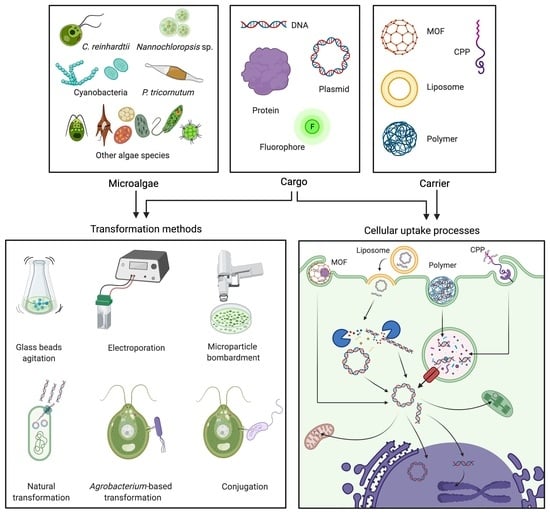
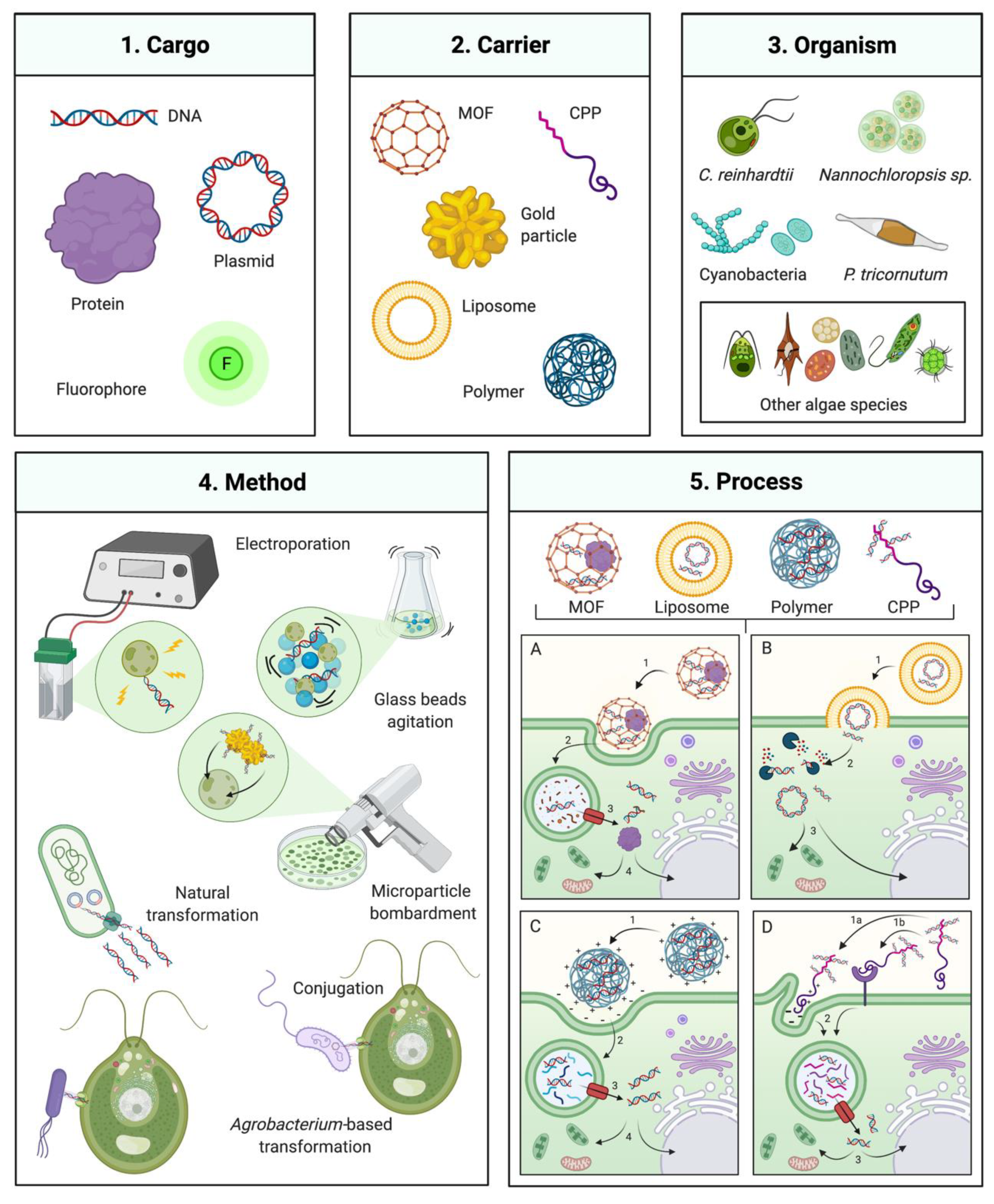
Gene Delivery Technologies with Applications in Microalgal Genetic Engineering
Abstract
Simple Summary
Abstract
1. Introduction
2. Traditional Algal Transformation Techniques
2.1. Agitation of Cells in the Presence of DNA and Non-Ionic Surfactants
2.2. Electroporation
2.3. Microparticle Bombardment
3. Natural Transformation, Bacterial Conjugation, and Agrobacterium-Mediated Transformation
3.1. Natural Transformation
3.2. Bacterial Conjugation
3.3. Agrobacterium-Mediated Transformation
4. Non-Traditional and Emerging Transformation Technologies
4.1. Cell-Penetrating Peptides
4.2. Cell-Penetrating Polymers
4.3. Metal-Organic Frameworks
4.4. Liposome-Mediated Transformation
5. Considerations for the Future of Algal Transformation
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallmann, A. Algal Transgenics and Biotechnology. Transgenic Plant J. 2007, 1, 81–98. [Google Scholar]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; Mccauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Nelson, D.R.; Hazzouri, K.M.; Lauersen, K.J.; Jaiswal, A.; Chaiboonchoe, A.; Mystikou, A.; Fu, W.; Daakour, S.; Dohai, B.; Alzahmi, A.; et al. Large-scale genome sequencing reveals the driving forces of viruses in microalgal evolution. Cell Host Microbe 2021, 29, 250–266.e8. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 1–21. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Wang, Q. Ten years of algal biofuel and bioproducts: Gains and pains. Planta 2019, 249, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chaiboonchoe, A.; Khraiwesh, B.; Nelson, D.R.; Al-Khairy, D.; Mystikou, A.; Alzahmi, A.; Salehi-Ashtiani, K. Algal cell factories: Approaches, applications, and potentials. Mar. Drugs 2016, 14, 225. [Google Scholar] [CrossRef]
- Wannathong, T.; Waterhouse, J.C.; Young, R.E.B.; Economou, C.K.; Purton, S. New tools for chloroplast genetic engineering allow the synthesis of human growth hormone in the green alga Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2016, 100, 5467–5477. [Google Scholar] [CrossRef]
- Coll, J.M. Review. Methodologies for transferring DNA into eukaryotic microalgae. Spanish J. Agric. Res. 2006, 4, 316–330. [Google Scholar] [CrossRef]
- Porter, R.D. Transformation in cyanobacteria. Crit. Rev. Microbiol. 1986, 13, 111–132. [Google Scholar] [CrossRef] [PubMed]
- León, R.; Fernández, E.; Leon, R.; Fernandez, E. Nuclear transformation of eukaryotic microalgae: Historical overview, achievements and problems. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007; Volume 616, pp. 1–11. ISBN 9780387755311. [Google Scholar]
- Klein, R.M.; Wolf, E.D.; Wu, R.; Sanford, J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. 1987. Biotechnology 1992, 24, 384–386. [Google Scholar] [PubMed]
- Kindle, K.L.; Schnell, R.A.; Fernandez, E.; Lefebvre, P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989, 109, 2589–2601. [Google Scholar] [CrossRef]
- Kindle, K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1990, 87, 1228–1232. [Google Scholar] [CrossRef]
- Sodeinde, O.A.; Kindle, K.L. Homologous recombination in the nuclear genome of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1993, 90, 9199–9203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, T.M.; Kopera, H.C.; Saunders, T.L. Principles of Genetic Engineering. Genes 2020, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.L.; Nakamura, M. Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999, 38, 335–341. [Google Scholar] [CrossRef]
- Zienkiewicz, M.; Krupnik, T.; Drożak, A.; Kania, K. PEG-mediated, Stable, Nuclear and Chloroplast Transformation of Cyanidioschizon merolae. Bio-Protocol 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Polle, J.E.W.; Melis, A. Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina. Biochim. Biophys. Acta-Bioenerg. 2001, 1506, 244–259. [Google Scholar] [CrossRef]
