J.; Fuentes, I.; Molina, I. Epidemiological and clinical profile of adult patients with Blastocystis hominis infection in Barcelona, Spain. Parasites Vectors 2016, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Stark, D.; Harkness, J.; Ellis, J. Subtype distribution of Blastocystis isolates identified in a Sydney population and pathogenic potential of Blastocystis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 32, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Cetin, E.S.; Aridogan, B.C.; Arikan, S.; Demirci, M. Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turk. J. Parasitol. 2007, 31, 184–187. [Google Scholar]
- Hidalgo, L.; Salvador, F.; Sulleiro, E.; López, I.; Balladares, M.; García, E.; Paz, C.; Sánchez-Montalvá, A.; Bosch-Nicolau, P.; Sao-Avilés, A.; et al. Evaluation of risk factors associated to detection of Blastocystis sp in fecal samples in population from Barcelona, Spain: A case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1241–1247. [Google Scholar] [CrossRef]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology. 2016, 150, 1393–1407. [Google Scholar] [CrossRef] [PubMed]
- Botschuijver, S.; Roeselers, G.; Levin, E.; Jonkers, D.M.; Welting, O.; Heinsbroek, S.E.; de Weerd, H.H.; Boekhout, T.; Fornai, M.; Masclee, A.A.; et al. Intestinal fungal dysbiosis is associated with visceral hypersensitivity in patients with irritable bowel syndrome and rats. Gastroenterology 2017, 153, 1026–1039. [Google Scholar] [CrossRef]
- Klem, F.; Wadhwa, A.; Prokop, L.J.; Sundt, W.J.; Farrugia, G.; Camilleri, M.; Singh, S.; Grover, M. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology 2017, 152, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gonzalez, D.E.; Martinez-Flores, W.A.; Reyes-Gordillo, J.; Ramirez-Miranda, M.E.; Arroyo-Escalante, S.; Romero-Valdovinos, M.; Stark, D.; Souza-Saldivar, V.; Martinez-Hernandez, F.; Flisser, A.; et al. Blastocystis infection is associated with irritable bowel syndrome in a Mexican patient population. Parasitol. Res. 2011, 110, 1269–1275. [Google Scholar] [CrossRef]
- Yakoob, J.; Beg, M.A.; Zaman, V.; Jafri, W.; Jafri, N.; Khan, R.; Islam, M. Irritable bowel syndrome: In search of an etiology: Role of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004, 70, 383–385. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: A population-based case-control study. Clin. Gastroenterol. Hepatol. 2015, 13, 507–513. [Google Scholar] [CrossRef]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Újszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: A six-country study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Rey, E.; Balboa, A. Functional and motor gastrointestinal disorders. Gastroenterol. Hepatol. 2016, 39 (Suppl. 1), 3–13. [Google Scholar] [CrossRef]
- Scicluna, S.M.; Tawari, B.; Clark, C.G. DNA barcoding of Blastocystis. Protist 2006, 157, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Not, A.; Salvador, F.; Goterris, L.; Sulleiro, E.; López, I.; Balladares, M.; García, E.; Paz, C.; Sánchez-Montalvá, A.; Bosch-Nicolau, P.; et al. Microscopic examination after concentration techniques for Blastocystis sp. detection in serial faecal samples: How many samples are needed? Parasite Epidemiol. Control. 2020, 9, e00137. [Google Scholar] [CrossRef] [PubMed]
