Induction of foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Splenocytes
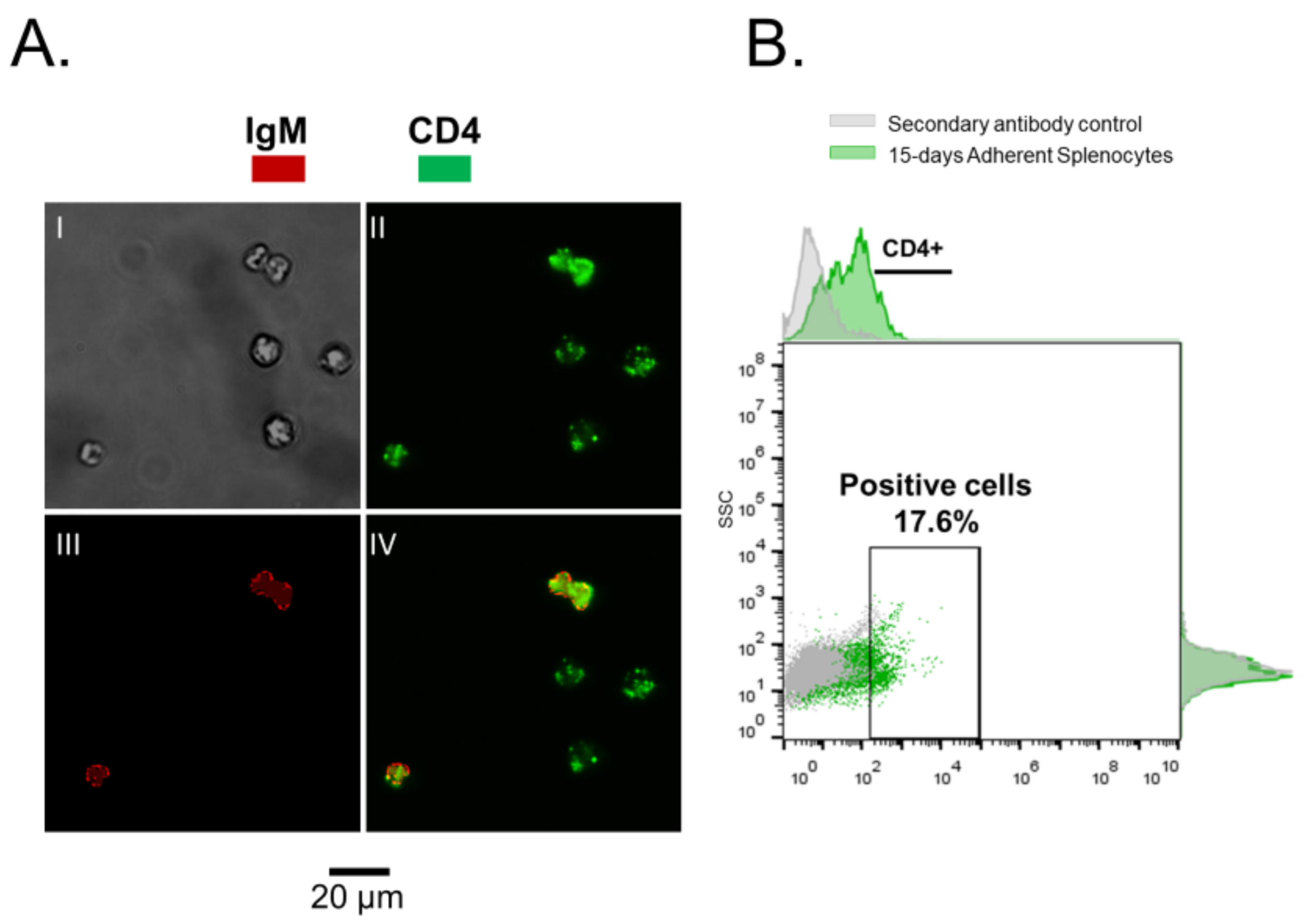
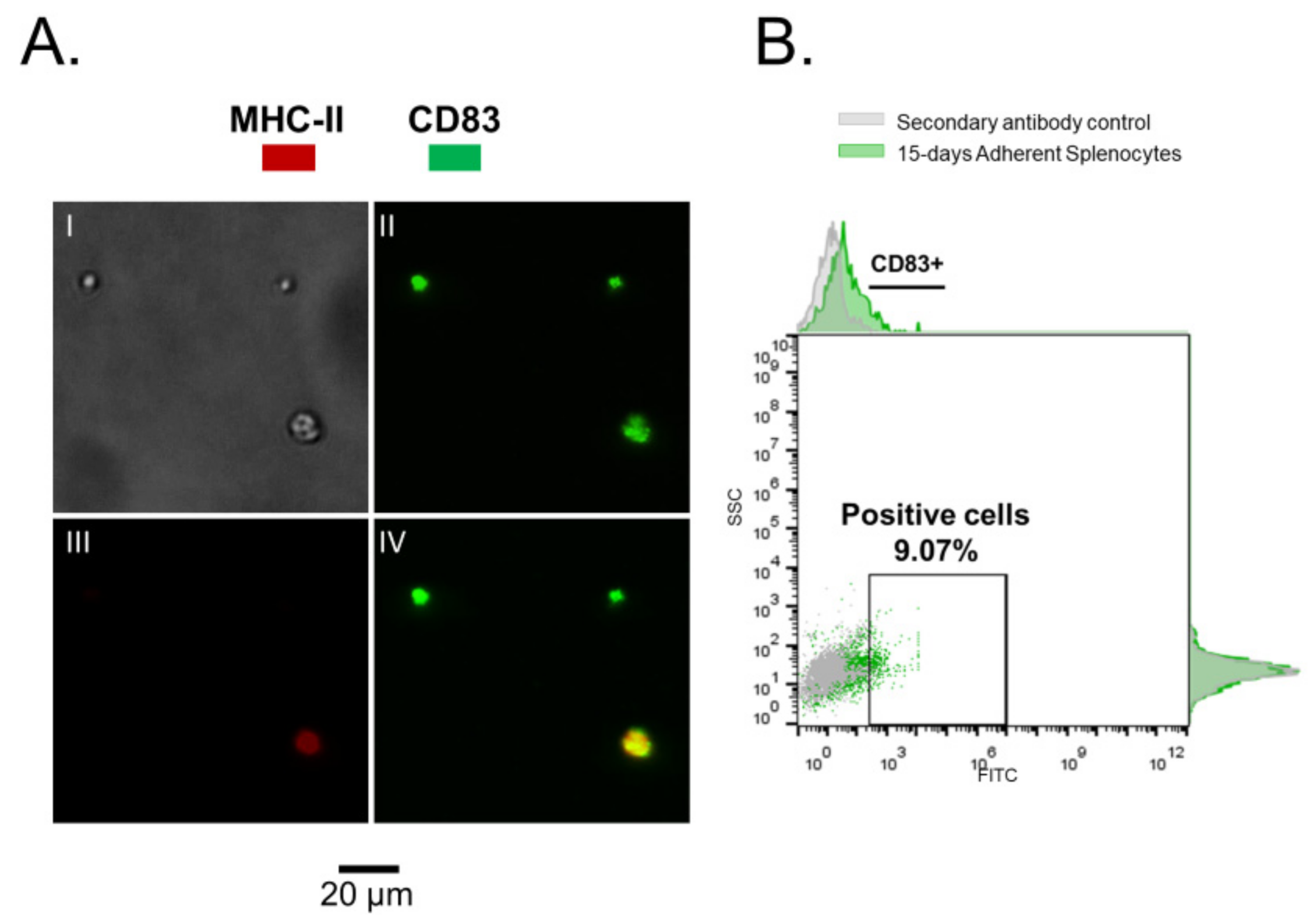
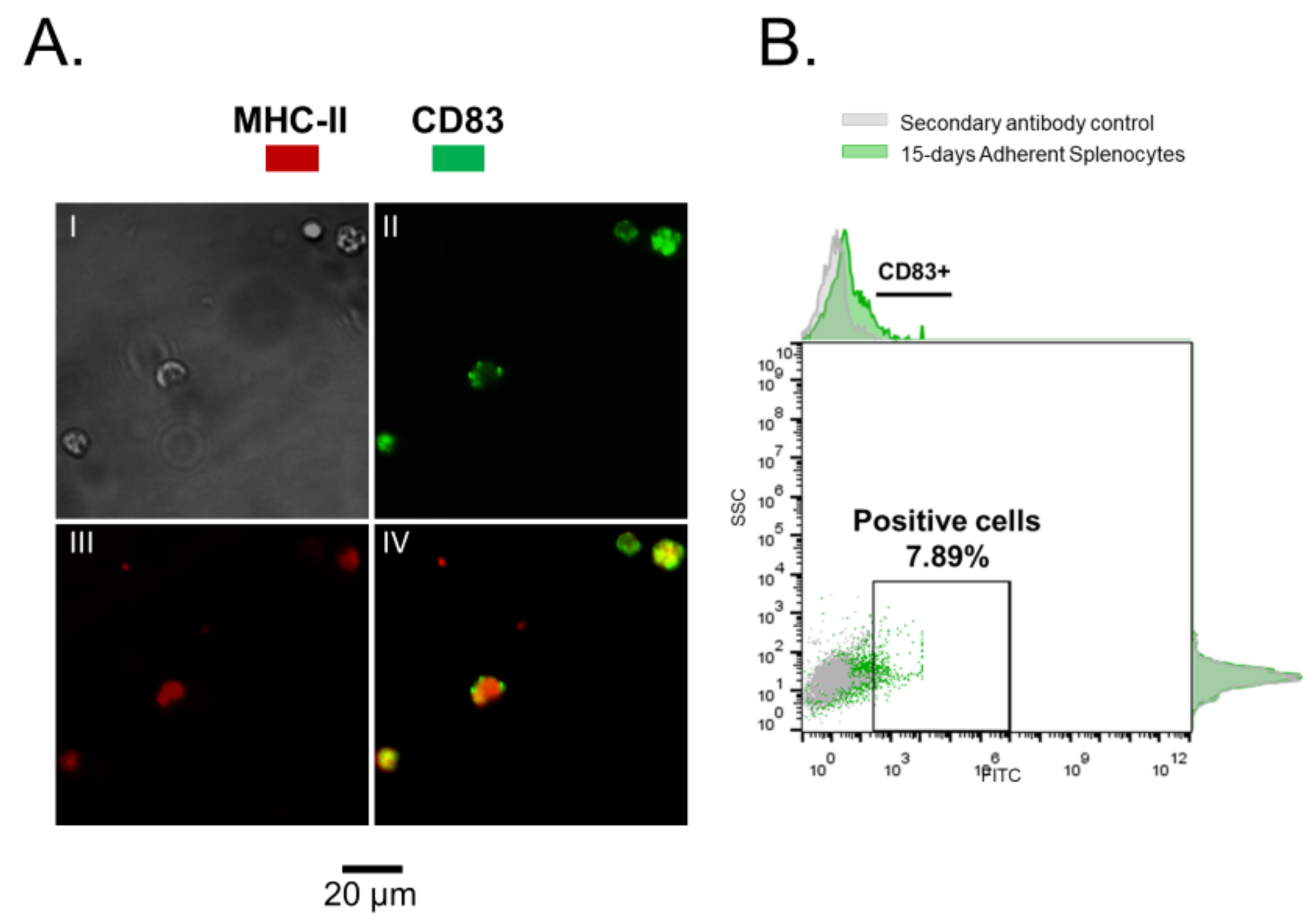
2.3. Immunofluorescence and Flow Cytometry
2.4. Co-Culture of Splenocytes from Rainbow Trout
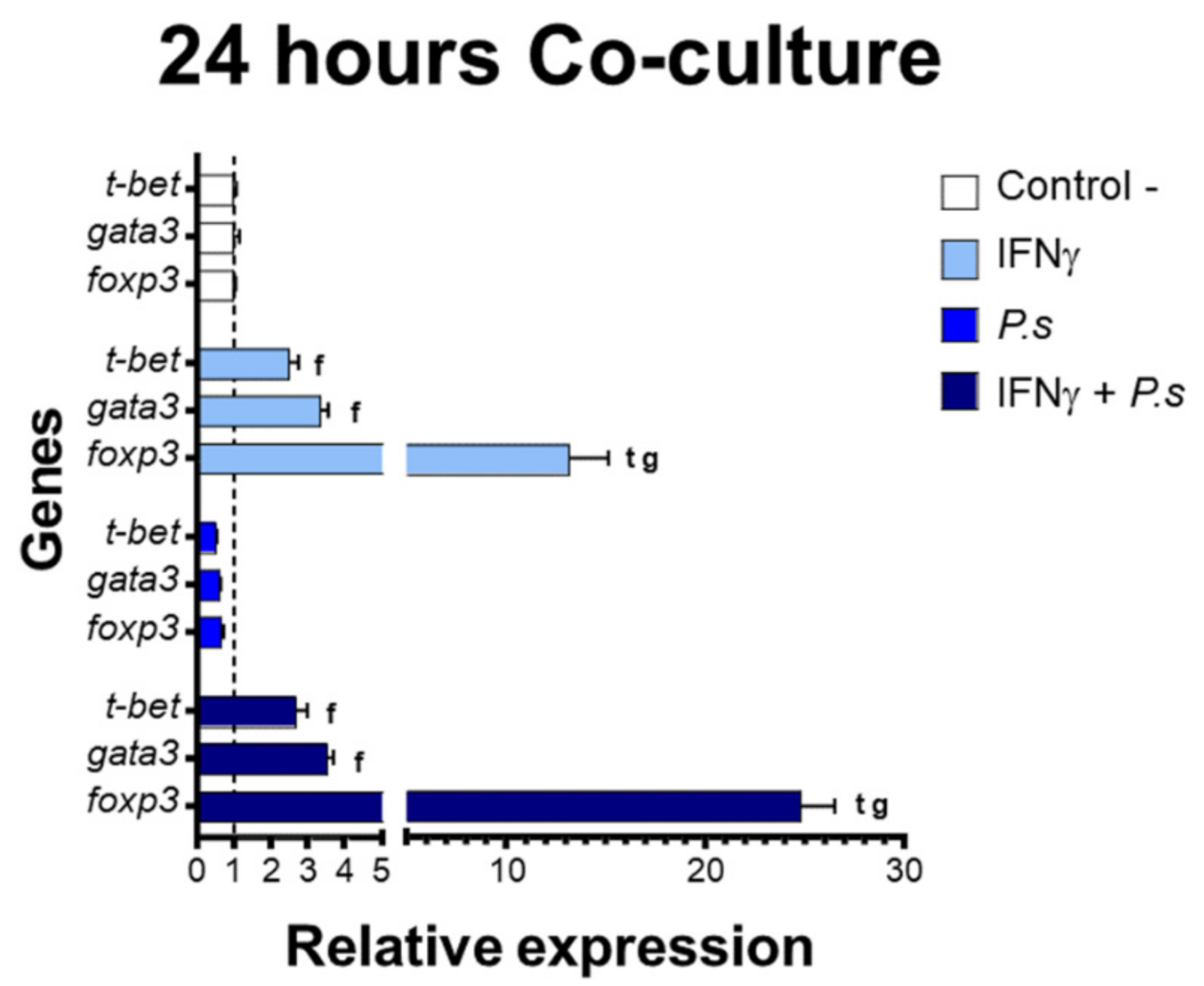
2.5. Gene Expression
2.6. Statistical Analysis
3. Results and Discussion
3.1. Rainbow Trout Splenocytes
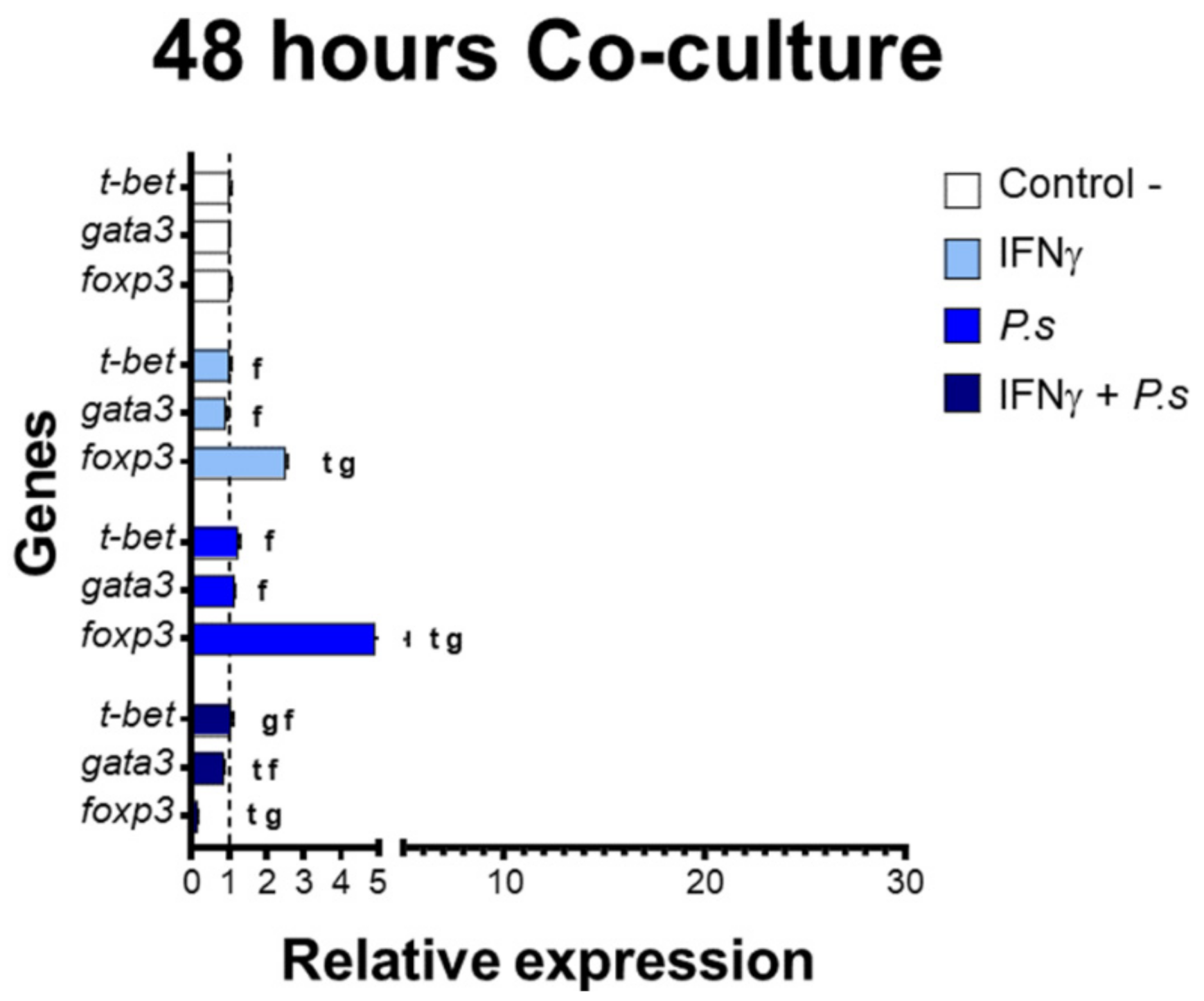
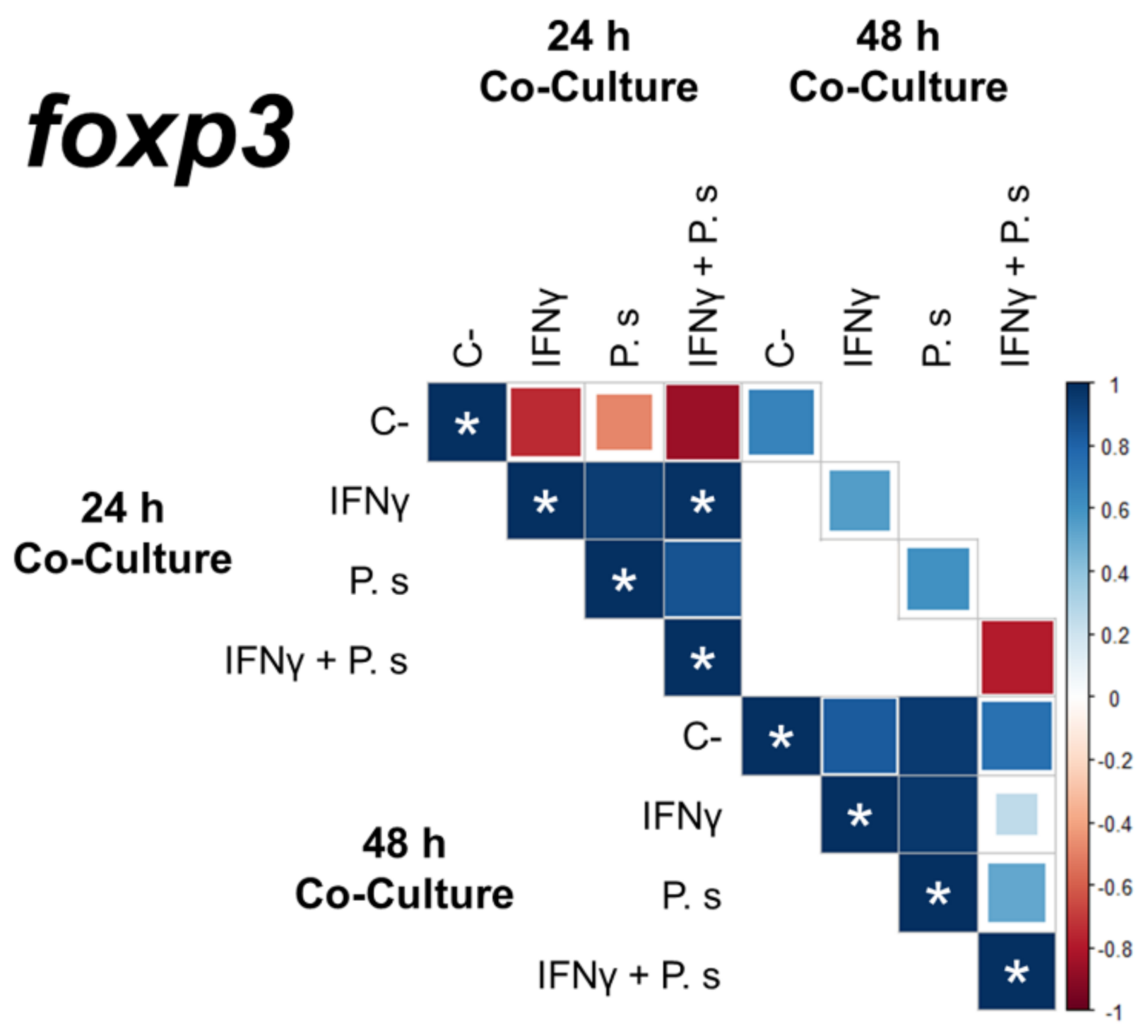
3.2. Gene Expression of Transcriptional Factors Related to T Cell Polarization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galezan, F.H.; Bayati, M.R.; Safari, O.; Rohani, A. Evaluating the Rearing condition of Rainbow Trout (Oncorhynchus Mykiss) Using Fuzzy Inference System. Aquac. Eng. 2020, 89, 102051. [Google Scholar] [CrossRef]
- Ojeda, N.; Cárdenas, C.; Guzmán, F.; Marshall, S.H. Chemical Synthesis and In Vitro Evaluation of a Phage Display-Derived Peptide Active against Infectious Salmon Anemia Virus. Appl. Environ. Microbiol. 2016, 82, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Koppang, E.O.; Nakanishi, T. Teleost T and NK cell immunity. Fish Shellfish. Immunol. 2013, 35, 197–206. [Google Scholar] [CrossRef]