- Economou, C.; Wannathong, T.; Szaub, J.; Purton, S. A simple, low-cost method for chloroplast transformation of the green alga Chlamydomonas reinhardtii. Methods Mol. Biol. 2014, 1132, 401–411. [Google Scholar] [CrossRef]
- Kindle, K.L.; Richards, K.L.; Stern, D.B. Engineering the chloroplast genome: Techniques and capabilities for chloroplast transformation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 1991, 88, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, M.J.; Snell, W.J. Biochemical studies on lysin, a cell wall degrading enzyme released during fertilization in Chlamydomonas. Exp. Cell Res. 1988. [Google Scholar] [CrossRef]
- Zorin, B.; Hegemann, P.; Sizova, I. Nuclear-gene targeting by using single-stranded DNA avoids illegitimate DNA integration in Chlamydomonas reinhardtii. Eukaryot. Cell 2005, 4, 1264–1272. [Google Scholar] [CrossRef]
- Rivera, A.L.; Magaña-Ortíz, D.; Gómez-Lim, M.; Fernández, F.; Loske, A.M. Physical methods for genetic transformation of fungi and yeast. Phys. Life Rev. 2014, 11, 184–203. [Google Scholar] [CrossRef]
- Dunahay, T.G. Transformation of Chlamydomonas reinhardtii with silicon carbide whiskers. Biotechniques 1993, 15, 452–460. [Google Scholar] [PubMed]
- Hiramatsu, S.; Ishihara, M.; Fujie, M.; Usami, S. Expression of a chitinase gene and lysis of the host cell wall during Chlorella virus CVK2 infection. Virology 1999, 260, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gurnon, J.R.; Adams, B.J.; Graves, M.V.; Van Etten, J.L. Characterization of a β-1,3-glucanase encoded by Chlorella virus PBCV-1. Virology 2000, 276, 27–36. [Google Scholar] [CrossRef]
- Sugimoto, I.; Hiramatsu, S.; Murakami, D.; Fujie, M.; Usami, S.; Yamada, T. Algal-lytic activities encoded by Chlorella virus CVK2. Virology 2000, 277, 119–126. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, Y.T.; Cho, J.J.; Bae, J.H.; Hur, S.B.; Hwang, I.; Choi, T.J. Stable integration and functional expression of flounder growth hormone gene in transformed microalga, Chlorella ellipsoidea. Mar. Biotechnol. 2002, 4, 63–73. [Google Scholar] [CrossRef]
- Maruyama, M.; Horáková, I.; Honda, H.; Xing, X.H.; Shiragami, N.; Unno, H. Introduction of foreign DNA into Chlorella saccharophila by electroporation. Biotechnol. Tech. 1994, 8, 821–826. [Google Scholar] [CrossRef]
- Jarvis, E.E.; Brown, L.M. Transient expression of firefly luciferase in protoplasts of the green alga Chlorella ellipsoidea. Curr. Genet. 1991, 19, 317–321. [Google Scholar] [CrossRef]
- Shimogawara, K.; Fujiwara, S.; Grossman, A.; Usuda, H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 1998, 148, 1821–1828. [Google Scholar] [CrossRef]
- Brown, L.E.; Sprecher, S.L.; Keller, L.R. Introduction of exogenous DNA into Chlamydomonas reinhardtii by electroporation. Mol. Cell. Biol. 1991, 11, 2328–2332. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation theory. Concepts and mechanisms. Methods Mol. Biol. 1995, 48, 3–28. [Google Scholar] [PubMed]
- Ladygin, V.G. Efficient transformation of mutant cells of Chlamydomonas reinhardtii by electroporation. Process Biochem. 2004, 39, 1685–1691. [Google Scholar] [CrossRef]
- Azencott, H.R.; Peter, G.F.; Prausnitz, M.R. Influence of the Cell Wall on Intracellular Delivery to Algal Cells by Electroporation and Sonication. Ultrasound Med. Biol. 2007, 33, 1805–1817. [Google Scholar] [CrossRef]
- Tang, D.K.H.; Qiao, S.Y.; Wu, M. Insertion mutagenesis of Chlamydomonas reinhardtii by electroporation and heterologous DNA. Biochem. Mol. Biol. Int. 1995, 36, 1025–1035. [Google Scholar] [PubMed]
- Chen, Y.; Hu, H. High efficiency transformation by electroporation of the freshwater alga Nannochloropsis limnetica. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, H. High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar. Genom. 2014, 16, 63–66. [Google Scholar] [CrossRef]
- Holmqvist, M.; Stensjö, K.; Oliveira, P.; Lindberg, P.; Lindblad, P. Characterization of the hupSL promoter activity in Nostoc punctiforme ATCC 29133. BMC Microbiol. 2009, 9. [Google Scholar] [CrossRef]