- Paulos, S.; Köster, P.C.; De Lucio, A.; Hernández-De-Mingo, M.; Cardona, G.A.; Fernández-Crespo, J.C.; Stensvold, C.R.; Carmena, D. Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses Public Health 2018, 65, 993–1002. [Google Scholar] [CrossRef]
- Muadica, A.S.; Köster, P.C.; Dashti, A.; Bailo, B.; Hernández-de-Mingo, M.; Reh, L.; Balasegaram, S.; Verlander, N.Q.; Ruiz Chércoles, E.; Carmena, D. Molecular diversity of Giardia duodenalis, Crysptosporidium spp. and Blastocystis sp. in asymptomatic school children in Leganés, Madrid (Spain). Microorganisms 2020, 8, 466. [Google Scholar] [CrossRef]
- Reh, L.; Muadica, A.S.; Köster, P.C.; Balasegaram, S.; Verlander, N.Q.; Chércoles, E.R.; Carmena, D. Substantial prevalence of enteroparasites Cryptosporidium spp., Giardia duodenalis and Blastocystis sp. in asymptomatic schoolchildren in Madrid, Spain, November 2017 to June 2018. Eurosurveillance 2019, 24, 1900241. [Google Scholar]
- Soriano, J.M.; Domènech, G.; Martínez, M.C.; Mañes, J.; Soriano, F. Intestinal parasitic infections in hosted Saharawi children. Trop. Biomed. 2011, 28, 557–562. [Google Scholar]
- El Safadi, D.; Cian, A.; Nourrisson, C.; Pereira, B.; Morelle, C.; Bastien, P.; Bellanger, A.P.; Botterel, F.; Candolfi, E.; Desoubeaux, G.; et al. Prevalence, risk factors for infection and subtype distribution of the intestinal parasite Blastocystis sp. from a large-scale multi-center study in France. BMC Infect. Dis. 2016, 16, 451. [Google Scholar] [CrossRef] [PubMed]
- Pipatsatitpong, D.; Rangsin, R.; Leelayoova, S.; Naaglor, T.; Mungthin, M. Incidence and risk factors of Blastocystis infection in an orphanage in Bangkok, Thailand. Parasites Vectors 2012, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Muadica, A.S.; Balasegaram, S.; Beebeejaun, K.; Köster, P.C.; Bailo, B.; Hernández-De-Mingo, M.; Dashti, A.; Dacal, E.; Saugar, J.M.; Fuentes, I.; et al. Risk associations for intestinal parasites in symptomatic and asymptomatic schoolchildren in central Mozambique. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Smith, H. Comparison of methods for detecting Blastocystis hominis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 509–511. [Google Scholar] [CrossRef]
- Shafiei, Z.; Esfandiari, F.; Sarkari, B.; Rezaei, Z.; Fatahi, M.R.; Asl, S.M.K.H. Parasitic infections in irritable bowel syndrome patients: Evidence to propose a possible link, based on a case–control study in the south of Iran. BMC Res. Notes 2020, 13, 264. [Google Scholar] [CrossRef]
- Rostami, A.; Riahi, S.M.; Haghighi, A.; Saber, V.; Armon, B.; Seyyedtabaei, S.J. The role of Blastocystis sp. and Dientamoeba fragilis in irritable bowel syndrome: A systematic review and meta-analysis. Parasitol. Res. 2017, 116, 2361–2371. [Google Scholar] [CrossRef]
- Kodio, A.; Coulibaly, D.; Koné, A.K.; Konaté, S.; Doumbo, S.; Guindo, A.; Bittar, F.; Gouriet, F.; Raoult, D.; Thera, M.A.; et al. Blastocystis colonization is associated with increased diversity and altered gut bacterial communities in healthy malian children. Microorganisms 2019, 7, 649. [Google Scholar] [CrossRef]
