Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Treatment
2.4. Clinical Data Collection
2.5. Blood Collection and Handling
2.6. Flow Cytometry
2.7. End-Points
2.8. Statistical Analysis
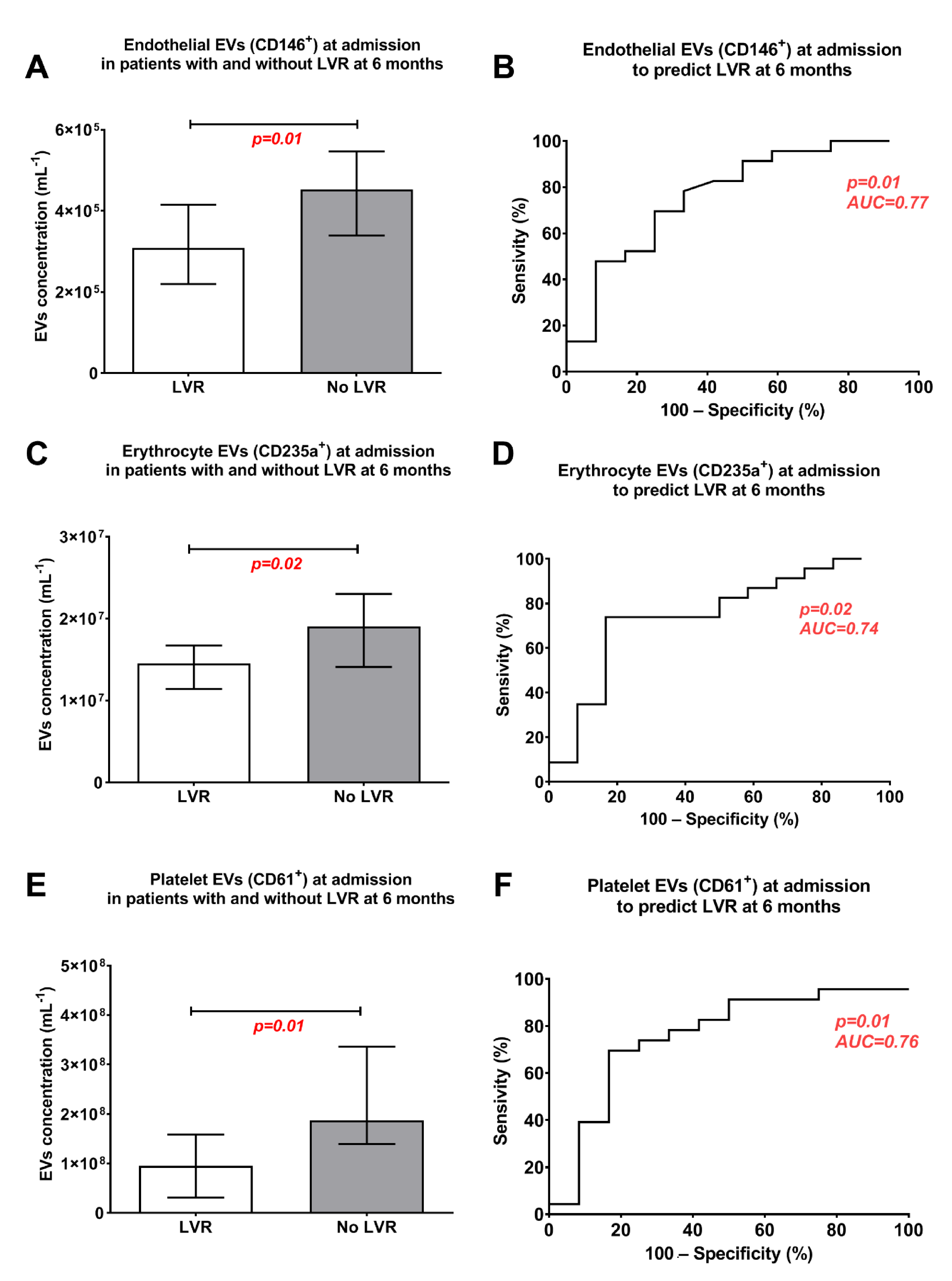
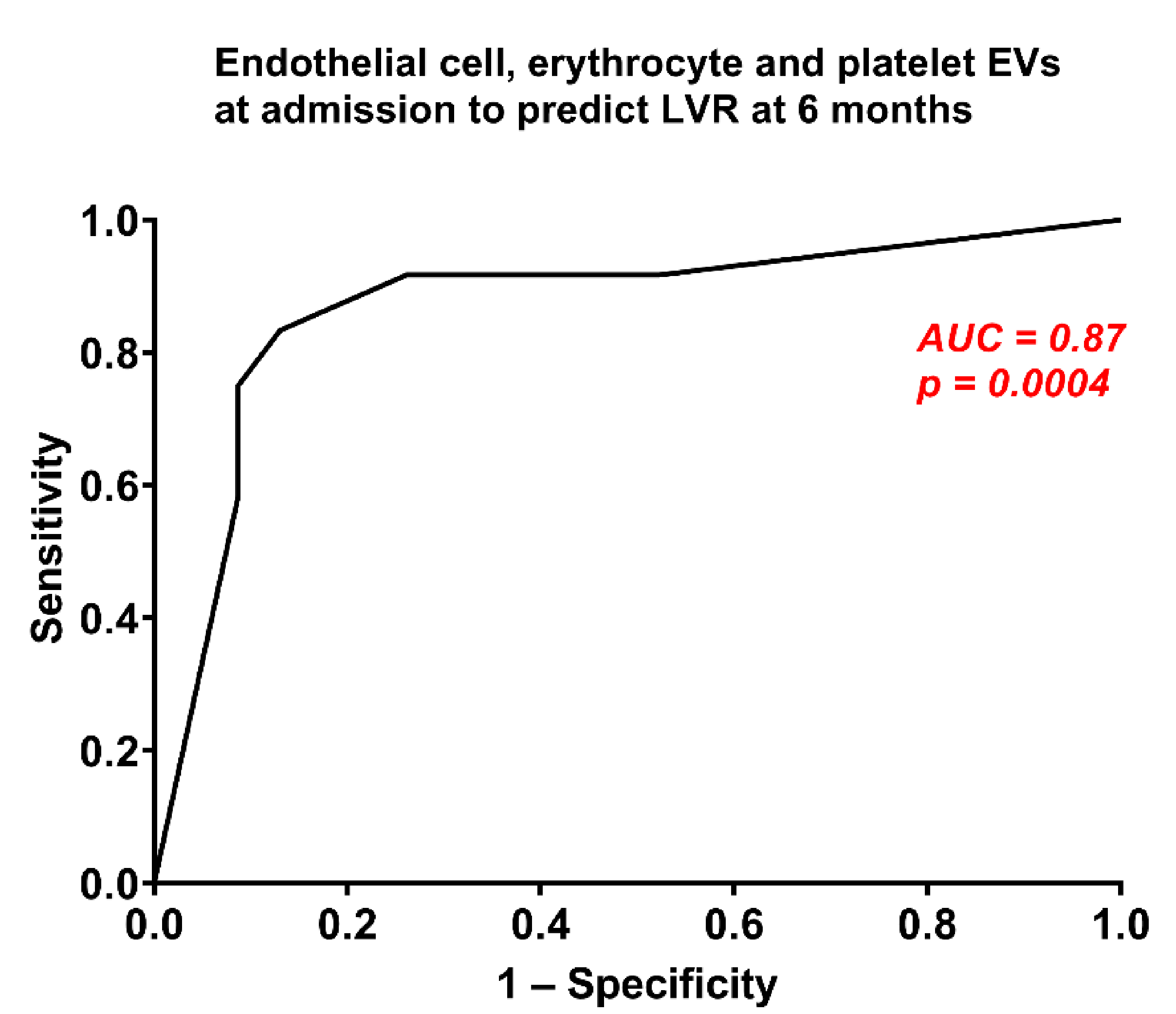
3. Results
Left-Ventricular Remodelling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ab Khan, M.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; Alkatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of Global Mortality and Burden of Disease from 2002 to 2030. PloS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.; Marshall, D.C.; Salciccioli, J.D.; Sikkel, M.B.; Maruthappu, M.; Shalhoub, J. Trends in Mortality from Ischemic Heart Disease and Cerebrovascular Disease in Europe. Circulation 2016, 133, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute myocardial infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- Flachskampf, F.; Schmid, M.; Rost, C.; Achenbach, S.; DeMaria, A.N.; Daniel, W.G. Cardiac imaging after myocardial infarction. Eur. Hear. J. 2010, 32, 272–283. [Google Scholar] [CrossRef]
- Ola, R.K.; Meena, C.B.; Ramakrishnan, S.; Agarwal, A.; Bhargava, S. Detection of Left Ventricular Remodeling in Acute ST Elevation Myocardial Infarction after Primary Percutaneous Coronary Intervention by Two Dimensional and Three Dimensional Echocardiography. J. Cardiovasc. Echogr. 2018, 28, e39. [Google Scholar] [CrossRef]
- Cokkinos, D.; Belogianneas, C. Left Ventricular Remodelling: A Problem in Search of Solutions. Eur. Cardiol. Rev. 2016, 11, 29–35. [Google Scholar] [CrossRef]
- Frigerio, M.; Roubina, E. Drugs for Left Ventricular Remodeling in Heart Failure. Am. J. Cardiol. 2005, 96, 10–18. [Google Scholar] [CrossRef]
- Van Der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, A.; Böing, A.N.; Filipiak, K.J.; Nieuwland, R. Platelet extracellular vesicles as biomarkers for arterial thrombosis. Platelets 2016, 28, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, A.; Nieuwland, R.; Budnik, M.; Dignat-George, F.; Eyileten, C.; Harrison, P.; Huczek, Z.; Kaplon-Cieslicka, A.; Lacroix, R.; Opolski, G.; et al. Randomized controlled trial protocol to investigate the antiplatelet therapy effect on extracellular vesicles (AFFECT EV) in acute myocardial infarction. Platelets 2018, 31, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Nieuwland, R.; Budnik, M.; Dignat-George, F.; Eyileten, C.; Harrison, P.; Lacroix, R.; Leroyer, A.; Opolski, G.; Pluta, K.; et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J. Thromb. Haemost. 