- Sales, C.F.; Silva, R.F.; Amaral, M.G.C.; Domingos, F.F.T.; Ribeiro, R.I.M.A.; Thomé, R.G.; Santos, H.B. Comparative histology in the liver and spleen of three species of freshwater teleost. Neotrop. Ichthyol. 2017, 15, e160041. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Nakanishi, T.; Shibasaki, Y.; Matsuura, Y. T Cells in Fish. Biology 2015, 4, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Tanaka, H.; Naoe, Y.; Taniuchi, I. Transcriptional control of T-cell development. Int. Immunol. 2011, 23, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, J.; Dong, M.; Zhang, K.; Tu, E.; Gao, Q.; Chen, W.; Zhang, C.; Zhang, Y. Regulatory T cells in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 167–179. [Google Scholar] [CrossRef]
- Wang, T.; Secombes, C.J. The cytokine networks of adaptive immunity in fish. Fish Shellfish. Immunol. 2013, 35, 1703–1718. [Google Scholar] [CrossRef]
- Mutoloki, S.; Jørgensen, J.B.; Evensen, Ø. The Adaptive Immune Response in Fish. In Fish Vaccination; Gudding, R., Lillehaug, A., Evensen, Ø., Eds.; Wiley-Blackwell: New York, NY, USA, 2014; pp. 104–115. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts 2018, 9, 64–79. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Wang, J.; Wang, Q.; Xia, D.; Sun, W.; Zhang, L.; Yu, H.; Liu, Y.; Cao, X. Interferon-γ is an autocrine mediator for dendritic cell maturation. Immunol. Lett. 2004, 94, 141–151. [Google Scholar] [CrossRef]
