The Application of Bicarbonate Recovers the Chemical-Physical Properties of Airway Surface Liquid in Cystic Fibrosis Epithelia Models
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Micro-Rheology
2.3. pH Measurement
2.4. Measurement of Fluid Re-Absorption
2.5. Chemicals
2.6. Data Analysis and Statistics
3. Results
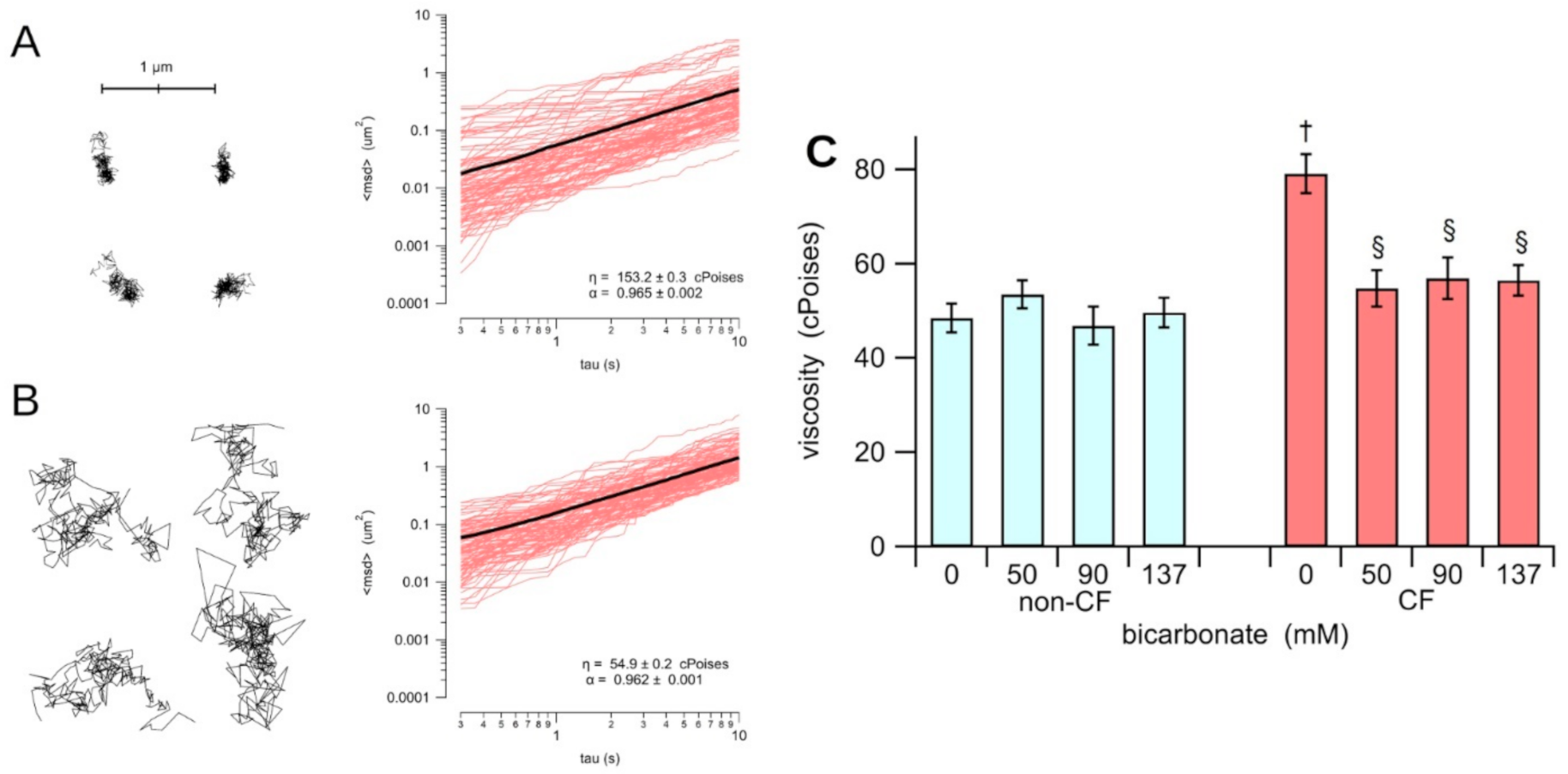
3.1. Rheological Properties
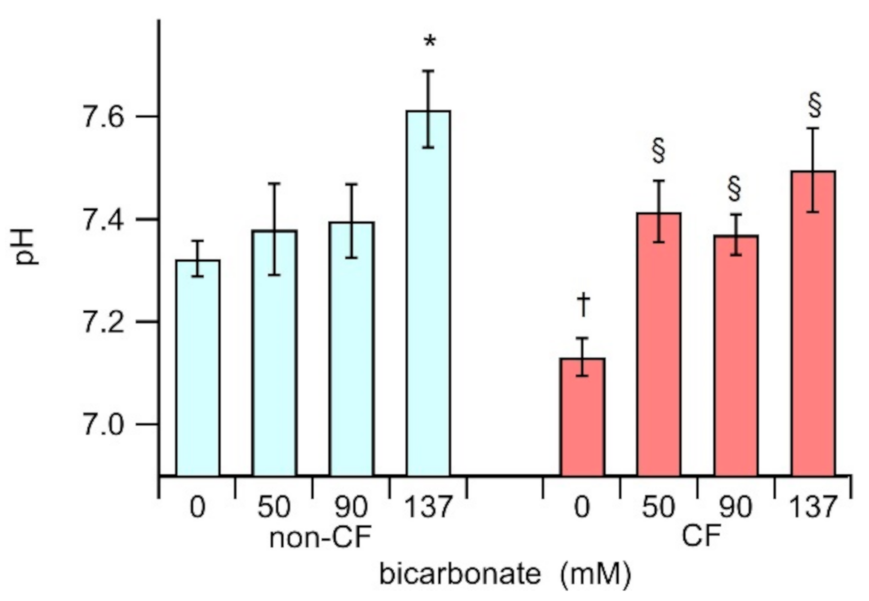
3.2. The ASL pH
3.3. Transepithelial Fluid Re-Absorption
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riordan, J.R. CFTR function and prospects for therapy. Annu. Rev. Biochem. 2008, 77, 701–726. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Assael, B.M. Cystic fibrosis: A clinical view. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.J.; Smith, A.E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993, 73, 1251–1254. [Google Scholar] [CrossRef]
- Farinha, C.M.; Canato, S. From the endoplasmic reticulum to the plasma membrane: Mechanisms of CFTR folding and trafficking. Cell. Mol. Life Sci. 2017, 74, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.C.; Turvey, S.E.; Alves, M.P.; Regamey, N.; Tümmler, B.; Hartl, D. Current concepts: Host-pathogen interactions in cystic fibrosis airways disease. Eur. Respir. Rev. 2014, 23, 320–332. [Google Scholar] [CrossRef]
- Hansson, G.C. Mucus and mucins in diseases of the intestinal and respiratory tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Galietta, L.J.V.; Moran, O. Identification of CFTR activators and inhibitors: Chance or design? Curr. Opin. Pharmacol. 2004, 4, 497–503. [Google Scholar] [CrossRef]
- Zegarra-Moran, O.; Galietta, L.J.V. CFTR pharmacology. Cell. Mol. Life Sci. 2017, 74, 117–128. [Google Scholar] [CrossRef]
- Clancy, J.P.; Rowe, S.M.; Accurso, F.J.; Aitken, M.L.; Amin, R.S.; Ashlock, M.A.; Ballmann, M.; Boyle, M.P.; Bronsveld, I.; Campbell, P.W.; et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012, 67, 12–18. [Google Scholar] [CrossRef]
- Shamsuddin, A.K.M.; Quinton, P.M. Concurrent absorption and secretion of airway surface liquids and bicarbonate secretion in human bronchioles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L953–L960. [Google Scholar] [CrossRef]
- Schultz, A.; Puvvadi, R.; Borisov, S.M.; Shaw, N.C.; Klimant, I.; Berry, L.J.; Montgomery, S.T.; Nguyen, T.; Kreda, S.M.; Kicic, A.; et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat. Commun. 2017, 8, 1409. [Google Scholar] [CrossRef]