- Tito, R.Y.; Chaffron, S.; Caenepeel, C.; Lima-Mendez, G.; Wang, J.; Vieira-Silva, S.; Falony, G.; Hildebrand, F.; Darzi, Y.; Rymenans, L.; et al. Population-level analysis of Blastocystis subtype prevalence and variation in the human gut microbiota. Gut 2019, 68, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Kesuma, Y.; Firmansyah, A.; Bardosono, S.; Sari, I.P.; Kurniawan, A. Blastocystis ST-1 is associated with irritable bowel syndrome-diarrhoea (IBS-D) in Indonesian adolescences. Par. Epid. Cont. 2019, 6, e112. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Sánchez, G.B.; Romero-Valdovinos, M.; Ramírez-Guerrero, C.; Vargas-Hernández, I.; Ramírez-Miranda, M.E.; Martínez-Ocaña, J.; Valadez, A.; Ximenez, C.; Lopez-Escamilla, E.; Hernandez-Campos, M.E.; et al. Blastocystis isolates from patients with irritable bowel syndrome and from asymptomatic carriers exhibit similar parasitological loads, but significantly different generation times and genetic variability across multiple subtypes. PLoS ONE 2015, 10, e0124006. [Google Scholar] [CrossRef] [PubMed]
| Healthy Controls (n = 36) | IBS patients (n = 36) | p Value | |
|---|---|---|---|
| Age, years | 30 (29–38.7) | 43 (29.5–50.5) | 0.094 |
| Gender, male | 19 (52.8%) | 16 (44.4%) | 0.479 |
| Living in rural environment | 3 (8.3%) | 1 (2.8%) | 0.614 |
| Regular contact with animals | 11 (30.6%) | 21 (58.3%) | 0.018 |
| Travelling abroad in the last 12 months | 26 (72.2%) | 8 (22.2%) | <0.001 |
| Travelling to low or middle income country in the last 12 months | 14 (38.8%) | 1 (2.7%) | <0.001 |
| Working in close contact with people | 20 (55.6%) | 10 (27.8%) | 0.017 |
| Healthy Controls (n = 36) | IBS Patients (n = 36) | p Value | |
|---|---|---|---|
| Blastocystis sp. detection (microscopy + PCR) | 13 (36.1%) | 7 (19.4%) | 0.114 |
| Blastocystis sp. detection by microscopic examination | 10 (27.8%) | 6 (16.7%) | 0.257 |
| Parasite burden (n = 16) * | |||
| Low (<1 parasite/high-power field ×400) | 4/10 (40%) | 2/6 (33.3%) | 1 |
| Medium (1–5 parasite/high-power field ×400) | 4/10 (40%) | 2/6 (33.3%) | 1 |
| High (>5 parasite/high-power field ×400) | 2 (20%) | 2/6 (33.3%) | 0.604 |
| Positive Blastocystis sp. PCR | 8 (22.2%) | 3 (8.3%) | 0.101 |
| Detection of other intestinal microbial eukaryotes | 1 (2.8%) | 3 (8.3%) | 0.614 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, F.; Lobo, B.; Goterris, L.; Alonso-Cotoner, C.; Santos, J.; Sulleiro, E.; Bailo, B.; Carmena, D.; Sánchez-Montalvá, A.; Bosch-Nicolau, P.; et al. Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established? Biology 2021, 10, 340. https://doi.org/10.3390/biology10040340
Salvador F, Lobo B, Goterris L, Alonso-Cotoner C, Santos J, Sulleiro E, Bailo B, Carmena D, Sánchez-Montalvá A, Bosch-Nicolau P, et al. Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established? Biology. 2021; 10(4):340. https://doi.org/10.3390/biology10040340
Chicago/Turabian StyleSalvador, Fernando, Beatriz Lobo, Lidia Goterris, Carmen Alonso-Cotoner, Javier Santos, Elena Sulleiro, Begoña Bailo, David Carmena, Adrián Sánchez-Montalvá, Pau Bosch-Nicolau, and et al. 2021. "Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established?" Biology 10, no. 4: 340. https://doi.org/10.3390/biology10040340
APA StyleSalvador, F., Lobo, B., Goterris, L., Alonso-Cotoner, C., Santos, J., Sulleiro, E., Bailo, B., Carmena, D., Sánchez-Montalvá, A., Bosch-Nicolau, P., Espinosa-Pereiro, J., Fuentes, I., & Molina, I. (2021). Blastocystis sp. Carriage and Irritable Bowel Syndrome: Is the Association Already Established? Biology, 10(4), 340. https://doi.org/10.3390/biology10040340








