Biodiscovery of Potential Antibacterial Diagnostic Metabolites from the Endolichenic Fungus Xylaria venustula Using LC–MS-Based Metabolomics
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Test Organisms: The Lichen Usnea and the Isolated Endolichenic Fungi (ELF)
2.2. Extraction of Secondary Metabolites Produced by Lichen Usnea and ELF
2.3. Antibacterial Activities of the Lichen and ELF Crude Extracts
2.4. Selection of Lichen and ELF Crude Extracts for Fractionation Work
2.5. Fractionation and Initial Characterization of Selected Lichen and ELF Crude Extracts
2.6. Metabolomics Profiling Studies
2.7. Multivariate Analyses
3. Results
3.1. Bioactivities of Lichen and ELF Erude Extracts
3.2. Metabolomic Profiles of Lichen and ELF Crude Extracts
3.3. Bioactivities of Lichen and ELF Fractions
3.4. Chemical Profile Differences between Host Lichens and Associated ELF
3.5. Predicting the Bioactive Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosanić, M.; Ranković, B. Lichen secondary metabolites: Bioactive properties and pharmaceutical potential. Lichen Second. Metab. Bioact. Prop. Pharm. Potential 2015, 1–202. [Google Scholar] [CrossRef]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Shrestha, G.; Clair, L.L.S. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013, 12, 229–244. [Google Scholar] [CrossRef]
- Jha, B.N.; Shrestha, M.; Pandey, D.P.; Bhattarai, T.; Bhattarai, H.D.; Paudel, B. Investigation of antioxidant, antimicrobial and toxicity activities of lichens from high altitude regions of Nepal. BMC Complement. Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Goel, M.; Sharmaa, P.K.; Dureja, P.; Rani, A.; Uniyal, P.L. Antifungal activity of extracts of the lichens Parmelia reticulata, Ramalina roesleri, Usnea longissima and stereocaulon himalayense. Arch. Phytopathol. Plant Prot. 2011, 44, 1300–1311. [Google Scholar] [CrossRef]
- Vanga, N.R.; Kota, A.; Sistla, R.; Uppuluri, M. Synthesis and anti-inflammatory activity of novel triazole hybrids of (+)-usnic acid, the major dibenzofuran metabolite of the lichen Usnea longissima. Mol. Divers. 2017, 21, 273–282. [Google Scholar] [CrossRef]