- Gianotti, A.; Capurro, V.; Scudieri, P.; Galietta, L.J.; Moran, O.; Zegarra-Moran, O. Pharmacological rescue of mutant CFTR protein improves the viscoelastic properties of CF mucus. J. Cyst. Fibros. 2016, 15, 295–301. [Google Scholar] [CrossRef][Green Version]
- Gustafsson, J.K.; Ermund, A.; Ambort, D.; Johansson, M.E.V.; Nilsson, H.E.; Thorell, K.; Hebert, H.; Sjövall, H.; Hansson, G.C. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012, 209, 1263–1272. [Google Scholar] [CrossRef]
- Yang, N.; Garcia, M.A.S.; Quinton, P.M. Normal mucus formation requires cAMP-dependent HCO3- secretion and Ca2+-mediated mucin exocytosis. J. Physiol. Lond. 2013, 591, 4581–4593. [Google Scholar] [CrossRef]
- Gray, M.A.; O’Reilly, C.; Winpenny, J.; Argent, B. Functional interactions of HCO3- with cystic fibrosis transmembrane conductance regulator. JOP 2001, 2, 207–211. [Google Scholar]
- Ferrera, L.; Baroni, D.; Moran, O. Lumacaftor-rescued F508del-CFTR has a modified bicarbonate permeability. J. Cyst. Fibros. 2019, 18, 602–605. [Google Scholar] [CrossRef]
- Fiore, M.; Picco, C.; Moran, O. Correctors modify the bicarbonate permeability of F508del-CFTR. Sci. Rep. 2020, 10, 8440. [Google Scholar] [CrossRef]
- Dobay, O.; Laub, K.; Stercz, B.; Kéri, A.; Balázs, B.; Tóthpál, A.; Kardos, S.; Jaikumpun, P.; Ruksakiet, K.; Quinton, P.M.; et al. Bicarbonate Inhibits Bacterial Growth and Biofilm Formation of Prevalent Cystic Fibrosis Pathogens. Front. Microbiol. 2018, 9, 2245. [Google Scholar] [CrossRef]
- Wardeh, A.; Conklin, J.; Ko, M. Case reports of observed significant improvement in patients with ARDS due to COVID-19 and maximum ventilatory support after inhalation of sodium bicarbonate. J. Clin. Intensive Care Med. 2020, 5, 016–019. [Google Scholar] [CrossRef]
- Stigliani, M.; Manniello, M.D.; Zegarra-Moran, O.; Galietta, L.; Minicucci, L.; Casciaro, R.; Garofalo, E.; Incarnato, L.; Aquino, R.P.; Del Gaudio, P.; et al. Rheological Properties of Cystic Fibrosis Bronchial Secretion and in Vitro Drug Permeation Study: The Effect of Sodium Bicarbonate. J. Aerosol. Med. Pulm. Drug. Deliv. 2016, 29, 337–345. [Google Scholar] [CrossRef]
- Gróf, I.; Bocsik, A.; Harazin, A.; Santa-Maria, A.R.; Vizsnyiczai, G.; Barna, L.; Kiss, L.; Fűr, G.; Rakonczay, Z., Jr.; Ambrus, R.; et al. The Effect of Sodium Bicarbonate, a Beneficial Adjuvant Molecule in Cystic Fibrosis, on Bronchial Epithelial Cells Expressing a Wild-Type or Mutant CFTR Channel. Int. J. Mol. Sci. 2020, 21, 4024. [Google Scholar] [CrossRef]