2020, 18, 609–623. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST -segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 1–66. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.A.; Hill, A.F.A.F.; et al. Methodological guidelines to study extracellular vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- van der Pol, E.; Van Gemert, M.J.C.; Sturk, A.; Nieuwland, R.; Van Leeuwen, T.G.; Nieuwl, R.; Van Leeuwen, T.G. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J. Thromb. Haemost. 2012, 10, 919–930. [Google Scholar] [CrossRef]
- van der Pol, E.; de Rond, L.; Coumans, F.A.W.; Gool, E.L.; Böing, A.N.; Sturk, A.; Nieuwland, R.; van Leeuwen, T.G. Absolute sizing and label-free identification of extracellular vesicles by flow cytometry. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 801–810. [Google Scholar] [CrossRef]
- de Rond, L.; Libregts, S.F.W.M.; Rikkert, L.G.; Hau, C.M.; van der Pol, E.; Nieuwland, R.; van Leeuwen, T.G.; Coumans, F.A.W. Refractive index to evaluate staining specificity of extracellular vesicles by flow cytometry. J. Extracell. Vesicles 2019, 8, 1643671. [Google Scholar] [CrossRef]
- De Rond, L.; Van Der Pol, E.; Hau, C.M.; Varga, Z.; Sturk, A. Comparison of Generic Fluorescent Markers for Detection of Extracellular Vesicles by Flow Cytometry. Clin. Chem. 2018, 64, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, W.-B.; Chen, Y.; Hu, H. Higher Plasma Concentrations of Platelet Microparticles in Patients with Acute Coronary Syndrome: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2016, 32, e1325. [Google Scholar] [CrossRef] [PubMed]
- Ørn, S.; Manhenke, C.; Anand, I.S.; Squire, I.; Nagel, E.; Edvardsen, T.; Dickstein, K. Effect of Left Ventricular Scar Size, Location, and Transmurality on Left Ventricular Remodeling With Healed Myocardial Infarction. Am. J. Cardiol. 2007, 99, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Hallén, J.; Jensen, J.K.; Fagerland, M.W.; Jaffe, A.S.; Atar, D. Cardiac troponin I for the prediction of functional recovery and left ventricular remodelling following primary percutaneous coronary intervention for ST-elevation myocardial infarction. Heart 2010, 96, 1892–1897. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular endothelial cell-derived vesicles: Emerging role in cardiac and vascular remodeling in heart failure. Front. Cardiovasc. Med. 2020, 7, 47. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Feistritzer, H.-J.; Reindl, M.; Klug, G.; Mayr, A.; Mair, J.; Jaschke, W.; Metzler, B. Combined biomarker testing for the prediction of left ventricular remodelling in ST-elevation myocardial infarction. Open Heart 2016, 3, e000485. [Google Scholar] [CrossRef]
