Simple Summary
The diagnosis of Anisakiasis is documented by the occasional finding of L3 larvae in the infected gastro-intestinal tract. Currently, about 14 allergens have been described, among which Ani s1 and Ani s4, both highly heat-resistant, appear central in Anisakiasis anaphylaxis and necessary to cause allergic reactions. Food has to be considered Anisakis-free only when heat-resistant Anisakis allergens are not present.
Abstract
Background: Anisakis simplex (A. simplex) infection, in humans, causes a series of clinical manifestations affecting the gastro-intestinal tract known as Anisakiasis/Anisakidosis. Patients may also present allergic manifestations such as hives and/or angioedema and even anaphylactic shock. The aim of this study was to investigate whether aquacultured fish could be considered A. simplex-free food and constitute a safe, alternative, wild-capture fish food for Gastro-Allergic Anisakiasis (GAA)-sensitized subjects. Methods: Protein extracts from A. simplex larvae in the third stage (L3) and from edible part of heavily infected horse mackerel (Trachurus trachurus) and aquacultured sea bream, have been tested for A. simplex allergens presence by immunological analysis. Western blot analysis using, as source of specific Anisakis allergens antibodies, serum samples from subjects referring allergic symptoms after raw fish ingestion, was performed. These subjects showed high levels of specific IgE anti A. simplex allergens determined by clinical laboratory tests (ISAC test). Results: Our data demonstrate the presence of Ani s4 allergen in both infected and aquacultured fish extracts, providing a possible interpretation for the allergic manifestations reported by subjects, already sensitized to A. simplex, who ate frozen or well-cooked or, even, aquacultured fish. Conclusions: The present data stimulate more accurate prophylaxis suggestions for Anisakis allergy and more specific controls of fishmeal used in aquaculture.
1. Introduction
There are numerous zoonotic diseases in humans that can be transmitted by ingestion of parasite-infected foods. Among the parasites responsible for indirectly transmitted zoonoses are nematodes which, found in a wide range of marine organisms, play a decisive role, in this context, especially for those populations accustomed to consuming marine products [1,2,3,4,5,6]. At the same time, the changes in food tastes that have characterized humans’ eating habits in recent years have increasingly directed consumption towards fresh and natural products, and the introduction of habits and culinary specialties from different countries has also led to an increase in the consumption of raw fish products [4].
Anisakis simplex (A. simplex) is a nematode which, at its third-stage larvae (L3), can infect humans [7] eating raw, undercooked and even smoked, salted or in brine parasitized fish (cod, tuna, sardines, anchovies, salmon etc.) or cephalopodos [8]. Parasite specimens have been detected also in a number of uncommon hosts including Grey petrel, Procellaria cinerea; Little penguin, Eudyptula minor; Blue-lipped sea krait, Laticauda laticaudata and Spinner shark, Carcharhinus brevipinna [9] as well Delphinus delphis, Tursiops truntatus and Kogia sima [10].
A. simplex infection causes a series of clinical manifestations affecting the gastro-intestinal tract known as Anisakiasis/Anisakidosis; A. simplex-infected patients may have, in addition to abdominal symptoms, allergic manifestations such as urticaria and/or angioedema and even anaphylactic shock [1]. Reports are increasingly numerous claiming the appearance of allergic symptoms, after ingestion of fish, such as canned fish, in subjects previously A. simplex-infected [5,6]. This indicates that the prophylaxis currently suggested, fish cooked at 60 °C for 10′ or frozen at −20 °C, for at least 24 hr, might not be sufficient to avoid the allergic effects caused by infested fish ingestion, probably due to the thermal-resistance of A. simplex allergens [5]. Indeed, it is known that several A. simplex allergens are heat stable, supporting the hypothesis that cooking or freezing procedures may not protect humans against allergic reactions as observed when the parasite is subjected to high temperature conditions or to the thermal procedures adopted for marine food preparation [11].