- Leal, A.; Rojas, J.L.; Valencia-Islas, N.A.; Castellanos, L. New β-orcinol depsides from Hypotrachyna caraccensis, a lichen from the páramo ecosystem and their free radical scavenging activity. Nat. Prod. Res. 2018, 32, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.H.; Le Lamer, A.C.; Lalli, C.; Samson, J.B.M.; Dévéhat, F.L.-L.; Le Seyec, J. Depsides: Lichen metabolites active against hepatitis C virus. PLoS ONE 2015, 10, e0120405. [Google Scholar] [CrossRef]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Varol, M. Lichens as a Promising Source of Unique and Functional Small Molecules for Human Health and Well-Being, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 60, ISBN 9780444641816. [Google Scholar]
- Arnold, A.E.; Miadlikowska, J.; Higgins, K.L.; Sarvate, S.D.; Gugger, P.; Way, A.; Hofstetter, V.; Kauff, F.; Lutzoni, F. A phylogenetic estimation of trophic transition networks for ascomycetous Fungi: Are lichens cradles of symbiotrophic Fungal diversification? Syst. Biol. 2009, 58, 283–297. [Google Scholar] [CrossRef] [PubMed]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Arnold, A.E. Community Analysis Reveals Close Affinities Between Endophytic and Endolichenic Fungi in Mosses and Lichens. Microb. Ecol. 2010, 60, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Petrini, O.; Hake, U.; Dreyfuss, M.M. An Analysis of Fungal Communities Isolated from Fruticose Lichens. Mycologia 1990, 82, 444–451. [Google Scholar] [CrossRef]
- Santiago, K.A.A.; dela Cruz, T.E.E.; Ting, A.S.Y. Diversity and bioactivity of endolichenic fungi in Usnea lichens from the Philippines. Czech Mycol. 2020, 73, 1–19. [Google Scholar] [CrossRef]
- Santiago, K.A.A.; dela Cruz, T.E.E.; Ting, A.S.Y. Diversity and bioactivities of culturable endolichenic fungi from three Usnea spp.: A case study in Bukit Larut, Malaysia. Saudi J. Biol. Sci. 2020. under review. [Google Scholar]