- Fertin, M.; Dubois-Deruy, E.; Belliard, A.; Amouyel, P.; Pinet, F.; Bauters, C. Usefulness of Circulating Biomarkers for the Prediction of Left Ventricular Remodeling After Myocardial Infarction. Am. J. Cardiol. 2012, 110, 277–283. [Google Scholar] [CrossRef]
- Waldenström, A.; Ronquist, G. Role of Exosomes in Myocardial Remodeling. Circ. Res. 2014, 114, 315–324. [Google Scholar] [CrossRef]
- Ailawadi, S.; Wang, X.; Guo-Chang, F.; Fan, G.-C. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1–11. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-Cardiomyocyte Interactions in Cardiac Development and Repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.-M. Platelet-derived extracellular vesicles. Platelets 2019, 401–416. [Google Scholar] [CrossRef]
- Deng, F.; Wang, S.; Zhang, L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: A literature review. J. Cell. Mol. Med. 2017, 21, 1698–1710. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller IV, T.C.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red blood cell function and dysfunction: Redox regulation, nitric oxide metabolism, anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Bhakuni, P.; Chandra, M.; Misra, M.K. Oxidative stress parameters in erythrocytes of post-reperfused patients with myocardial infarction. J. Enzym. Inhib. Med. Chem. 2005, 20, 377–381. [Google Scholar] [CrossRef]
- Shiomi, T.; Tsutsui, H.; Matsusaka, H.; Murakami, K.; Hayashidani, S.; Ikeuchi, M.; Wen, J.; Kubota, T.; Utsumi, H.; Takeshita, A. Overexpression of Glutathione Peroxidase Prevents Left Ventricular Remodeling and Failure After Myocardial Infarction in Mice. Circulation 2004, 109, 544–549. [Google Scholar] [CrossRef]
- Willekens, F.L.A.; Werre, J.M.; Groenen-Döpp, Y.A.M.; Roerdinkholder-Stoelwinder, B.; De Pauw, B.; Bosman, G.J.C.G.M. Erythrocyte vesiculation: A self-protective mechanism? Br. J. Haematol. 2008, 141, 549–556. [Google Scholar] [CrossRef]
- Das, A.; Ziegler, O.; Danielson, K.; Simonson, B.; Lu, S.; Toxavidis, V.; John, T.; Ghiran, I.; Das, S. Role of Red Blood Cell Derived Extracellular Vesicles in Cardiac Remodeling After Myocardial Infarction in a Transgenic Murine Model. Circ. Res. 2017, 121, A414. [Google Scholar]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.-M.; Fang, L.; Jennings, N.L.; Su, Y.; Q, X.; Samson, A.L.; Kiriazis, H.; Wang, X.-F.; Shan, L.; et al. Novel Role of Platelets in Mediating Inflammatory Responses and Ventricular Rupture or Remodeling Following Myocardial Infarction. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 834–841. [Google Scholar] [CrossRef]