- Thiel, T.; Poo, H. Transformation of a filamentous cyanobacterium by electroporation. J. Bacteriol. 1989, 171, 5743–5746. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, D.; Hübner, W.; Huser, T.; Mussgnug, J.H.; Kruse, O. Nuclear transformation and functional gene expression in the oleaginous microalga Monoraphidium neglectum. J. Biotechnol. 2017, 249, 10–15. [Google Scholar] [CrossRef]
- Doron, L.; Segal, N.; Shapira, M. Transgene expression in microalgae—from tools to applications. Front. Plant Sci. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Iguchi, H.; Fukuzawa, H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J. Biosci. Bioeng. 2013, 115, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Wen, X.; Chen, Z.; Liang, Q.; Li, J.; Wang, W. Rapid and high efficiency transformation of Chlamydomonas reinhardtii by square-wave electroporation. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Angstenberger, M.; De Signori, F.; Vecchi, V.; Dall’Osto, L.; Bassi, R. Cell Synchronization Enhances Nuclear Transformation and Genome Editing via Cas9 Enabling Homologous Recombination in Chlamydomonas reinhardtii. ACS Synth. Biol. 2020, 9, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Im, D.J.; Jeong, S.-N.; Yoo, B.S.; Kim, B.; Kim, D.-P.; Jeong, W.-J.; Kang, I.S. Digital Microfluidic Approach for Efficient Electroporation with High Productivity: Transgene Expression of Microalgae without Cell Wall Removal. Anal. Chem. 2015, 87, 6592–6599. [Google Scholar] [CrossRef]
- Bodénès, P.; Wang, H.-Y.; Lee, T.-H.; Chen, H.-Y.; Wang, C.-Y. Microfluidic techniques for enhancing biofuel and biorefinery industry based on microalgae. Biotechnol. Biofuels 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Boynton, J.E.; Gillham, N.W.; Harris, E.H.; Hosler, J.P.; Johnson, A.M.; Jones, A.R.; Randolph-Anderson, B.L.; Robertson, D.; Klein, T.M.; Shark, K.B.; et al. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 1988, 240, 1534–1538. [Google Scholar] [CrossRef]
- Day, A.; Debuchy, R.; van Dillewijn, J.; Purton, S.; Rochaix, J.-D. Studies on the maintenance and expression of cloned DNA fragments in the nuclear genome of the green alga Chlamydomonas reinhardtii. Physiol. Plant. 1990, 78, 254–260. [Google Scholar] [CrossRef]
- Remacle, C.; Cardol, P.; Coosemans, N.; Gaisne, M.; Bonnefoy, N. High-efficiency biolistic transformation of Chlamydomonas mitochondria can be used to insert mutations in complex I genes. Proc. Natl. Acad. Sci. USA 2006, 103, 4771–4776. [Google Scholar] [CrossRef]
- Altpeter, F.; Baisakh, N.; Beachy, R.; Bock, R.; Capell, T.; Christou, P.; Daniell, H.; Datta, K.; Datta, S.; Dix, P.J.; et al. Particle bombardment and the genetic enhancement of crops: Myths and realities. Mol. Breed. 2005, 15, 305–327. [Google Scholar] [CrossRef]
- Li, Z.; Bock, R. Replication of bacterial plasmids in the nucleus of the red alga Porphyridium purpureum. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Matamoros, M.F.; Villanueva, M.A.; Islas-Flores, T. Genetic transformation of cell-walled plant and algae cells: Delivering DNA through the cell wall. Brief. Funct. Genom. 2018, 17, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Schiedlmeier, B.; Schmitt, R.; Müller, W.; Kirk, M.M.; Gruber, H.; Mages, W.; Kirk, D.L. Nuclear transformation of Volvox carteri. Proc. Natl. Acad. Sci. USA 1994, 91, 5080–5084. [Google Scholar] [CrossRef]
- Feng, S.; Xue, L.; Liu, H.; Lu, P. Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol. Biol. Rep. 2009, 36, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Apt, K.E.; Grossman, A.R.; Kroth-Pancic, P.G. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. MGG 1996, 252, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Randolph-Anderson, B.; Boynton, J.E.; Dawson, J.; Dunder, E.; Eskes, R.; Gillham, N.W.; Johnson, A.; Perlman, P.S.; Suttie, J.; Heiser, W.C. Sub-Micron Gold Particles are Superior to Larger Particles for Efficient Biolistic Transformation of Organelles and Some Cell Types. 1995. Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_2015.pdf (accessed on 31 January 2021).