- Tripathi, M.; Joshi, Y. Endolichenic Fungi: Present and Future Trends. In Endolichenic Fungi: Present and Future Trends; Springer: Berlin, Germany, 2019; pp. 27–47. ISBN 9789811372681. [Google Scholar]
- Singh, B.N.; Upreti, D.K.; Gupta, V.K.; Dai, X.F.; Jiang, Y. Endolichenic Fungi: A Hidden Reservoir of Next Generation Biopharmaceuticals. Trends Biotechnol. 2017, 35, 808–813. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wijeratne, E.M.K.; Burns, A.M.; Marron, M.T.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.A.L. Heptaketides from Corynespora sp. inhabiting the cavern beard lichen, Usnea cavernosa: First report of metabolites of an endolichenic fungus. J. Nat. Prod. 2007, 70, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Chen, G.D.; Gao, H.; Yang, F.; Li, X.X.; Peng, T.; Guo, L.D.; Yao, X.S. Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 2012, 83, 1087–1091. [Google Scholar] [CrossRef]
- Wang, Q.X.; Bao, L.; Yang, X.L.; Liu, D.L.; Guo, H.; Dai, H.Q.; Song, F.H.; Zhang, L.X.; Guo, L.D.; Li, S.J.; et al. Ophiobolins P-T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia 2013, 90, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Samanthi, K.A.U.; Wickramarachchi, S.; Wijeratne, E.M.K.; Paranagama, P.A. Two new bioactive polyketides from Curvularia trifolii, an endolichenic fungus isolated from Usnea sp., in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2015, 43, 217–224. [Google Scholar] [CrossRef]
- Xie, F.; Chang, W.; Zhang, M.; Li, Y.; Li, W.; Shi, H.; Zheng, S.; Lou, H. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Padhi, S.; Das, D.; Panja, S.; Tayung, K. Molecular Characterization and Antimicrobial Activity of an Endolichenic Fungus, Aspergillus sp. Isolated from Parmelia caperata of Similipal Biosphere Reserve, India. Interdiscip. Sci. Comput. Life Sci. 2016, 9, 237–246. [Google Scholar] [CrossRef]
- Covington, B.C.; McLean, J.A.; Bachmann, B.O. Comparative mass spectrometry-based metabolomics strategies for the investigation of microbial secondary metabolites. Nat. Prod. Rep. 2017, 34, 6–24. [Google Scholar] [CrossRef]