- Wan, E.; Yeap, X.Y.; Dehn, S.; Terry, R.L.; Novak, M.L.; Zhang, S.; Iwata, S.; Han, X.; Homma, S.; Drosatos, K.; et al. Enhanced Efferocytosis of Apoptotic Cardiomyocytes Through Myeloid-Epithelial-Reproductive Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair After Infarction. Circ. Res. 2013, 113, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, B.; Trivedi, A.; Togarrati, P.P.; Potter, D.; Baimukanova, G.; Vivona, L.; Lin, M.; Lopez, E.; Callcut, R.; Srivastava, A.K.; et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 86, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Wu, Y.-W.; Blyth, C.; Lichtfuss, G.; Goubran, H.; Burnouf, T. Prospective Therapeutic Applications of Platelet Extracellular Vesicles. Trends Biotechnol. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Li, Y.; Chen, L.; Wang, X.; Guo, W.; Zhang, X.; Qin, G.; He, S.-H.; Zimmerman, A.; et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int. J. Cardiol. 2015, 192, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; Van Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.-M.; Mornet, S.; Brisson, A.R. Extracellular vesicles from blood plasma: Determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]

| Characteristic | LVR (n = 12) | No LVR (n = 43) | p-Value | ||
|---|---|---|---|---|---|
| N | SD, range, % | N | SD, range, % | ||
| Age, years–mean ± SD | 59.5 | 10.3 | 65.6 | 9.3 | 0.06 |
| Male gender–number (%) | 8 | 67 | 32 | 74 | 0.72 |
| BMI–median (IQR) | 28.1 | 25.5–29.9 | 27.9 | 25.4–31.8 | 0.73 |
| Diagnosis at admission–number (%) | |||||
| STEMI | 11 | 92 | 31 | 72 | 0.26 |
| Anterior AMI | 2 | 17 | 8 | 19 | 1.00 |
| Cardiovascular risk factors–number (%) | |||||
| Arterial hypertension | 8 | 67 | 26 | 60 | 0.75 |
| Diabetes mellitus | 3 | 35 | 7 | 16 | 0.67 |
| Dyslipidaemia | 8 | 67 | 29 | 67 | 1.00 |
| Smoking | 5 | 41 | 18 | 41 | 1.00 |
| Laboratory characteristics at baseline | |||||
| Creatinine, mg/dL–median (IQR) | 0.91 | 0.73–1.02 | 0.95 | 0.81–1.05 | 0.39 |
| C-reactive protein–median (IQR) | 3.75 | 1.73–6.08 | 3.00 | 1.7–5.9 | 0.16 |
| Haemoglobin, g/dL–mean ± SD | 14.0 | 1.3 | 13.9 | 1.3 | 0.89 |
| INR–mean ± SD | 1.14 | 0.16 | 1.07 | 0.08 | 0.06 |
| NT-proBNP–median (IQR) | 696 | 386–1936 | 888 | 192–1978 | 0.49 |
| Platelet count, 103/μL–mean (SD) | 262.3 | 75.4 | 235.6 | 69.2 | 0.27 |
| Troponin I, ng/mL–median (IQR) | 16.2 | 5.2–42.6 | 14.7 | 2.5–35.5 | 0.46 |