Furthermore, much debated is the question of whether, for the induction of allergic manifestations, the viable L3 larvae ingestion is necessary [12] or if just the exposure to parasite allergens can trigger adverse reactions even when L3 larvae are killed by freezing and/or cooking the fish [13]. Some A. simplex allergens show to be relatively resistant to enzyme digestion or heat treatment: Ani s1 [14]; cystatin Ani s4 [15] and allergens belonging to the SXP/Ral family such as Ani s5 [16], Ani s8 [17] and Ani s9 [18]. These proteins could account for the occurrence of hypersensitivity reactions to fishery products contaminated by A. simplex proteins. The accidental presence of these allergens in food would recommend to A. simplex-sensitized patients to avoid the use of fishery products resulting from (i) losing the beneficial nutritional effects (ω-3 lipids and proteins with high biological effect) [19,20] and (ii) causing negative commercial relevance to high income populations through marine products. Two possibilities seem to circumvent such a problem: (i) removal of A. simplex allergens from infected fish or (ii) eating A. simplex allergens-free fish. Olivares et al. reported that in order to remove A. simplex allergens from infected fish, for example in the preparation of surimi following the presence of the heat resistant allergen Ani s4, several washing steps with water and strong buffers are required, making this process impractical [21]. Therefore, the removal of A. simplex allergens from marine products not being convenient or safe, for previously sensitized patients by L3 A. simplex allergens, eating A. simplex allergens-free aquacultured fish seems to be the only suitable way to avoid the allergic manifestations caused by them [4,22]. On the other hand, as stated by Fæste et al., the detection of immunoreactive Anisakis peptides in the tissue of zebrafish exposed to high amounts of A. simplex in the feed can be regarded as a proof-of-principle that allergenic peptides may be transferred from animal feed into the final food products [22].
The growth of the fish aquaculture industry has outpaced production of wild-capture fisheries for over 2 decades, currently producing nearly 50% of all seafood consumed globally. As wild-capture fisheries continue to decline, aquaculture’s role in food production will grow, and it will produce an estimated 62% of all seafood consumed in 2020 [20,21]. The feeding of fish in aquaculture usually consists of flours obtained by adding fishmeal in order to increase the efficiency and growth of the animals [23]. The balanced amino acid composition of fishmeal integrates and quickly promotes growth and reduces feeding costs. In addition, fishmeal provides a balanced amount of all essential amino acids, phospholipids and fatty acids (docosahecsenic acid-DHA and eicosapentaenoic acid-EPA) [23].
Previous studies reported a minor presence of Anisakide parasites in farmed fish, suggesting that consumption of these fish species carries almost no risk of exposure to these nematodes [24,25,26,27]. The aim of the present study was to evaluate the presence of heat-resistant allergens in aquacultured fish, fed with a standard fishmeal-based diet. The data obtained were compared with similar data from wild-capture L3 A. simplex larvae-infected fish and, as positive control, protein extracts from L3 A. simplex larvae. An immunological procedure, Western blot analysis, was set up, using as A. simplex allergens antibodies, serum samples from subjects previously accidentally sensitized by A. simplex allergens against which high sIgE levels were evidenced by a commercial microarray immunoassay.
2. Materials and Methods
The study was conducted in agreement with the ethical guidelines of the Declaration of Helsinki and received the approval of the Ethical Committee of the University Elbasan (INTL_ALITMKCOOP/HealthMicroPath/HMM2019_IPM).