- Gomez, C.C.S.; Parazzi, P.L.F.; Clinckspoor, K.J.; Mauch, R.M.; Pessine, F.B.T.; Levy, C.E.; Peixoto, A.O.; Gonçalves Oliveira Ribeiro, M.A.; Ribeiro, A.F.; Conrad, D.; et al. Safety, Tolerability, and Effects of Sodium Bicarbonate Inhalation in Cystic Fibrosis. Clin. Drug. Investig. 2019, 40, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, A.; Delpiano, L.; Caci, E. In vitro Methods for the Development and Analysis of Human Primary Airway Epithelia. Front. Pharmacol. 2018, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Lantero, S.; Gazzolo, A.; Sacco, O.; Romano, L.; Rossi, G.A.; Zegarra-Moran, O. An improved method to obtain highly differentiated monolayers of human bronchial epithelial cells. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, D. Particle-tracking microrheology of living cells: Principles and applications. Annu. Rev. Biophys. 2009, 38, 301–326. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tarran, R.; Trout, L.; Donaldson, S.H.; Boucher, R.C. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J. Gen. Physiol. 2006, 127, 591–604. [Google Scholar] [CrossRef]
- Harvey, P.R.; Tarran, R.; Garoff, S.; Myerburg, M.M. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 592–599. [Google Scholar] [CrossRef]
- Gianotti, A.; Melani, R.; Caci, E.; Sondo, E.; Ravazzolo, R.; Galietta, L.J.V.; Zegarra-Moran, O. Epithelial sodium channel silencing as a strategy to correct the airway surface fluid deficit in cystic fibrosis. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 445–452. [Google Scholar] [CrossRef]
- Gianotti, A.; Capurro, V.; Delpiano, L.; Mielczarek, M.; García-Valverde, M.; Carreira-Barral, I.; Ludovico, A.; Fiore, M.; Baroni, D.; Moran, O.; et al. Small Molecule Anion Carriers Correct Abnormal Airway Surface Liquid Properties in Cystic Fibrosis Airway Epithelia. Int. J. Mol. Sci. 2020, 21, 1488. [Google Scholar] [CrossRef]
- Matsui, H.; Randell, S.H.; Peretti, S.W.; Davis, C.W.; Boucher, R.C. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J. Clin. Investig. 1998, 102, 1125–1131. [Google Scholar] [CrossRef]
- Widdicombe, J.H. Regulation of the depth and composition of airway surface liquid. J. Anat. 2002, 201, 313–318. [Google Scholar] [CrossRef]
- Song, Y.; Thiagarajah, J.; Verkman, A.S. Sodium and Chloride Concentrations, pH, and Depth of Airway Surface Liquid in Distal Airways. J. Gen. Physiol. 2003, 122, 511–519. [Google Scholar] [CrossRef]
- Sondo, E.; Caci, E.; Galietta, L.J.V. The TMEM16A Chloride Channel as an Alternative Therapeutic Target in Cystic Fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 73–76. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Boucher, R.C. Purinergic Receptors in Airway Hydration. Biochem. Pharmacol. 2021, 114387. [Google Scholar] [CrossRef]