| Echocardiography at baseline | |||||
| LVEDV, mL–median (IQR) | 104 | 92–120 | 105 | 95–123 | 0.58 |
| LVESV, mL–median (IQR) | 41 | 38–52 | 41 | 40–65 | 0.38 |
| LVEF, mL–median (IQR) | 53 | 47-57 | 51 | 45–54 | 0.43 |
| Echocardiography at 6 months | |||||
| LVEDV, mL–median (IQR) | 107 | 97–126 | 83 | 62–93 | 0.03 |
| LVESV, mL–median (IQR) | 59 | 43–63 | 57 | 27–48 | 0.04 |
| LVEF, mL–median (IQR) | 56 | 50–58 | 60 | 52–63 | 0.10 |
| Pharmacotherapy at discharge–number (%) | |||||
| Aspirin | 12 | 100 | 43 | 100 | 1.00 |
| P2Y12 inhibitor | 12 | 100 | 43 | 100 | 1.00 |
| Atorvastatin | 11 | 92 | 42 | 98 | 0.39 |
| β-blocker | 10 | 83 | 39 | 90 | 0.60 |
| ACE-inhibitor or ARB | 11 | 92 | 41 | 95 | 0.53 |
| Aldosterone receptor antagonist | 3 | 25 | 11 | 26 | 1.00 |
| Proton pump inhibitor | 11 | 92 | 40 | 93 | 1.00 |
| Pharmacotherapy at follow-up–number (%) | |||||
| Aspirin | 12 | 100 | 43 | 100 | 1.00 |
| P2Y12 inhibitor | 12 | 92 | 43 | 100 | 1.00 |
| Atorvastatin | 11 | 92 | 41 | 95 | 0.53 |
| β-blocker | 10 | 83 | 38 | 88 | 0.64 |
| ACE-inhibitor or ARB | 11 | 92 | 42 | 98 | 0.40 |
| Aldosterone receptor antagonist | 4 | 33 | 11 | 26 | 0.72 |
| Proton pump inhibitor | 10 | 83 | 41 | 95 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsecka, A.; Pluta, K.; Solarska, K.; Rydz, B.; Eyileten, C.; Postula, M.; van der Pol, E.; Nieuwland, R.; Budnik, M.; Kochanowski, J.; et al. Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling. Biology 2021, 10, 97. https://doi.org/10.3390/biology10020097
Gąsecka A, Pluta K, Solarska K, Rydz B, Eyileten C, Postula M, van der Pol E, Nieuwland R, Budnik M, Kochanowski J, et al. Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling. Biology. 2021; 10(2):97. https://doi.org/10.3390/biology10020097
Chicago/Turabian StyleGąsecka, Aleksandra, Kinga Pluta, Katarzyna Solarska, Bartłomiej Rydz, Ceren Eyileten, Marek Postula, Edwin van der Pol, Rienk Nieuwland, Monika Budnik, Janusz Kochanowski, and et al. 2021. "Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling" Biology 10, no. 2: 97. https://doi.org/10.3390/biology10020097
APA StyleGąsecka, A., Pluta, K., Solarska, K., Rydz, B., Eyileten, C., Postula, M., van der Pol, E., Nieuwland, R., Budnik, M., Kochanowski, J., Jaguszewski, M. J., Szarpak, Ł., Mazurek, T., Kapłon-Cieślicka, A., Opolski, G., & Filipiak, K. J. (2021). Plasma Concentrations of Extracellular Vesicles Are Decreased in Patients with Post-Infarct Cardiac Remodelling. Biology, 10(2), 97. https://doi.org/10.3390/biology10020097










