Assessment of Agreement between Two Difference Prostate-Specific Antigen Assay Modalities
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. PSA Assay Modalities
2.3. Statistical Analysis
2.4. Ethics Statement
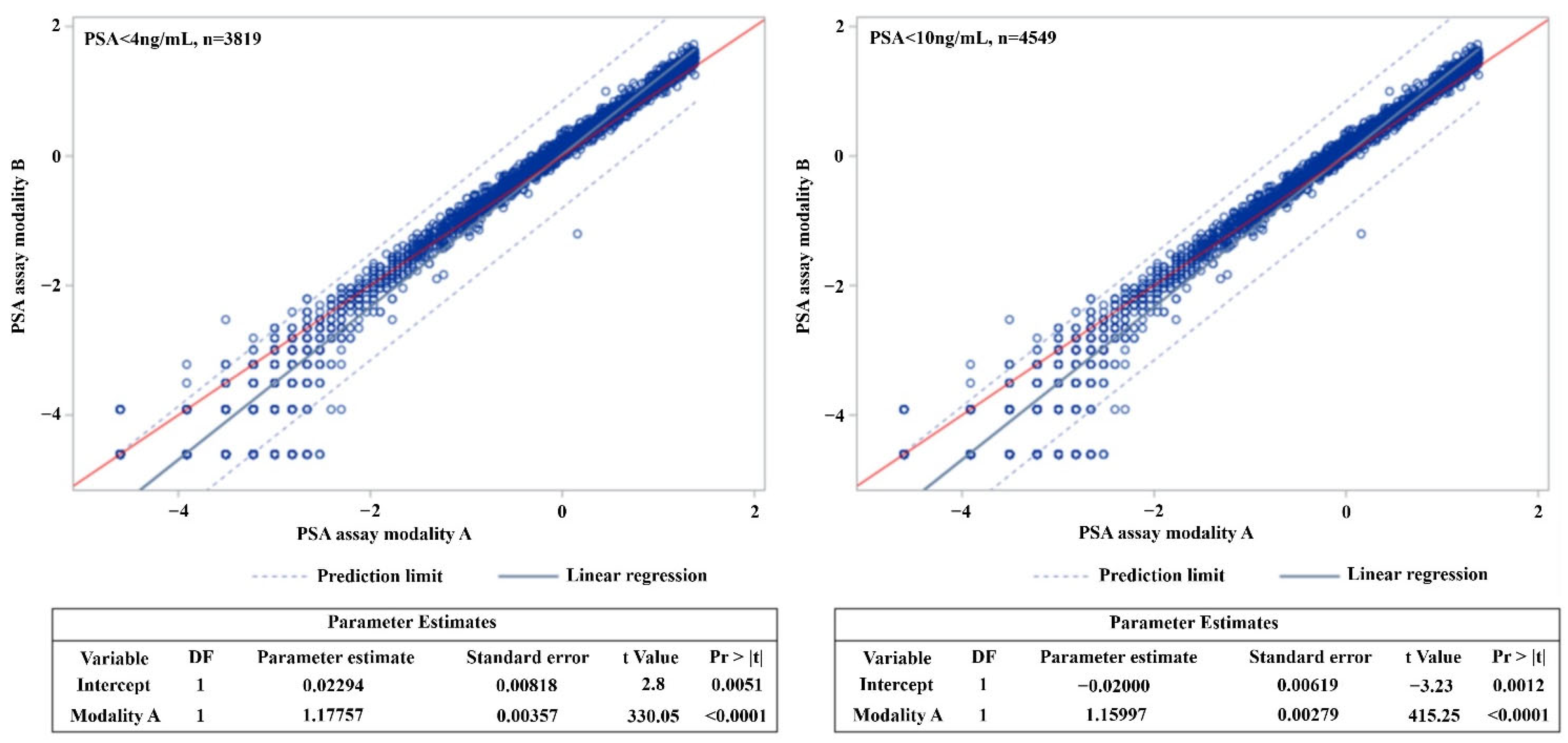
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.C.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Purification of a human prostate specific antigen. Investig. Urol. 1979, 17, 159–163. [Google Scholar]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.E.; Freiha, F.S.; Redwine, E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N. Engl. J. Med. 1991, 324, 1156–1161. [Google Scholar] [CrossRef]
- Andriole, G.L.; Crawford, E.D.; Grubb, R.L., 3rd; Buys, S.S.; Chia, D.; Church, T.R.; Fouad, M.N.; Isaacs, C.; Kvale, P.A.; Reding, D.J.; et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality results after 13 years of follow-up. J. Natl. Cancer Inst. 2012, 104, 125–132. [Google Scholar] [CrossRef]
- Littrup, P.J.; Kane, R.A.; Mettlin, C.J.; Murphy, G.P.; Lee, F.; Toi, A.; Badalament, R.; Babaian, R. Cost-effective prostate cancer detection. Reduction of low-yield biopsies. Investigators of the American Cancer Society National Prostate Cancer Detection Project. Cancer 1994, 74, 3146–3158. [Google Scholar] [CrossRef]
- Catalona, W.J.; Smith, D.S.; Ornstein, D.K. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA 1997, 277, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; van der Cruijsen-Koeter, I.; de Koning, H.J.; Vis, A.N.; Hoedemaeker, R.F.; Kranse, R. Prostate cancer detection at low prostate specific antigen. J. Urol. 2000, 163, 806–812. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, S.S.; Choi, J.D.; Kim, W.; Han, D.H.; Jeong, B.C.; Seo, S.I.; Lee, K.S.; Lee, S.W.; Lee, H.M.; et al. Detection rates of nonpalpable prostate cancer in Korean men with prostate-specific antigen levels between 2.5 and 4.0 ng/mL. Urology 2010, 76, 919–922. [Google Scholar] [CrossRef]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; DeKernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6630 men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef]
- Killian, C.S.; Emrich, L.J.; Vargas, F.P.; Yang, N.; Wang, M.C.; Priore, R.L.; Murphy, G.P.; Chu, T.M. Relative reliability of five serially measured markers for prognosis of progression in prostate cancer. J. Natl. Cancer Inst. 1986, 76, 179–185. [Google Scholar]