- Martin-Ortigosa, S.; Wang, K. Proteolistics: A biolistic method for intracellular delivery of proteins. Transgenic Res. 2014, 23, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Serif, M.; Dubois, G.; Finoux, A.L.; Teste, M.A.; Jallet, D.; Daboussi, F. One-step generation of multiple gene knock-outs in the diatom Phaeodactylum tricornutum by DNA-free genome editing. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, S.P.; Kindle, K.L. Stable nuclear transformation of Chlamydomonas reinhardtii by using a C. reinhardtii by using a C. reinhardtii gene as the selectable marker. Proc. Natl. Acad. Sci. USA 1990, 87, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.M.; Bingham, S.E.; Webber, A.N. A simple method for chloroplast transformation in Chlamydomonas reinhardtii. Methods Mol. Biol. 2011, 684, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.E.; Porter, R.D. Heterospecific transformation among cyanobacteria. J. Bacteriol. 1986, 167, 1074–1076. [Google Scholar] [CrossRef]
- Stucken, K.; Koch, R.; Dagan, T. Cyanobacterial defense mechanisms against foreign DNA transfer and their impact on genetic engineering. Biol. Res. 2013, 46, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, S.V.; Khyen, N.T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. MGG Mol. Gen. Genet. 1970, 107, 372–375. [Google Scholar] [CrossRef]
- Grigorieva, G.; Shestakov, S. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Onai, K.; Morishita, M.; Kaneko, T.; Tabata, S.; Ishiura, M. Natural transformation of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1: A simple and efficient method for gene transfer. Mol. Genet. Genom. 2004, 271, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Dubnau, D. DNA uptake during bacterial transformation. Nat. Rev. Microbiol. 2004, 2, 241–249. [Google Scholar] [CrossRef]
- Williams, J.G.K.; Szalay, A.A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 1983, 24, 37–51. [Google Scholar] [CrossRef]
- Yoshihara, S.; Geng, X.X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73. [Google Scholar] [CrossRef]
- Nakasugi, K.; Svenson, C.J.; Neilan, B.A. The competence gene, comF, from Synechocystis sp. strain PCC 6803 is involved in natural transformation, phototactic motility and piliation. Microbiology 2006, 152, 3623–3631. [Google Scholar] [CrossRef]
- Wendt, K.E.; Pakrasi, H.B. Genomics approaches to deciphering natural transformation in cyanobacteria. Front. Microbiol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Proels, R. Stable Transformation of Cyanobacterium Synechocystis sp. Bio-Protocol 2014, 4. [Google Scholar] [CrossRef]
- Nagarajan, A.; Winter, R.; Eaton-Rye, J.; Burnap, R. A synthetic DNA and fusion PCR approach to the ectopic expression of high levels of the D1 protein of photosystem II in Synechocystis sp. PCC 6803. J. Photochem. Photobiol. B Biol. 2011, 104, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.V.; Martens, S.B.B.; Lanes, C.F.C.; Marins, L.F. Improved genetic transformation of Synechococcus elongatus PCC 7942 using linear DNA fragments in association with a DNase inhibitor. Biotechnol. Res. Innov. 2017, 1, 123–128. [Google Scholar] [CrossRef]
- Vioque, A. Transformation of cyanobacteria. Adv. Exp. Med. Biol. 2007, 616, 12–22. [Google Scholar] [PubMed]
- Karas, B.J.; Diner, R.E.; Lefebvre, S.C.; McQuaid, J.; Phillips, A.P.R.; Noddings, C.M.; Brunson, J.K.; Valas, R.E.; Deerinck, T.J.; Jablanovic, J.; et al. Designer diatom episomes delivered by bacterial conjugation. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Currier, T.C.; Wolk, C.P. Characteristics of Anabaena variabilis influencing plaque formation by cyanophage N-1. J. Bacteriol. 1979. [Google Scholar] [CrossRef]
- Thiel, T.; Peter Wolk, C. Conjugal Transfer of Plasmids to Cyanobacteria. Methods Enzymol. 1987, 153, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Wolk, C.P.; Vonshak, A.; Kehoe, P.; Elhai, J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Isotopenpraxis 1984, 20, 1561–1565. [Google Scholar] [CrossRef]
- Brahamsha, B. A Genetic Manipulation System for Oceanic Cyanobacteria of the Genus Synechococcus. Appl. Environ. Microbiol. 1996, 62, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.C.; Liszt, G.B.; Hess, W.R. Genetic Manipulation of Prochlorococcus Strain MIT9313: Green Fluorescent Protein Expression from an RSF1010 Plasmid and Tn5 Transposition. Appl. Environ. Microbiol. 2006, 72, 7607–7613. [Google Scholar] [CrossRef]
- Marraccini, P.; Bulteau, S.; Cassier-Chauvat, C.; Mermet-Bouvier, P.; Chauvat, F. A conjugative plasmid vector for promoter analysis in several cyanobacteria of the genera Synechococcus and Synechocystis. Plant Mol. Biol. 1993, 23, 905–909. [Google Scholar] [CrossRef]