- He, T.; Tang, C.; Xu, S.; Moyana, T.; Xiang, J. Interferon gamma stimulates cellular maturation of dendritic cell line DC2.4 leading to induction of efficient cytotoxic T cell responses and antitumor immunity. Cell Mol. Immunol. 2007, 2, 105–111. [Google Scholar]
- Wang, J.; Wang, T.; Benedicenti, O.; Collins, C.; Wang, K.; Secombes, C.J.; Zou, J. Characterisation of ZBTB46 and DC-SCRIPT/ZNF366 in rainbow trout, transcription factors potentially involved in dendritic cell maturation and activation in fish. Dev. Comp Immunol. 2018, 80, 2–14. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.Y.; Kumar, S.; Puerta MD, M.; Jo, H.; Rezvan, A. ZBTB46 is a shear-sensitive transcription factor inhibiting endothelial cell proliferation via gene expression regulation of cell cycle proteins. Lab. Investig. 2019, 99, 305–318. [Google Scholar] [CrossRef]
- Cortés, J.; Alvarez, C.; Santana, P.; Torres, E.; Mercado, L. Indoleamine 2,3-dioxygenase: First evidence of expression in rainbow trout (Oncorhynchus mykiss). Dev. Comp Immunol. 2016, 65, 73–78. [Google Scholar] [CrossRef]
- Carril, G.P.; Gómez, F.A.; Marshall, S.H. Expression of flagellin and key regulatory flagellar genes in the non-motile bacterium Piscirickettsia salmonis. Dis. Aquat. Org. 2017, 123, 29–43. [Google Scholar] [CrossRef]