- Killian, C.S.; Yang, N.; Emrich, L.J.; Vargas, F.P.; Kuriyama, M.; Wang, M.C.; Slack, N.H.; Papsidero, L.D.; Murphy, G.P.; Chu, T.M. Prognostic importance of prostate-specific antigen for monitoring patients with stages B2 to D1 prostate cancer. Cancer Res. 1985, 45, 886–891. [Google Scholar]
- Stamey, T.A.; Kabalin, J.N. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. I. Untreated patients. J. Urol. 1989, 141, 1070–1075. [Google Scholar] [PubMed]
- Lightner, D.J.; Lange, P.H.; Reddy, P.K.; Moore, L. Prostate specific antigen and local recurrence after radical prostatectomy. J. Urol. 1990, 144, 921–926. [Google Scholar] [CrossRef]
- Arai, Y.; Okubo, K.; Aoki, Y.; Maekawa, S.; Okada, T.; Maeda, H. Ultrasensitive assay of prostate-specific antigen for early detection of residual cancer after radical prostatectomy. Int. J. Urol. 1998, 5, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Ankerst, D.P.; Chi, C.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Feng, Z.; Parnes, H.L.; Coltman, C.A., Jr. Assessing prostate cancer risk: Results from the Prostate Cancer Prevention Trial. J. Natl. Cancer Inst. 2006, 98, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Togo, Y.; Yamamoto, S. Prevention of infectious complications after prostate biopsy procedure. Int. J. Urol. 2017, 24, 486–492. [Google Scholar] [CrossRef]
- Christensson, A.; Bjork, T.; Nilsson, O.; Dahlen, U.; Matikainen, M.T.; Cockett, A.T.; Abrahamsson, P.A.; Lilja, H. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J. Urol. 1993, 150, 100–105. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/mL prostate specific antigen range. J. Urol. 2011, 185, 1650–1655. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Aus, G.; Pihl, C.G.; Becker, C.; Pettersson, K.; Scardino, P.T.; Hugosson, J.; Lilja, H. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: Data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med. 2008, 6, 19. [Google Scholar] [CrossRef]
- Bussemakers, M.J.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar]
- Leyten, G.H.; Hessels, D.; Smit, F.P.; Jannink, S.A.; de Jong, H.; Melchers, W.J.; Cornel, E.B.; de Reijke, T.M.; Vergunst, H.; Kil, P.; et al. Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Clin. Cancer Res. 2015, 21, 3061–3070. [Google Scholar] [CrossRef]