Serum samples for Abs anti-A. simplex allergens were obtained from patients with allergic and/or gastrointestinal symptoms within 12 hrs after ingestion of fish. Seventeen subjects were enrolled during the study period (March–November 2019). Specific immunoglobulin (sIgE) to A. simplex allergens was evaluated by an allergen microarray immunoassay (ImmunoCAP® Specific IgE Phadia, (ThermoFisher Scientific, Milan, Italy), a fluoro-immunoassay, repeatable and reproducible in vitro diagnostic tool for sIgE determination that allows detection of sIgE to 112 molecular components from 51 allergenic sources. All selected serum samples for the study showed undetectable sIgE to fish and in particular to cod, shrimp and dust mite. The negative limit for A. simplex sIgE, for ImmunoCAP® is ≤0.3 Standardized Units (ISU-E), and all the serum samples selected showed sIgE levels mainly between 1 and 15 ISU-E. The serum samples induced a strong positivity when used, preliminarily, as Abs source in a dot-blot analysis performed with L3 A. simplex larvae as antigens (data not reported). All subjects included in the study were asked to sign an informed consent form for the use of their serum samples which, alternatively, would have been safely eliminated.
2.1. L3 Anisakis Simplex Larvae
- (a)
- Extraction of L3 larvae. A. simplex L3 larvae were obtained from heavily infected Trachurus trachurus, called horse mackerel, from a fish market in Bari (Italy). Even if it has been reported that Trachurus trachurus is infected with Hysterothylacium larvae [28,29], in our study, A. simplex L3 larvae were identified. For each protein preparation, 400 to 500 L3 larvae were used. The L3 larvae were washed in PBS, collected and stored, in PBS, frozen at −20 °C until use. The L3 larvae were, morphologically, identified as Anisakis simplex sensu lato by one of the investigators (L.D.).
- (b)
- Protein preparation. A. simplex L3 larvae were subsequently ground in a Potter-ELV homogenizer in a RIPA-buffer (TRIS HCl 25 mM, NaCl 150 mM, 1% Triton x-100, Sodium Deoxycholate 1%, SDS 0.1%) with anti-proteases and sonicated at 103 18 w for 5 s. The homogenate was then centrifuged at 16,000× g for 10 min. The protein extract was then aliquoted in 200 µL Eppendorf tubes and frozen at −20 °C until the use, mainly on the day after protein preparation. The protein concentration was determined by using Quick Start 105 Bradford Protein Assay (Bio-Rad Laboratories S.r.l., Milan, Italy) and using bovine serum albumin (BSA) as protein standard.
2.2. Aquacultured and Infected Fish
- (c)
- Protein preparation. Five aquacultured sea bream and five L3 A. simplex larvae-infected horse mackerel, previously stored at −20 °C for 24 h, were utilized for the study. Protein preparation was conducted mainly following the procedure used for L3 A. simplex larvae. Of each fish, 2 g of edible part was weighed and homogenized with 7 mL of RIPA buffer (Sigma-Aldrich, Milan, Italy), containing protease inhibitors cocktail tablets (Roche Applied Science, Milan, Italy) and anti-phosphatases (sodium orthovanadate 2 mM; Sigma-Aldrich, Milan, Italy). The homogenate was then centrifuged at 16,000× g for 30 min and separately aliquoted and stored at −20 °C until the use.
2.3. Western Blot Analysis
The procedure was done mainly as suggested by Rodriguez-Mahillo et al. [15]. Briefly, proteins samples, respectively from 3 protein extracts of A. simplex larvae, 5 protein extracts of sea bream and 5 protein extracts of Trachurus trachurus were used. Aliquots of 20 µg were separated on 4–12% sodium dodecyl sulphate polyacrylamide gels (Invitrogen S.r.l., Milan, Italy), transferred onto a nitrocellulose membrane, later incubated for 1 h in a blocking solution (5% of non-fat dry milk) (Bio-Rad Laboratories S.r.l., Milan, Italy) in TBS-T and then incubated, overnight at 4 °C, under shaking, with a pool of A. simplex allergic patients’ sera diluted 1:10 in blocking solution. The primary antibody was identified by an HRP-conjugated secondary antibody, anti-human immunoglobulin (Bio-Rad Laboratories S.r.l., Milan, Italy), diluted 1:20,000 in TBS-T and subsequently detected by a chemiluminescent substrate of HRP (Pierce Biotechnology, Inc., Rockford, IL, USA). The chemiluminescence analysis of each signal on nitrocellulose membranes was evaluated by Molecular Image Chemidoc XRS+ (Bio-Rad Laboratories S.r.l.) as by us previously reported [30]. Each analysis was done, at least, three times, using, each time, a different protein extract.