- Maisey, K.; Montero, R.; Corripio-Miyar, Y.; Toro-Ascuy, D.; Valenzuela, B.; Reyes-Cerpa, S.; Sandino, A.M.; Zou, J.; Wang, T.; Secombes, C.J.; et al. Isolation and Characterization of Salmonid CD4+ T Cells. J. Immunol. 2016, 196, 4150–4163. [Google Scholar] [CrossRef]
- Nombela, I.; Requena-Platek, R.; Morales-Lange, B.; Chico, V.; Puente-Marin, S.; Ciordia, S.; Mena, M.C.; Coll, J.; Perez, L.; Mercado, L.; et al. Rainbow Trout Red Blood Cells Exposed to Viral Hemorrhagic Septicemia Virus Up-Regulate Antigen-Processing Mechanisms and MHC I & II, CD86, and CD83 Antigen-presenting Cell Markers. Cells 2019, 8, 386. [Google Scholar] [CrossRef]
- Chico, V.; Gomez, N.; Estepa, A.; Perez, L. Rapid detection and quantitation of viral hemorrhagic septicemia virus in experimentally challenged rainbow trout by real-time RT-PCR. J. Virol. Methods 2006, 132, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Kasheta, M.; Painter, C.A.; Moore, F.E.; Lobbardi, R.; Bryll, A.; Freiman, E.; Stachura, D.; Rogers, A.B.; Houvras, Y.; Langenau, D.M.; et al. Identification and characterization of T reg–like cells in zebrafish. J. Exp. Med. 2017, 214, 3519–3530. [Google Scholar] [CrossRef] [PubMed]
- Aerts-Toegaert, C.; Heirman, C.; Tuyaerts, S.; Corthals, J.; Aerts, J.L.; Bonehill, A.; Thielemans, K.; Breckpot, K. CD83 expression on dendritic cells and T cells: Correlation with effective immune responses. Eur. J. Immunol. 2007, 37, 686–695. [Google Scholar] [CrossRef]