- Nagana Gowda, G.; Raftery, D. Biomarker Discovery and Translation in Metabolomics. Curr. Metabolomics 2013, 1, 227–240. [Google Scholar] [CrossRef]
- Ito, T.; Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. 2014, 67, 353–360. [Google Scholar] [CrossRef]
- Hubert, J.; Nuzillard, J.M.; Renault, J.H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 2017, 16, 55–95. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, A.S. Oleoresins of three Pinus species from Malaysian pine plantations. ASEAN Rev. Biodivers. Environ. Conserv. 1999, 1–9. [Google Scholar] [CrossRef]
- Santiago, K.A.A.; Sangvichien, E.; Boonpragob, K.; dela Cruz, T.E.E. Secondary metabolic profiling and antibacterial activities of different species of Usnea collected in Northern Philippines. Mycosphere 2013, 4, 267–280. [Google Scholar] [CrossRef]
- Wu, W.; Dai, H.; Bao, L.; Ren, B.; Lu, J.; Luo, Y.; Guo, L.; Zhang, L.; Liu, H. Isolation and structural elucidation of proline-containing cyclopentapeptides from an endolichenic Xylaria sp. J. Nat. Prod. 2011, 74, 1303–1308. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Clinical and Laboratory Standards Institute: Pennsylvania, PA, USA, 2012; Volume 32, ISBN 1562387855. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, 1–128. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, L.; Zhang, T.; Viegelmann, C.; Martinez, I.J.; Cheng, C.; Dowdells, C.; Abdelmohsen, U.R.; Gernert, C.; Hentschel, U.; Edrada-Ebel, R.A. Metabolomic tools for secondary metabolite discovery from marine microbial symbionts. Mar. Drugs 2014, 12, 3416–3448. [Google Scholar] [CrossRef] [PubMed]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013. [Google Scholar] [CrossRef]
- Davis, V.W.; Schiller, D.E.; Eurich, D.; Sawyer, M.B. Urinary metabolomic signature of esophageal cancer and Barrett’s esophagus. World J. Surg. Oncol. 2012, 10, 1–12. [Google Scholar] [CrossRef]