- Bertrand, C.A.; Mitra, S.; Mishra, S.K.; Wang, X.; Zhao, Y.; Pilewski, J.M.; Madden, D.R.; Frizzell, R.A. The CFTR Trafficking Mutation F508del Inhibits the Constitutive Activity of SLC26A9. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L912–L925. [Google Scholar] [CrossRef]
- Rose, M.C.; Voynow, J.A. Respiratory Tract Mucin Genes and Mucin Glycoproteins in Health and Disease. Physiol. Rev. 2006, 86, 245–278. [Google Scholar] [CrossRef]
- Boucher, R.C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef]
- Quinton, P.M. Cystic fibrosis: Impaired bicarbonate secretion and mucoviscidosis. Lancet 2008, 372, 415–417. [Google Scholar] [CrossRef]
- Collier, D.M.; Snyder, P.M. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J. Biol. Chem. 2009, 284, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.R.; Luckie, D.B. Dissection of a Mechanistic Controversy in Cystic Fibrosis. JSM Genet. Genom. 2016, 3, 1017. [Google Scholar]
- Cho, D.Y.; Hwang, P.H.; Illek, B.; Fischer, H. Acid and base secretion in freshly excised nasal tissue from cystic fibrosis patients with ΔF508 mutation. Int. Forum Allergy Rhinol. 2011, 1, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.; Toth, L.A.; Kunos, L.; Vasas, S.; Losonczy, G.; Mendes, E.; Wanner, A.; Horvath, G. The Effect of Airway Alkalization by Nebulized Sodium Bicarbonate on Airway Blood Flow. Eur. Respir. J. 2012, 40, P2143. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrera, L.; Capurro, V.; Delpiano, L.; Gianotti, A.; Moran, O. The Application of Bicarbonate Recovers the Chemical-Physical Properties of Airway Surface Liquid in Cystic Fibrosis Epithelia Models. Biology 2021, 10, 278. https://doi.org/10.3390/biology10040278
Ferrera L, Capurro V, Delpiano L, Gianotti A, Moran O. The Application of Bicarbonate Recovers the Chemical-Physical Properties of Airway Surface Liquid in Cystic Fibrosis Epithelia Models. Biology. 2021; 10(4):278. https://doi.org/10.3390/biology10040278
Chicago/Turabian StyleFerrera, Loretta, Valeria Capurro, Livia Delpiano, Ambra Gianotti, and Oscar Moran. 2021. "The Application of Bicarbonate Recovers the Chemical-Physical Properties of Airway Surface Liquid in Cystic Fibrosis Epithelia Models" Biology 10, no. 4: 278. https://doi.org/10.3390/biology10040278
APA StyleFerrera, L., Capurro, V., Delpiano, L., Gianotti, A., & Moran, O. (2021). The Application of Bicarbonate Recovers the Chemical-Physical Properties of Airway Surface Liquid in Cystic Fibrosis Epithelia Models. Biology, 10(4), 278. https://doi.org/10.3390/biology10040278