- Prechtel, A.T.; Steinkasserer, A. CD83: An update on functions and prospects of the maturation marker of dendritic cells. Arch. Dermatol. Res. 2007, 299, 59–69. [Google Scholar] [CrossRef]
- Braden, L.M.; Rasmussen, K.J.; Purcell, S.L.; Ellis, L.; Mahony, A.; Cho, S.; Whyte, S.K.; Jones, S.R.M.; Fast, M.D. Acquired protective immunity in Atlantic salmon Salmo salar against the myxozoan Kudoa thyrsites involves induction of MHIIβ+CD83+antigen-presenting cells. Infect. Immun. 2018, 86, e00556-17. [Google Scholar] [CrossRef]
- Grimholt, U. MHC and Evolution in Teleosts. Biology 2016, 5, 6. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front Immunol. 2019, 10, 360. [Google Scholar] [CrossRef]
- Wang, T.; Holland, J.W.; Martin, S.A.M.; Secombes, C.J. Sequence and expression analysis of two T helper master transcription factors, T-bet and GATA3, in rainbow trout Oncorhynchus mykiss and analysis of their expression during bacterial and parasitic infection. Fish Shellfish Immunol. 2010, 29, 705–715. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef]
- Vitry, M.A.; De Trez, C.; Goriely, S.; Dumoutier, L.; Akira, S.; Ryffel, B.; Carlier, Y.; Letesson, J.J.; Muraille, E. Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect. Immun. 2012, 80, 4271–4280. [Google Scholar] [CrossRef]