- Maciag-Dorszyńska, M.; Wegrzyn, G.; Guzow-Krzemińska, B. Antibacterial activity of lichen secondary metabolite usnic acid is primarily caused by inhibition of RNA and DNA synthesis. FEMS Microbiol. Lett. 2014, 353, 57–62. [Google Scholar] [CrossRef]
- Santiago, K.A.A.; Borricano, J.N.C.; Canal, J.N.; Marcelo, D.M.A.; Perez, M.C.P.; dela Cruz, T.E.E. Antibacterial activities of fruticose lichens collected from selected sites in Luzon Island, Philippines. Philipp. Sci. Lett. 2010, 3, 18–29. [Google Scholar]
- Guo, L.; Shi, Q.; Fang, J.-L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.A.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Heal. Part C 2008, 26, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Fahselt, D. Individuals and populations of lichens. In Lichen Biology; Nash, T., III, Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 252–273. [Google Scholar]
- Padhi, S.; Tayung, K. In vitro antimicrobial potentials of endolichenic fungi isolated from thalli of Parmelia lichen against some human pathogens. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 299–306. [Google Scholar] [CrossRef]
- Nwodo, U.U.; Ngene, A.A.; Iroegbu, C.U.; Obiiyeke, G. Effects of fractionation on antibacterial activity of crude extracts of Tamarindus indica. African J. Biotechnol. 2010, 9, 7108–7113. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Lu, C.; Lin, T.; Hu, P.; Shen, Y. Seven Novel Linear Polyketides from Xylaria sp. NCY2 jackii Chun, collected from Jiangshi Nature Reserve Zone of Fujian Province, China, during November of 2004. This strain was determined to be Xylaria sp. based on its complete ITS1-5. 8S-ITS2 gen. Helv. Chim. Acta 2010, 93, 925–933. [Google Scholar] [CrossRef]
- Elias, L.M.; Fortkamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Brazilian J. Microbiol. 2018, 49, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Yang, X.; Schwartz, M.; Strobel, G.; Clardy, J. The relationship between an endangered North American tree and an endophytic fungus. Chem. Biol. 1995, 2, 721–727. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, Y.; Fukuda, T.; Hagimori, K.; Tomoda, H.; Omura, S. Tensyuic acids, new antibiotics produced by Aspergillus niger FKI-2342. Chem. Pharm. Bull. 2007, 55, 1338–1341. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett. 1977, 18, 61–64. [Google Scholar] [CrossRef]