- Donovan, M.J.; Noerholm, M.; Bentink, S.; Belzer, S.; Skog, J.; O’Neill, V.; Cochran, J.S.; Brown, G.A. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015, 18, 370–375. [Google Scholar] [CrossRef]
- Futterer, J.J. Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer. Korean J. Radiol. 2017, 18, 597–606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esen, T.; Kilic, M.; Seymen, H.; Acar, O.; Demirkol, M.O. Can Ga-68 PSMA PET/CT replace conventional imaging modalities for primary lymph node and bone staging of prostate cancer? Eur. Urol. Focus 2020, 6, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Yu, J.; Song, W.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Lee, H.H.; Jeon, S.S. Strategy for prostate cancer patients with low prostate specific antigen level (2.5 to 4.0 ng/mL). J. Korean Med. Sci. 2020, 35, e342. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [PubMed]
- Shariat, S.F.; Karakiewicz, P.I.; Roehrborn, C.G.; Kattan, M.W. An updated catalog of prostate cancer predictive tools. Cancer 2008, 113, 3075–3099. [Google Scholar] [CrossRef]
- Kang, J.J.; Reiter, R.E.; Steinberg, M.L.; King, C.R. First Postprostatectomy Ultrasensitive Prostate-specific Antigen Predicts Survival in Patients with High-risk Prostate Cancer Pathology. Eur. Urol. Oncol. 2018, 1, 378–385. [Google Scholar] [CrossRef]
- Skove, S.L.; Howard, L.E.; Aronson, W.J.; Terris, M.K.; Kane, C.J.; Amling, C.L.; Cooperberg, M.R.; Moreira, D.M.; Freedland, S.J. Timing of Prostate-specific Antigen Nadir After Radical Prostatectomy and Risk of Biochemical Recurrence. Urology 2017, 108, 129–134. [Google Scholar] [CrossRef]
- Kirby, R.; Moul, J.W. Is the PSA test useless? Prostate Cancer Prostatic Dis. 2004, 7, 271–272. [Google Scholar] [CrossRef][Green Version]
| PSA Modality A | N | ICC | 95% CI | p Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| 0.01~0.05 | 1012 | 0.092 | −0.045 | 0.228 | 0.1063 |
| 0.05~0.1 | 377 | 0.281 | −0.085 | 0.558 | 0.0860 |
| 0.1~0.15 | 119 | 0.492 | 0.333 | 0.622 | <0.0001 |
| 0.15~0.2 | 86 | 0.288 | 0.081 | 0.471 | 0.0036 |
| 0.2~0.5 | 382 | 0.862 | 0.705 | 0.923 | <0.0001 |
| 0.5~1.0 | 531 | 0.774 | 0.130 | 0.913 | 0.0103 |
| 1.0~1.5 | 315 | 0.488 | −0.064 | 0.754 | 0.0478 |
| 1.5~2.0 | 249 | 0.360 | −0.095 | 0.660 | 0.0860 |