- Zou, J.; Carrington, A.; Collet, B.; Dijkstra, J.M.; Yoshiura, Y.; Bols, N.; Secombes, C. Identification and Bioactivities of IFN-γ in Rainbow Trout Oncorhynchus mykiss: The First Th1-Type Cytokine Characterized Functionally in Fish. J. Immunol. 2005, 175, 2484–2494. [Google Scholar] [CrossRef]
- Gan, Z.; Chen, S.N.; Huang, B.; Zou, J.; Nie, P. Fish type I and type II interferons: Composition, receptor usage, production and function. Rev. Aquacult. 2020, 12, 773–804. [Google Scholar] [CrossRef]
- Reyes-López, F.E.; Romeo, J.S.; Vallejos-Vidal, E.; Reyes-Cerpa, S.; Sandino, A.M.; Tort, L.; Mackenzie, S.; Imarai, M. Differential immune gene expression profiles in susceptible and resistant full-sibling families of Atlantic salmon (Salmo salar) challenged with infectious pancreatic necrosis virus (IPNV). Dev. Comp Immunol. 2015, 53, 210–221. [Google Scholar] [CrossRef]
- Figueroa, C.; Veloso, P.; Espin, L.; Dixon, B.; Torrealba, D.; Elalfy, I.S.; Afonso, J.M.; Soto, C.; Conejeros, P.; Gallardo, J.A. Host genetic variation explains reduced protection of commercial vaccines against Piscirickettsia salmonis in Atlantic salmon. Sci. Rep. 2020, 10, 18252. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Savelkoul, H.F.J.; Pietretti, D.; Wiegertjes, G.F.; Forlenza, M. Carp Il10 Has Anti-Inflammatory Activities on Phagocytes, Promotes Proliferation of Memory T Cells, and Regulates B Cell Differentiation and Antibody Secretion. J. Immunol. 2014, 194, 187–199. [Google Scholar] [CrossRef]