- Tsinoremas, N.F.; Kutach, A.K.; Strayer, C.A.; Golden, S.S. Efficient gene transfer in Synechococcus sp. strains PCC 7942 and PCC 6301 by interspecies conjugation and chromosomal recombination. J. Bacteriol. 1994, 176, 6764–6768. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.F.; Sturme, M.H.J.; D’Adamo, S.; Weusthuis, R.A.; Wijffels, R.H. Stable transformation of the green algae Acutodesmus obliquus and Neochloris oleoabundans based on E. coli conjugation. Algal Res. 2019, 39, 101453. [Google Scholar] [CrossRef]
- Fabris, M.; George, J.; Kuzhiumparambil, U.; Lawson, C.A.; Jaramillo-Madrid, A.C.; Abbriano, R.M.; Vickers, C.E.; Ralph, P. Extrachromosomal Genetic Engineering of the Marine Diatom Phaeodactylum tricornutum Enables the Heterologous Production of Monoterpenoids. ACS Synth. Biol. 2020. [Google Scholar] [CrossRef]
- Dunahay, T.G.; Jarvis, E.E.; Roessler, P.G. Genetic Transformation of the Diatoms Cyclotella Cryptica and Navicula Saprophila. J. Phycol. 1995, 31, 1004–1012. [Google Scholar] [CrossRef]
- Falciatore, A.; Casotti, R.; Leblanc, C.; Abrescia, C.; Bowler, C. Transformation of Nonselectable Reporter Genes in Marine Diatoms. Mar. Biotechnol. 1999, 1, 239–251. [Google Scholar] [CrossRef]
- Miyagawa, A.; Okami, T.; Kira, N.; Yamaguchi, H.; Ohnishi, K.; Adachi, M. Research note: High efficiency transformation of the diatom Phaeodactylum tricornutum with a promoter from the diatom Cylindrotheca fusiformis. Phycol. Res. 2009, 57, 142–146. [Google Scholar] [CrossRef]
- Diner, R.E.; Bielinski, V.A.; Dupont, C.L.; Allen, A.E.; Weyman, P.D. Refinement of the diatom episome maintenance sequence and improvement of conjugation-based DNA delivery methods. Front. Bioeng. Biotechnol. 2016, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.F.; Wallis, J.G.; Campbell, E.L.; Meeks, J.C. Transposon mutagenesis of Nostoc sp. strain ATCC 29133, a filamentous cyanobacterium with multiple cellular differentiation alternatives. Microbiology 1994, 140, 3233–3240. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nymark, M.; Sparstad, T.; Bones, A.M.; Winge, P. Transgene-free genome editing in marine algae by bacterial conjugation—comparison with biolistic CRISPR/Cas9 transformation. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Poliner, E.; Clark, E.; Cummings, C.; Benning, C.; Farre, E.M. A high-capacity gene stacking toolkit for the oleaginous microalga, Nannochloropsis oceanica CCMP1779. Algal Res. 2020, 45, 101664. [Google Scholar] [CrossRef]
- Poliner, E.; Takeuchi, T.; Du, Z.Y.; Benning, C.; Farre, E.M. Non-transgenic marker-free gene disruption by an episomal CRISPR system in the oleaginous microalga, Nannochloropsis oceanica CCMP1779 Eric. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Tzfira, T.; Citovsky, V. Agrobacterium-mediated genetic transformation of plants: Biology and biotechnology. Curr. Opin. Biotechnol. 2006, 17, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kunik, T. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. USA 2001, 98, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Van Montagu, M. The Ti-plasmid of Agrobacterium tumefaciens, a natural vector for the introduction of nif genes in plants? Basic Life Sci. 1977, 9, 159–179. [Google Scholar] [PubMed]
- Zambryski, P.; Joos, H.; Genetello, C.; Leemans, J.; Van Montagu, M.; Schell, J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983, 2, 2143–2150. [Google Scholar] [CrossRef]
- Hamilton, C.M.; Frary, A.; Lewis, C.; Tanksley, S.D. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl. Acad. Sci. USA 1996, 93, 9975–9979. [Google Scholar] [CrossRef] [PubMed]
- Valvekens, D.; Montagu, M.V.; Lijsebettens, M.V. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 1988, 85, 5536–5540. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Misquitta, R.W.; Reddy, V.S.; Rao, B.J.; Rajam, M.V. Genetic transformation of the green alga - Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci. 2004, 166, 731–738. [Google Scholar] [CrossRef]
- Kathiresan, S.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Agrobacterium-mediated transformation in the green alga Haematococcus pluvialis (chlorophyceae, volvocales). J. Phycol. 2009, 45, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.S.; Yee, W.; Aziz, A. Assessment of factors affecting Agrobacterium-mediated genetic transformation of the unicellular green alga, Chlorella vulgaris. World J. Microbiol. Biotechnol. 2012, 28, 1771–1779. [Google Scholar] [CrossRef]
- Rathod, J.P.; Prakash, G.; Pandit, R.; Lali, A.M. Agrobacterium-mediated transformation of promising oil-bearing marine algae Parachlorella kessleri. Photosynth. Res. 2013, 118, 141–146. [Google Scholar] [CrossRef]
- Anila, N.; Chandrashekar, A.; Ravishankar, G.A.; Sarada, R. Establishment of Agrobacterium tumefaciens -mediated genetic transformation in Dunaliella bardawil. Eur. J. Phycol. 2011, 46, 36–44. [Google Scholar] [CrossRef]