| 2.0~2.5 | 184 | 0.303 | −0.090 | 0.592 | 0.0868 |
| 2.5~4.0 | 564 | 0.594 | −0.085 | 0.845 | 0.0620 |
| 4.0~ | 991 | 0.999 | 0.999 | 0.999 | <0.0001 |
| Total | 4810 | 0.999 | 0.999 | 0.999 | <0.0001 |
| Modality B | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | G11 | Sum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Modality A | |||||||||||||
| G1 | 1008 (99.6) | 4 (0.4) | 1012 | ||||||||||
| G2 | 241 (63.93) | 126 (33.42) | 10 (2.65) | 377 | |||||||||
| G3 | 2 (1.68) | 34 (28.57) | 71 (59.66) | 12 (10.08) | 119 | ||||||||
| G4 | 3 (3.49) | 16 (18.6) | 51 (59.3) | 16 (18.6) | 86 | ||||||||
| G5 | 12 (3.14) | 314 (82.2) | 56 (14.66) | 382 | |||||||||
| G6 | 6 (1.13) | 402 (75.71) | 123 (23.16) | 531 | |||||||||
| G7 | 1 (0.32) | 1 (0.32) | 231 (73.33) | 80 (25.4) | 2 (0.63) | 315 | |||||||
| G8 | 4 (1.61) | 133 (53.41) | 109 (43.78) | 3 (1.2) | 249 | ||||||||
| G9 | 2 (1.09) | 90 (48.91) | 92 (50) | 184 | |||||||||
| G10 | 378 (67.02) | 186 (32.98) | 564 | ||||||||||
| G11 | 1 (0.1) | 990 (99.9) | 991 | ||||||||||
| Sum | 1251 | 167 | 97 | 75 | 337 | 459 | 215 | 201 | 474 | 1176 | 4810 | ||
| Modality B, PSA Value | Modality A Estimated Value | ||
|---|---|---|---|
| Fitted | 95% CI (Lower, Upper) | ||
| 0.01 | 0.021 | 0.020 | 0.021 |
| 0.02 | 0.037 | 0.037 | 0.038 |
| 0.03 | 0.052 | 0.052 | 0.053 |
| 0.04 | 0.067 | 0.066 | 0.068 |
| 0.05 | 0.080 | 0.079 | 0.081 |
| 0.06 | 0.094 | 0.093 | 0.095 |
| 0.07 | 0.107 | 0.105 | 0.108 |
| 0.08 | 0.119 | 0.118 | 0.121 |
| 0.09 | 0.132 | 0.130 | 0.133 |
| 0.1 | 0.144 | 0.142 | 0.146 |
| 0.11 | 0.156 | 0.154 | 0.158 |
| 0.12 | 0.168 | 0.166 | 0.170 |
| 0.13 | 0.180 | 0.178 | 0.181 |
| 0.14 | 0.191 | 0.189 | 0.193 |
| 0.15 | 0.202 | 0.200 | 0.205 |
| 0.16 | 0.214 | 0.212 | 0.216 |
| 0.17 | 0.225 | 0.223 | 0.227 |
| 0.18 | 0.236 | 0.234 | 0.238 |
| 0.19 | 0.247 | 0.244 | 0.249 |
| 0.2 | 0.258 | 0.255 | 0.260 |
| 0.3 | 0.362 | 0.359 | 0.366 |
| 0.4 | 0.462 | 0.457 | 0.466 |
| 0.5 | 0.557 | 0.551 | 0.562 |
| 0.6 | 0.649 | 0.642 | 0.655 |
| 0.7 | 0.738 | 0.731 | 0.746 |
| 0.8 | 0.826 | 0.818 | 0.834 |
| 0.9 | 0.912 | 0.903 | 0.921 |
| 1.0 | 0.996 | 0.986 | 1.007 |
| 1.1 | 1.079 | 1.068 | 1.091 |
| 1.2 | 1.161 | 1.149 | 1.174 |
| 1.3 | 1.242 | 1.229 | 1.255 |
| 1.4 | 1.322 | 1.307 | 1.336 |
| 1.5 | 1.401 | 1.385 | 1.416 |
| 1.6 | 1.479 | 1.462 | 1.495 |