- Sugimoto, K.; Hui, S.P.; Sheng, D.Z.; Nakayama, M.; Kikuchi, K. Zebrafish FOXP3 is required for the maintenance of immune tolerance. Dev. Comp Immunol. 2017, 73, 156–162. [Google Scholar] [CrossRef]
- Devaud, C.; Darcy, P.K.; Kershaw, M.H. Foxp3 expression in T regulatory cells and other cell lineages. Cancer Immunol. Immunother. 2014, 63, 869–876. [Google Scholar] [CrossRef]
- Vadasz, Z.; Toubi, E. FoxP3 Expression in Macrophages, Cancer, and B Cells—Is It Real? Clin. Rev. Allergy Immunol. 2016, 52, 364–372. [Google Scholar] [CrossRef]
- Jia, C.; Zhou, Y.; Huang, X.; Peng, X.; Liu, L.; Zhou, L.; Jin, L.; Shi, H.; Wei, J.; Wang, D. The cellular protein expression of Foxp3 in lymphoid and non-lymphoid organs of Nile tilapia. Fish Shellfish Immunol. 2015, 45, 300–306. [Google Scholar] [CrossRef]
| Molecule | Type | Source | Dilution | Reference |
|---|---|---|---|---|
| CD4-1 | IgG Polyclonal | Rabbit | 1:200 | [19] |
| CD83 | IgG Polyclonal | Rabbit | 1:100 | [20] |
| IgM | IgG Monoclonal | Mouse | 1:100 | [19] |
| MHC-II | IgG Polyclonal | Mouse | 1:200 | [20] |
| Gene | Primer | Sequence | Tm | Reference (GenBank) |
|---|---|---|---|---|
| t-bet | Forward | GAAGTGAAGGAGGATGGTTCTG | 55 °C | GU979861.1 |
| Reverse | GTGATGTCTGCGTTCTGATAGG | 55 °C | ||
| gata3 | Forward | CGACGATGTGGATGTACTGTTTA | 55 °C | EU418015.1 |