- Pratheesh, P.T.; Vineetha, M.; Kurup, G.M. An efficient protocol for the Agrobacterium-mediated genetic transformation of microalga Chlamydomonas reinhardtii. Mol. Biotechnol. 2014, 56, 507–515. [Google Scholar] [CrossRef]
- Mini, P.; Demurtas, O.C.; Valentini, S.; Pallara, P.; Aprea, G.; Ferrante, P.; Giuliano, G. Agrobacterium-mediated and electroporation-mediated transformation of Chlamydomonas reinhardtii: A comparative study. BMC Biotechnol. 2018, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Lein, W.; Thiyam, G.; Lindenberger, C.P.; Buchholz, R.; Vadakedath, N. Stable nuclear transformation of rhodophyte species Porphyridium purpureum: Advanced molecular tools and an optimized method. Photosynth. Res. 2019, 140, 173–188. [Google Scholar] [CrossRef]
- Michielse, C.B.; Hooykaas, P.J.J.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Curr. Genet. 2005, 48, 1–17. [Google Scholar] [CrossRef]
- Lauersen, K.J. Eukaryotic microalgae as hosts for light-driven heterologous isoprenoid production. Planta 2019, 249, 155–180. [Google Scholar] [CrossRef]
- Zhang, R.; Patena, W.; Armbruster, U.; Gang, S.S.; Blum, S.R.; Jonikas, M.C. High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell 2014, 26, 1398–1409. [Google Scholar] [CrossRef]
- Lin, H.; Cliften, P.F.; Dutcher, S.K. MAPINS, a highly efficient detection method that identifies insertional mutations and complex DNA rearrangements. Plant Physiol. 2018, 178, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Rádis-Baptista, G.; Campelo, I.S.; Morlighem, J.É.R.L.; Melo, L.M.; Freitas, V.J.F. Cell-penetrating peptides (CPPs): From delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. J. Biotechnol. 2017, 252, 15–26. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; EL Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef]
- Margus, H.; Padari, K.; Pooga, M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol. Ther. 2012, 20, 525–533. [Google Scholar] [CrossRef]
- Mishra, V.K.; Anantharamaiah, G.M.; Segrest, J.P.; Palgunachari, M.N.; Chaddha, M.; Sham, S.W.S.; Krishna, N.R. Association of a Model Class A (Apolipoprotein) Amphipathic α Helical Peptide with Lipid. J. Biol. Chem. 2006, 281, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carneado, J.; Kogan, M.J.; Pujals, S.; Giralt, E. Amphipathic Peptides and Drug Delivery. Proc. Biopolym. Pept. Sci. Sect. 2004, 76, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Vidal, P.; Chaloin, L.; Heitz, F.; Divita, G. A new peptide vector for efficient delivery of oligonucleotides into mammalian cells. Nucleic Acids Res. 1997, 25, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Eudes, F. Cellular uptake of cell-penetrating peptides pVEC and transportan in plants. J. Pept. Sci. 2008, 14, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Oehlke, J.; Scheller, A.; Wiesner, B.; Krause, E.; Beyermann, M.; Klauschenz, E.; Melzig, M.; Bienert, M. Cellular uptake of an α-helical amphipathic model peptide with the potential to deliver polar compounds into the cell interior non-endocytically. Biochim. Biophys. Acta-Biomembr. 1998. [Google Scholar] [CrossRef]
- Gräslund, A.; Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar]
- Pooga, M.; Langel, Ü. Classes of cell-penetrating peptides. In Cell-Penetrating Peptides: Methods and Protocols; Springer: New York, NY, USA, 2015; pp. 3–28. ISBN 9781493928064. [Google Scholar]
- Fuselier, T.; Wimley, W.C. Spontaneous Membrane Translocating Peptides: The Role of Leucine-Arginine Consensus Motifs. Biophys. J. 2017, 113, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Rojas, M.; Donahue, J.P.; Tan, Z.; Lin, Y.Z. Genetic engineering of proteins with cell membrane permeability. Nat. Biotechnol. 1998, 16, 370–375. [Google Scholar] [CrossRef]
- Liu, B.R.; Huang, Y.W.; Lee, H.J. Mechanistic studies of intracellular delivery of proteins by cell-penetrating peptides in cyanobacteria. BMC Microbiol. 2013, 13, 57. [Google Scholar] [CrossRef]
- Wei, Y.; Niu, J.; Huan, L.; Huang, A.; He, L.; Wang, G. Cell penetrating peptide can transport dsRNA into microalgae with thin cell walls. Algal Res. 2015, 8, 135–139. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Hyman, J.M.; Geihe, E.I.; Trantow, B.M.; Parvin, B.; Wender, P.A. A molecular method for the delivery of small molecules and proteins across the cell wall of algae using molecular transporters. Proc. Natl. Acad. Sci. USA 2012, 109, 13225–13230. [Google Scholar] [CrossRef] [PubMed]
- Gadamchetty, P.; Mullapudi, P.L.V.; Sanagala, R.; Markandan, M.; Polumetla, A.K. Genetic transformation of Chlorella vulgaris mediated by HIV-TAT peptide. 3 Biotech 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Kim, Y.C. Translocation of cell penetrating peptides on Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2013, 110, 2795–2801. [Google Scholar] [CrossRef]