- Bialonska, D.; Zjawiony, J.K. Aplysinopsins-Marine indole alkaloids: Chemistry, bioactivity and ecological significance. Mar. Drugs 2009, 7, 166–183. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.-W.; Gu, Y. Secondary Metabolites from the South China Sea Invertebrates: Chemistry and Biological Activity. Curr. Med. Chem. 2006, 13, 2041–2090. [Google Scholar] [CrossRef]
- Lambert, P.A.; Smith, A.R.W. The mode of action of N (n Dodecyl)diethanolamine with particular reference to the effect of protonation on uptake by Escherichia coli. J. Gen. Microbiol. 1977, 103, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Reátegui, R.F.; Gloer, J.B.; Campbell, J.; Shearer, C.A. Ophiocerins A-D and ophioceric acid: Tetrahydropyran derivatives and an africane sesquiterpenoid from the freshwater aquatic fungus Ophioceras venezuelense. J. Nat. Prod. 2005, 68, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, Å.M.; Wheelock, C.E. Trials and tribulations of omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef]
- Yu, N.H.; Park, S.-Y.; Kim, J.A.; Park, C.-H.; Jeong, M.-H.; Oh, S.-O.; Hong, S.G.; Talavera, M.; Divakar, P.K.; Hur, J.S. Endophytic and endolichenic fungal diversity in maritime Antarctica based on cultured material and their evolutionary position among Dikarya. Fungal Syst. Evol. 2018, 2, 263–272. [Google Scholar] [CrossRef]
- Dandapat, M.; Paul, S. Secondary metabolites from lichen Usnea longissima and its pharmacological relevance. Pharmacognosy Res. 2019, 11, 103–109. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
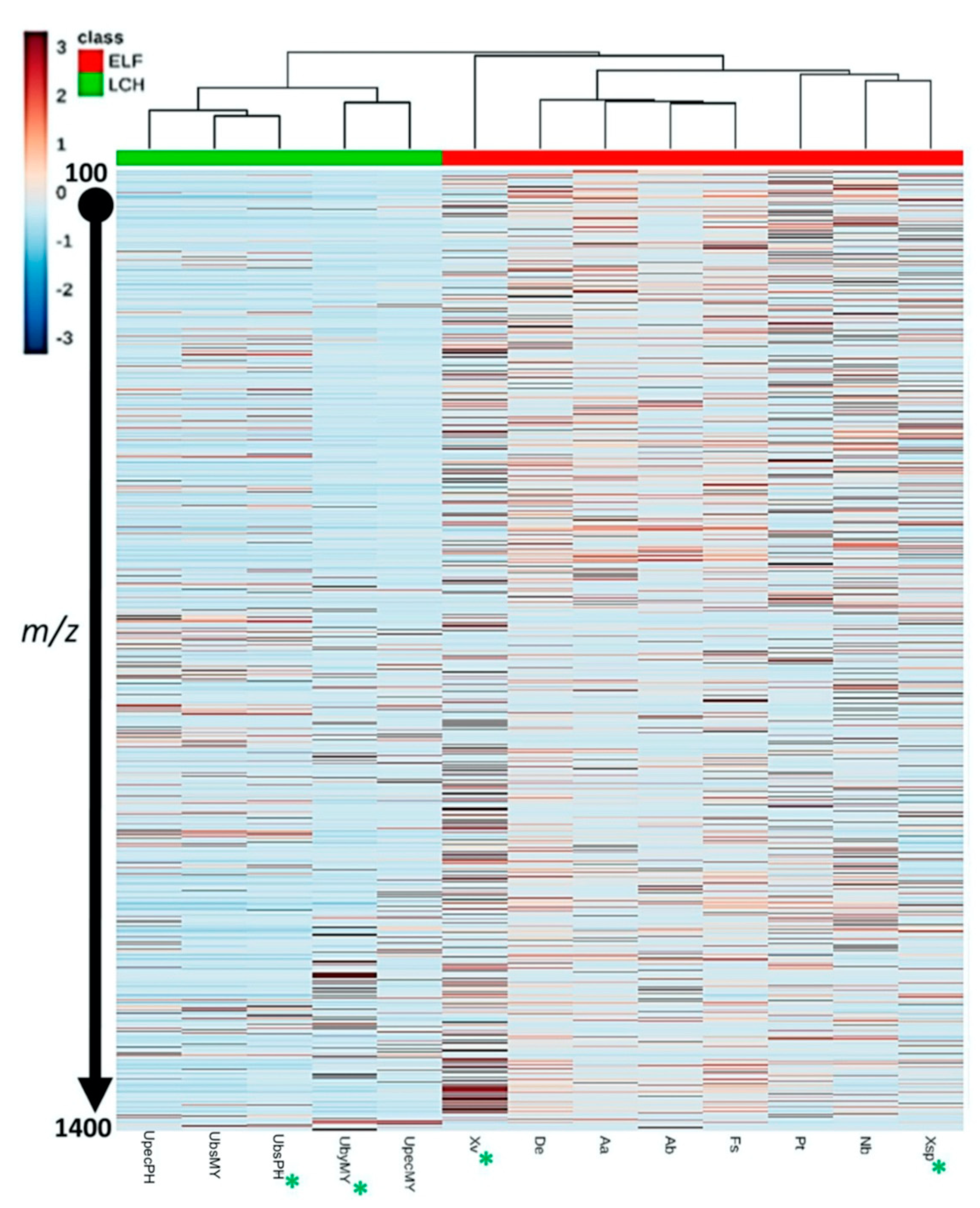

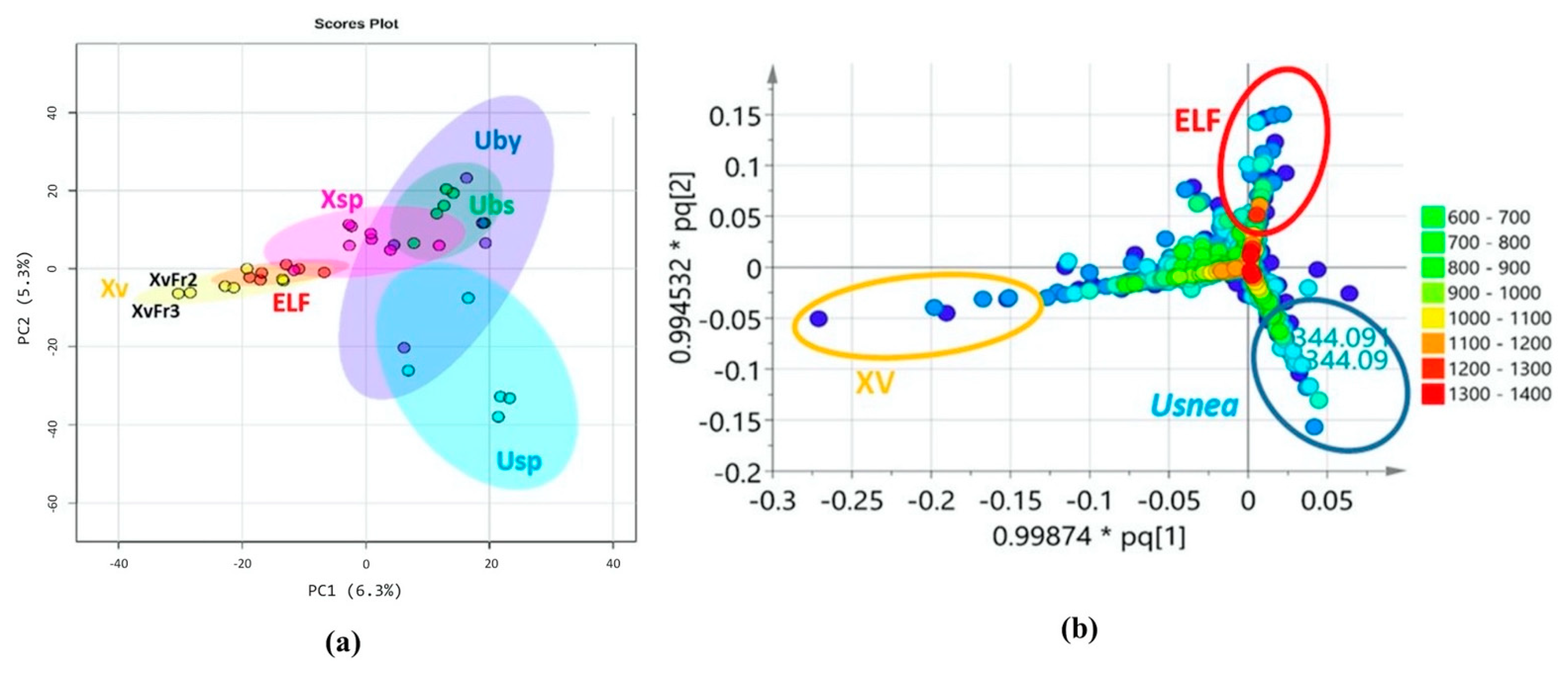
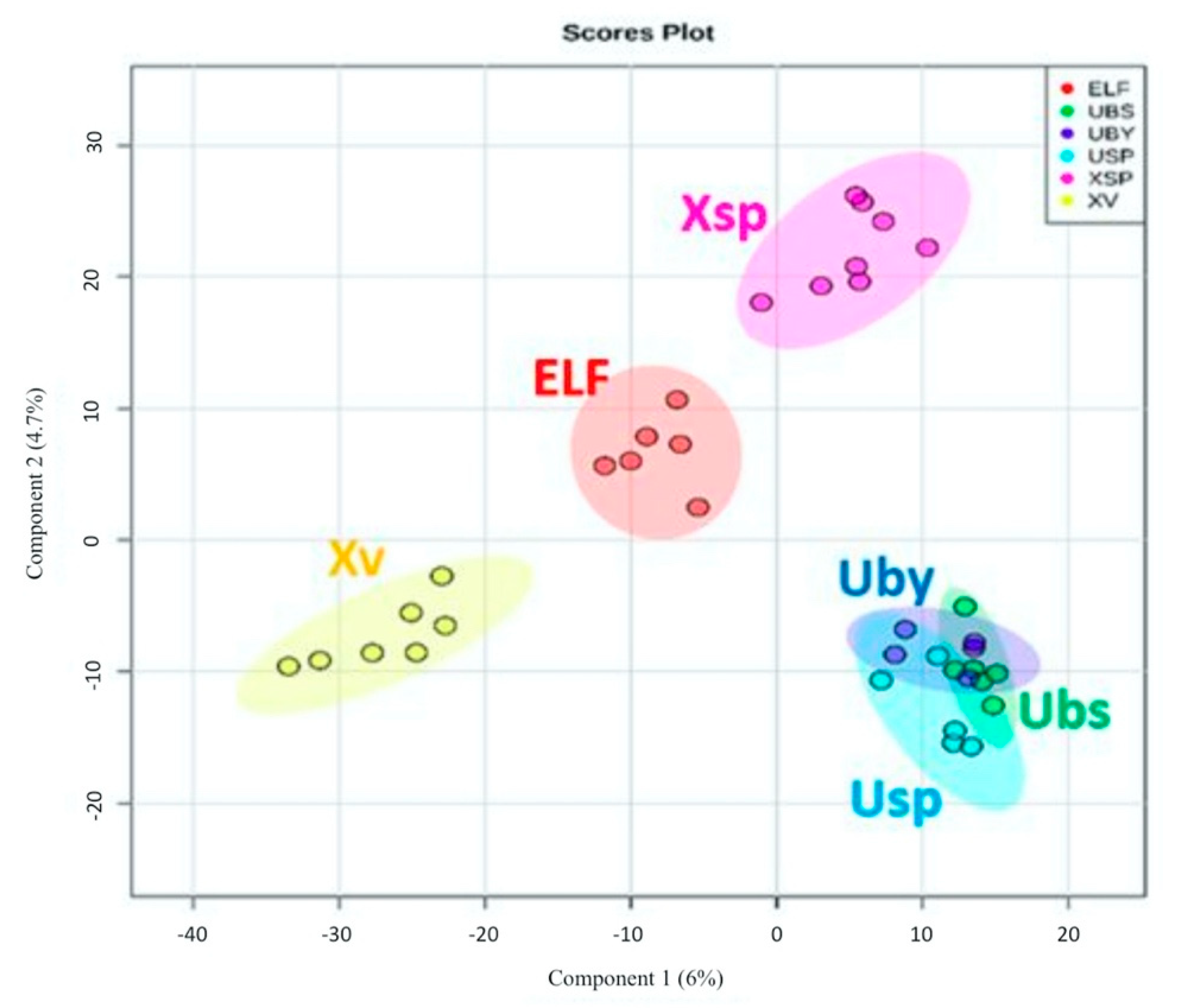
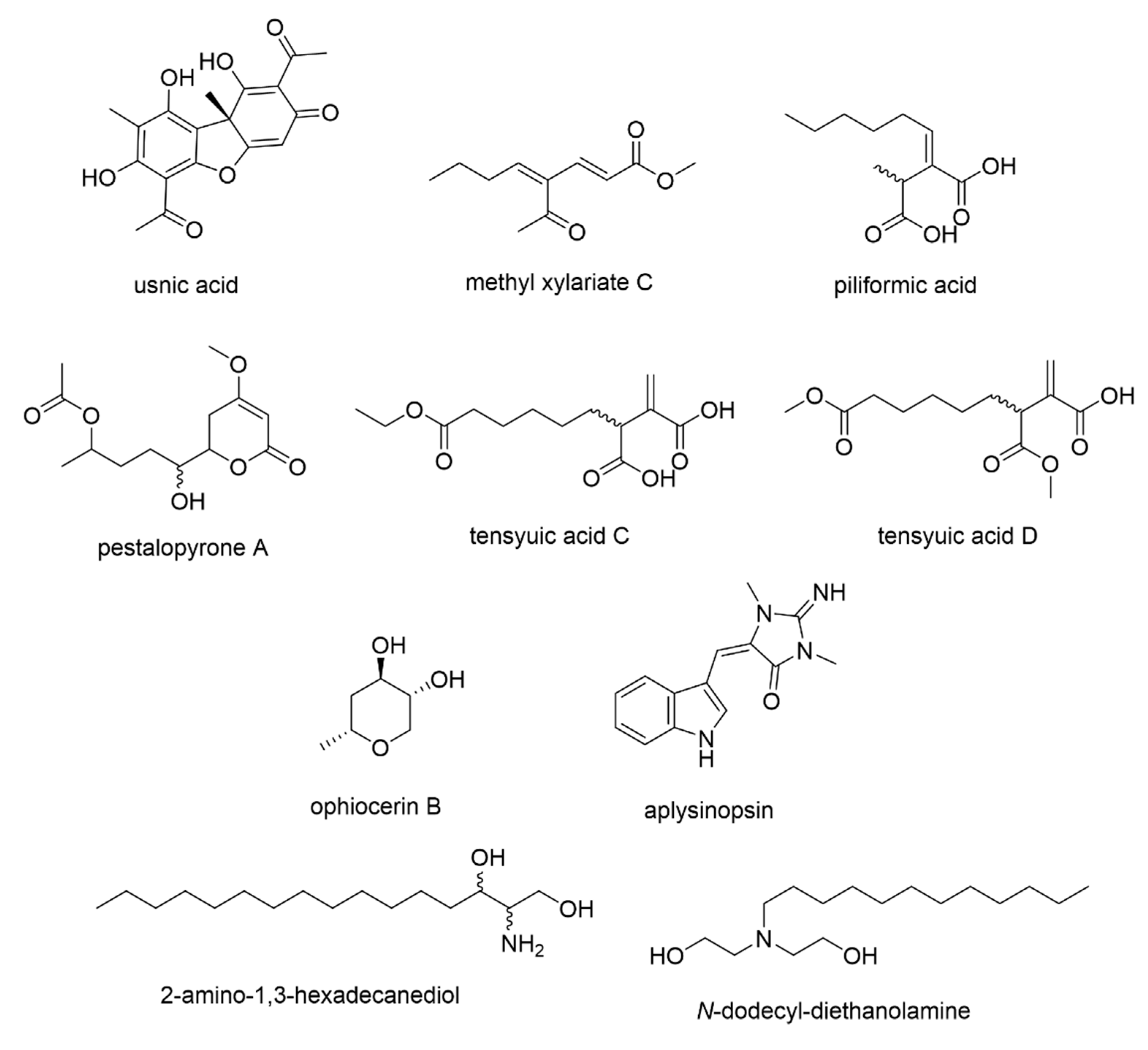

| ELF Species | GenBank Accession Number | Lichen Host | Criterion for Selection | Reference 1 |