| 1.7 | 1.556 | 1.538 | 1.574 |
| 1.8 | 1.632 | 1.614 | 1.651 |
| 1.9 | 1.708 | 1.689 | 1.728 |
| 2.0 | 1.784 | 1.763 | 1.804 |
| 2.1 | 1.858 | 1.836 | 1.880 |
| 2.2 | 1.932 | 1.909 | 1.955 |
| 2.3 | 2.006 | 1.982 | 2.030 |
| 2.4 | 2.079 | 2.054 | 2.104 |
| 2.5 | 2.151 | 2.125 | 2.177 |
| 2.6 | 2.223 | 2.196 | 2.251 |
| 2.7 | 2.295 | 2.267 | 2.323 |
| 2.8 | 2.366 | 2.337 | 2.396 |
| 2.9 | 2.437 | 2.406 | 2.467 |
| 3.0 | 2.507 | 2.476 | 2.539 |
| 3.1 | 2.577 | 2.545 | 2.610 |
| 3.2 | 2.647 | 2.613 | 2.681 |
| 3.3 | 2.716 | 2.681 | 2.751 |
| 3.4 | 2.785 | 2.749 | 2.821 |
| 3.5 | 2.854 | 2.817 | 2.891 |
| 3.6 | 2.922 | 2.884 | 2.960 |
| 3.7 | 2.990 | 2.951 | 3.030 |
| 3.8 | 3.058 | 3.018 | 3.098 |
| 3.9 | 3.125 | 3.084 | 3.167 |
| 4.0 | 3.192 | 3.150 | 3.235 |
| 4.5 | 3.524 | 3.477 | 3.573 |
| 5.0 | 3.851 | 3.797 | 3.905 |
| 5.5 | 4.171 | 4.112 | 4.231 |
| 6.0 | 4.488 | 4.423 | 4.553 |
| 6.5 | 4.800 | 4.730 | 4.871 |
| 7.0 | 5.108 | 5.032 | 5.185 |
| 7.5 | 5.413 | 5.331 | 5.496 |
| 8.0 | 5.714 | 5.627 | 5.803 |
| 8.5 | 6.013 | 5.920 | 6.107 |
| 9.0 | 6.309 | 6.210 | 6.409 |
| 9.5 | 6.602 | 6.497 | 6.708 |
| 10.0 | 6.892 | 6.782 | 7.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, J.H.; Park, M.; Cho, H.; Song, W.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Lee, H.M.; et al. Assessment of Agreement between Two Difference Prostate-Specific Antigen Assay Modalities. Biology 2021, 10, 297. https://doi.org/10.3390/biology10040297
Chung JH, Park M, Cho H, Song W, Kang M, Sung HH, Jeon HG, Jeong BC, Seo SI, Lee HM, et al. Assessment of Agreement between Two Difference Prostate-Specific Antigen Assay Modalities. Biology. 2021; 10(4):297. https://doi.org/10.3390/biology10040297
Chicago/Turabian StyleChung, Jae Hoon, Minsu Park, Hyun Cho, Wan Song, Minyong Kang, Hyun Hwan Sung, Hwang Gyun Jeon, Byong Chang Jeong, Seong IL Seo, Hyun Moo Lee, and et al. 2021. "Assessment of Agreement between Two Difference Prostate-Specific Antigen Assay Modalities" Biology 10, no. 4: 297. https://doi.org/10.3390/biology10040297
APA StyleChung, J. H., Park, M., Cho, H., Song, W., Kang, M., Sung, H. H., Jeon, H. G., Jeong, B. C., Seo, S. I., Lee, H. M., & Jeon, S. S. (2021). Assessment of Agreement between Two Difference Prostate-Specific Antigen Assay Modalities. Biology, 10(4), 297. https://doi.org/10.3390/biology10040297