- Holm, T.; Netzereab, S.; Hansen, M.; Langel, Ü.; Hällbrink, M. Uptake of cell-penetrating peptides in yeasts. FEBS Lett. 2005, 579, 5217–5222. [Google Scholar] [CrossRef] [PubMed]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef]
- Kang, S.; Jeon, S.; Kim, S.; Chang, Y.K.; Kim, Y.C. Development of a pVEC peptide-based ribonucleoprotein (RNP) delivery system for genome editing using CRISPR/Cas9 in Chlamydomonas reinhardtii. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Suresh, A.; Kim, Y.C. A highly efficient cell penetrating peptide pVEC-mediated protein delivery system into microalgae. Algal Res. 2017, 24, 360–367. [Google Scholar] [CrossRef]
- Boussif, O.; LezoualC’H, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Haensler, J.; Szoka, F.C. Polyamidoamine Cascade Polymers Mediate Efficient Transfection of Cells in Culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Klibanov, A.M. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14640–14645. [Google Scholar] [CrossRef] [PubMed]
- Forrest, M.L.; Meister, G.E.; Koerber, J.T.; Pack, D.W. Partial Acetylation of Polyethylenimine Enhances In Vitro Gene Delivery. Pharm. Res. 2004, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Carboni, V.; Maaliki, C.; Alyami, M.; Alsaiari, S.; Khashab, N. Synthetic Vehicles for Encapsulation and Delivery of CRISPR/Cas9 Gene Editing Machinery. Adv. Ther. 2019, 2, 1800085. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef]
- Alsaiari, S.K.; Patil, S.; Alyami, M.; Alamoudi, K.O.; Aleisa, F.A.; Merzaban, J.S.; Li, M.; Khashab, N.M. Endosomal Escape and Delivery of CRISPR/Cas9 Genome Editing Machinery Enabled by Nanoscale Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2018, 140, 143–146. [Google Scholar] [CrossRef]
- Alyami, M.Z.; Alsaiari, S.K.; Li, Y.; Qutub, S.S.; Aleisa, F.A.; Sougrat, R.; Merzaban, J.S.; Khashab, N.M. Cell-Type-Specific CRISPR/Cas9 Delivery by Biomimetic Metal Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 1715–1720. [Google Scholar] [CrossRef]
- Patil, A.J.; Muthusamy, E.; Mann, S. Synthesis and Self-Assembly of Organoclay-Wrapped Biomolecules. Angew. Chemie Int. Ed. 2004, 43, 4928–4933. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.J.; Li, M.; Dujardin, E.; Mann, S. Novel bioinorganic nanostructures based on mesolamellar intercalation or single-molecule wrapping of DNA using organoclay building blocks. Nano Lett. 2007, 7, 2660–2665. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Self-assembly and transformation of hybrid nano-objects and nanostructures under equilibrium and non-equilibrium conditions. Nat. Mater. 2009, 8, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.-C.; Cho, D.-H.; Lee, H.U.; Huh, Y.S.; Kim, G.-J.; Kim, H.-S. A Simple and Non-Invasive Method for Nuclear Transformation of Intact-walled Chlamydomonas reinhardtii. PLoS ONE 2014, 9, e101018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mann, S.; Burkett, S.L.; Davis, S.A.; Fowler, C.E.; Mendelson, N.H.; Sims, S.D.; Walsh, D.; Whilton, N.T. Sol-Gel Synthesis of Organized Matter. Chem. Mater. 1997, 9, 2300–2310. [Google Scholar] [CrossRef]
- Han, H.K.; Lee, Y.C.; Lee, M.Y.; Patil, A.J.; Shin, H.J. Magnesium and calcium organophyllosilicates: Synthesis and in vitro cytotoxicity study. ACS Appl. Mater. Interfaces 2011, 3, 2564–2572. [Google Scholar] [CrossRef]
- Kim, J.; Grate, J.W. Single-Enzyme Nanoparticles Armored by a Nanometer-Scale Organic/Inorganic Network. Nano Lett. 2003, 3, 1219–1222. [Google Scholar] [CrossRef]
- Ichinose, I.; Hashimoto, Y.; Kunitake, T. Wrapping of Bio-macromolecules (Dextran, Amylopectin, and Horse Heart Cytochrome c) with Ultrathin Silicate Layer. Chem. Lett. 2004, 33, 656–657. [Google Scholar] [CrossRef]
- Numata, M.; Li, C.; Bae, A.H.; Kaneko, K.; Sakurai, K.; Shinkai, S. Β-1,3-Glucan Polysaccharide Can Act As a One-Dimensional Host To Create Novel Silica Nanofiber Structures. Chem. Commun. 2005, 4655–4657. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal- organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; Okeeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef]
- Shieh, F.K.; Wang, S.C.; Yen, C.I.; Wu, C.C.; Dutta, S.; Chou, L.Y.; Morabito, J.V.; Hu, P.; Hsu, M.H.; Wu, K.C.W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal-Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Newman, C.; Briggs, S.; Seymour, L.; Sheridan, P.J. Nonviral gene delivery: Techniques and implications for molecular medicine. Expert Rev. Mol. Med. 2003, 5. [Google Scholar] [CrossRef]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hug, P.; Sleight, R.G. Liposomes for the transformation of eukaryotic cells. BBA-Mol. Basis Dis. 1991, 1097, 1–17. [Google Scholar] [CrossRef]
- Gad, A.E.; Rosenberg, N.; Altman, A. Liposome-mediated gene delivery into plant cells. Physiol. Plant. 1990, 79, 177–183. [Google Scholar] [CrossRef]