|---|---|---|---|---|
| Xylaria sp. | MN071376 | U. bismolliuscula | Abundance | Santiago et al. [15] |
| Fusarium solani (Mart.) Sacc. | MG881825 | U. pectinata | Unique morphology | |
| Pseudopestalotiopsis theae (Sawada) Maharachch., K.D. Hyde and Crous | MG881833 | U. pectinata | ||
| Xylariaceae sp. | MN071378 | U. pectinata | ||
| Astrocystis bambusae (Henn.) Lćssře et Spooner | MH370741 | U. bismolliuscula | Unique morphology | Santiago et al. [14] |
| Annulohypoxylon albidiscum J.F. Zhang, J.K. Liu, K.D. Hyde and Z.Y. Liu | MH370738 | U. pectinata | Short incubation period (fast growth) | |
| Daldinia eschscholtzii (Ehrenb.) Rehm | MN071367 | U. pectinata | ||
| Nemania bipapillata (Berk. and M.A. Curtis) Pouzar | MN071354 | U. baileyi | Abundance | |
| Xylaria venustula Sacc. | MH370742 | Unique morphology |
| ELF Species | Sample Code | Extract Yield 1 (g) | S. aureus ATCC 25923 (mg/mL) | E. coli ATCC 25922 (mg/mL) | References 3 |
|---|---|---|---|---|---|
| Xylaria sp. | Xsp | 3.873 (0.48%) | MIC and MBC: 10 | MIC and MBC: 10 | Santiago et al. [15] |
| Fusarium solani | Fs | 3.196 (0.40%) | MIC and MBC: 10 | No activity | |
| Pseudopestalotiopsis theae | Pt | 1.4909 (0.18%) | MIC and MBC: 10 | MIC and MBC: 10 | |
| Xylariaceae sp. 2 | Xcsp | 2.357 (0.29%) | MIC: 1.25; MBC: 2.5 | No activity | |
| Astrocystis bambusae | Ab | 3.368 (0.42%) | MIC: 10; MBC: >10 | No activity | Santiago et al. [14] |
| Annulohypoxylon albidiscum | Aa | 3.630 (0.45%) | MIC: 2.5; MBC: 5 | No activity | |
| Daldinia eschscholtzii | De | 2.755 (0.34%) | MIC and MBC: 10 | No activity | |
| Nemania bipapillata | Nb | 2.300 (0.29%) | MIC and MBC: 10 | MIC and MBC: 10 | |
| Xylaria venustula | Xv | 9.767 (1.22%) | MIC and MBC: 2.5 | MIC and MBC: 5 |
| MZMine ID 1 | m/z | Retention Time | Molecular Weight | Molecular Formula | Dereplicated Identity | Reported Source 2 | p-Value |
|---|---|---|---|---|---|---|---|
| P_60 | 197.117 | 11.55 | 196.11 | C11H16O3 | methyl xylariate C | Xylaria NCY2 | 0.05 |