| Reverse | TACTATGTGGAGGAGGTGGATAC | 55 °C | ||
| foxp3 | Forward | AGACTGAGAGTGAGAGGTTGA | 55 °C | HQ270469.1 |
| Reverse | TAGAGCCGAGGTGAGAAAGA | 55 °C | ||
| ef1α | Forward | TGGAGACTGGCACCCTGAAG | 55 °C | AF498320.1 |
| Reverse | CCAACATTGTCACCAGGCATGG | 55 °C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Lange, B.; Nombela, I.; Ortega-Villaizán, M.D.M.; Imarai, M.; Schmitt, P.; Mercado, L. Induction of foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout. Biology 2021, 10, 324. https://doi.org/10.3390/biology10040324
Morales-Lange B, Nombela I, Ortega-Villaizán MDM, Imarai M, Schmitt P, Mercado L. Induction of foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout. Biology. 2021; 10(4):324. https://doi.org/10.3390/biology10040324
Chicago/Turabian StyleMorales-Lange, Byron, Ivan Nombela, María Del Mar Ortega-Villaizán, Mónica Imarai, Paulina Schmitt, and Luis Mercado. 2021. "Induction of foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout" Biology 10, no. 4: 324. https://doi.org/10.3390/biology10040324
APA StyleMorales-Lange, B., Nombela, I., Ortega-Villaizán, M. D. M., Imarai, M., Schmitt, P., & Mercado, L. (2021). Induction of foxp3 during the Crosstalk between Antigen Presenting Like-Cells MHCII+CD83+ and Splenocytes CD4+IgM− in Rainbow Trout. Biology, 10(4), 324. https://doi.org/10.3390/biology10040324