- Thierry, A.R.; Rabinovich, P.; Peng, B.; Mahan, L.C.; Bryant, J.L.; Gallo, R.C. Characterization of liposome-mediated gene delivery: Expression, stability and pharmacokinetics of plasmid DNA. Gene Ther. 1997, 4, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Ohta, K.; Ichihashi, N. Liposome fragment-mediated introduction of multiple plasmids into Bacillus subtilis. Biochem. Biophys. Reports 2019, 18, 100646. [Google Scholar] [CrossRef] [PubMed]
- Yachi, K.; Harashima, H.; Kikuchi, H.; Sudo, R.; Yamauchi, H.; Ebihara, K.; Matsuo, H.; Funato, K.; Kiwada, H. Biopharmaceutical evaluation of the liposomes prepared by rehydration of freeze-dried empty liposomes (FDELs) with an aqueous solution of a drug. Biopharm. Drug Dispos. 1996, 17, 589–605. [Google Scholar] [CrossRef]
- Mannino, R.J.; Gould-Fogerite, S. Liposome mediated gene transfer. Biotechniques 1988, 6, 682–690. [Google Scholar] [CrossRef]
| Method | Species | Advantage | Disadvantage | Transformation Efficiency (cells/µg DNA) | Initial Cell Concentration (cells/mL) | DNA Added (µg) | Ref. |
|---|---|---|---|---|---|---|---|
| Glass bead agitation and PEG mediated DNA delivery | C. reinhardtii | Simple; inexpensive; fast | Requires cell wall removal/deficiency; occasional genome lesions | 103 | 108 | 2 | [14,15] |
| C. merolae | |||||||
| C. vulgaris | |||||||
| C. ellipsoidea | |||||||
| D. salina | |||||||
| protoplasts | |||||||
| Electroporation | C. reinhardtii | Not affected by cell wall presence; Occasional genome lesions | Specialized equipment | 105 | 108 | 2.5 | [34,37,46,48] |
| M. neglectum | |||||||
| Nannochloropsis sp. | |||||||
| P. tricornutum | |||||||
| Anabaena sp. | |||||||
| N. punctiforme | |||||||
| N. limnetica | |||||||
| Digital microfluidic electroporation (DME) | C. reinhardtii | Not affected by cell wall presence; occasional genome lesions | Specialized equipment | 104 | 106 | 1 | [49] |
| Square electric pulse electroporation | C. reinhardtii | Not affected by cell wall presence; occasional genome lesions | Specialized equipment | 103 | 107 | 0.1 | [47] |
| Microparticle bombardment (gene gun) | C. reinhardtii | Plastid target; not affected by cell wall | Cell viability compromise; specialized equipment | 102 | 105 | 0.1 | [23,51,52,53,59] |
| P. purpureum | |||||||
| D. salina | |||||||
| V. carteri | |||||||
| P. tricornutum | |||||||
| Natural transformation | Anacystis nidulans | Straightforward method for extensive genetic engineering | Limited to some species | 104 | 107 | 5 | [67,68,75] |
| Synechocystis sp. | |||||||
| Synechococcus sp. | |||||||
| T. elongatus | |||||||
| Bacterial conjugation | Anabaena | Low non-target insertions/knockouts; independent episome replication; allows delivery of large DNA fragments | Relies on target species characteristics based on recipient capability to integrate or maintain the vector | 104–106 | 107–109 | 30–50 | [81,82,84,85,86] |
| Nostoc sp. | |||||||
| Prochlorococcus sp. | |||||||
| Synechococcus sp. | |||||||
| Synechocystis sp. | |||||||
| N. punctiforme | |||||||
| P. tricornutum | |||||||
| T. pseudonana | |||||||
| A. obliquus | |||||||
| N. oleoabundans | |||||||
| N. oceanica | |||||||
| Agrobacterium-mediated transformation | C. reinhardtii | Low gene rearrangements; low foreign transcript silencing | Labor-intensive; no higher gene expression reported | 10 | 108 | 30 | [103,109] |
| H. lacustris | |||||||
| Chlorella sp. | |||||||
| D. bardawil | |||||||
| Symbiodinium sp. | |||||||
| Nannochloropsis sp. | |||||||
| P. kessleri | |||||||
| Cell-Penetrating Peptides | Synechocystis sp. | High cargo stability; internalized efficiently | Requires cell wall removal/deficiency; optimized for mammalian cells | 104 | 105–106 | 10–50 | [129,130,132] |
| S. elongatus | |||||||
| C. reinhardtii | |||||||
| C. vulgaris | |||||||
| P. tricornutum | |||||||
| D. salina | |||||||
| N. oleoabundans | |||||||
| S. dimorphus | |||||||
| Botrycoccus braunii | |||||||
| Metal-Organic Frameworks (MOF) | C. reinhardtii | High aqueous stability pH-buffering capacity, versatile | Not yet optimized requires cell wall removal/deficiency | 102 | 106 | 0.7 | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, S.; Lauersen, K.J. Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology 2021, 10, 265. https://doi.org/10.3390/biology10040265
Gutiérrez S, Lauersen KJ. Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology. 2021; 10(4):265. https://doi.org/10.3390/biology10040265
Chicago/Turabian StyleGutiérrez, Sergio, and Kyle J. Lauersen. 2021. "Gene Delivery Technologies with Applications in Microalgal Genetic Engineering" Biology 10, no. 4: 265. https://doi.org/10.3390/biology10040265
APA StyleGutiérrez, S., & Lauersen, K. J. (2021). Gene Delivery Technologies with Applications in Microalgal Genetic Engineering. Biology, 10(4), 265. https://doi.org/10.3390/biology10040265