| P_1117 | 215.128 | 11.41 | 214.121 | C11H18O4 | piliformic acid | Poronia piliformis, Xylaria longipes, X. polymorpha, X. hypoxylon, X. mali | 0.07 |
| P_844 | 274.274 | 11.74 | 273.267 | C16H35NO2 | 2-amino-1,3-hexadecanediol N-dodecyl-diethabolamine (DDE) | Various sponges marine-derived bacteria | 0.09 |
| N_592 | 195.103 | 13.29 | 196.11 | C11H16O3 | methyl xylariate C | Xylaria NCY2 | 0.11 |
| P_1588 | 273.134 | 10.68 | 272.126 | C13H20O6 | pestalopyrone A tensyuic acid C tensyuic acid D | Pestalotiopsis microspora Aspergillus niger FKI-2342 | 0.11 |
| P_1587 | 255.123 | 10.83 | 254.116 | C14H14N4O | aplysinopsin | various marine invertebrates | 0.11 |
| N_215 | 131.071 | 7.25 | 132.079 | C6H12O3 | ophiocerin B | Ophioceras venezuelense | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago, K.A.A.; Edrada-Ebel, R.; dela Cruz, T.E.E.; Cheow, Y.L.; Ting, A.S.Y. Biodiscovery of Potential Antibacterial Diagnostic Metabolites from the Endolichenic Fungus Xylaria venustula Using LC–MS-Based Metabolomics. Biology 2021, 10, 191. https://doi.org/10.3390/biology10030191
Santiago KAA, Edrada-Ebel R, dela Cruz TEE, Cheow YL, Ting ASY. Biodiscovery of Potential Antibacterial Diagnostic Metabolites from the Endolichenic Fungus Xylaria venustula Using LC–MS-Based Metabolomics. Biology. 2021; 10(3):191. https://doi.org/10.3390/biology10030191
Chicago/Turabian StyleSantiago, Krystle Angelique A., RuAngelie Edrada-Ebel, Thomas Edison E. dela Cruz, Yuen Lin Cheow, and Adeline Su Yien Ting. 2021. "Biodiscovery of Potential Antibacterial Diagnostic Metabolites from the Endolichenic Fungus Xylaria venustula Using LC–MS-Based Metabolomics" Biology 10, no. 3: 191. https://doi.org/10.3390/biology10030191
APA StyleSantiago, K. A. A., Edrada-Ebel, R., dela Cruz, T. E. E., Cheow, Y. L., & Ting, A. S. Y. (2021). Biodiscovery of Potential Antibacterial Diagnostic Metabolites from the Endolichenic Fungus Xylaria venustula Using LC–MS-Based Metabolomics. Biology, 10(3), 191. https://doi.org/10.3390/biology10030191