2.4. Dot-Blot Analysis
The aim of this procedure was initially adopted to identify serum samples from A. simplex sIgE positive subjects, evaluated by ImmunoCAP® Specific IgE analysis, able to ensure their use for Western blot analysis. Afterwards, this procedure was used to test the immunological presence of Anisakis allergens in commercial flours normally utilized in aquaculture. Protein extracts, carried out three times from two different flours, were loaded on nitrocellulose membranes contemporary to protein extracts of A. simplex L3 larvae. The nitrocellulose membranes were then treated with blocking solution (TBS-T, TRIS-HCl 20 mM, NaCl 150 mM, Tween 20 0.05%, pH 7.5) for 30 min, incubated with the human serum cocktail in TBS-T (Tris Buffer Saline Tween 20) overnight at 4 °C, washed with TBS-T and finally incubated with an anti-IgE antibody solution in TBS-T for 1 hr at room temperature. To verify the antigen-primary antibody-secondary antibody reaction, the membranes were incubated with a chemiluminescent substrate for 5 min, and the chemiluminescence on nitrocellulose membrane evaluated by ChemiDoc XRS+ (Bio-Rad Laboratories S.r.l., Milan, Italy) as above described and by us reported (30). Each analysis was done at least three times, using, each time, a different protein extract.
3. Results
Figure 1 reports Western blot analysis of protein extracts from aquacultured (sea bream), L3 larvae-infected fish (horse mackerel) and A. simplex L3 larvae. In both fish extracts it is possible to observe an immunological protein signal below 10 Kd MW, that parallels the L3 larvae signal with similar MW. As it is well known, this MW characterizes Ani s4 Anisakis allergen, a heat and pepsin-resistant allergen. The Figure is an example of WB analyses carried out running all protein extracts. Every time, an immunological signal below 10 Kd MW, not always with the same intensity, was evidenced.
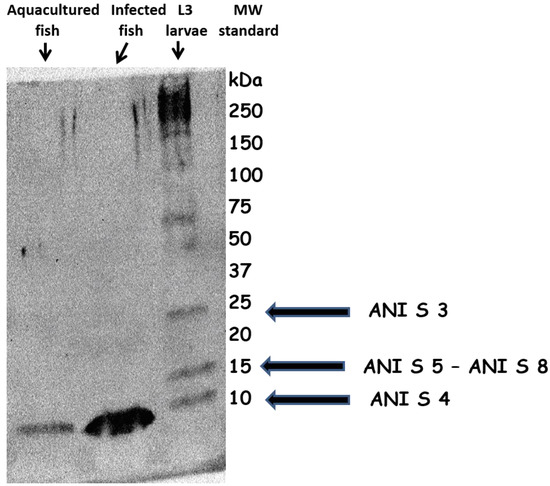
Figure 1.
WB analysis of protein extracts from aquacultured fish, A. simplex L3 larvae-infected fish and A. simplex L3 larvae. MW identification shows, in the edible part of both fishes, the presence of an immunodetected protein signal similar to the Ani s4 allergen of A. simplex L3 larvae.
Figure 2 reports dot blot analysis of two commercial flours widely used as animal feed for human food, such as farmed fish or poultry. In the Figure the immunological analysis using protein extract from A. simplex L3 larvae is also reported. A positive signal was detected in all three protein samples, evidencing the possibility of the presence of antigenic proteins recognized by the sera of A. simplex allergens positive patients. Constantly, a positive immunological signal was highlighted, testing, each time, a different protein extract of the two flours.
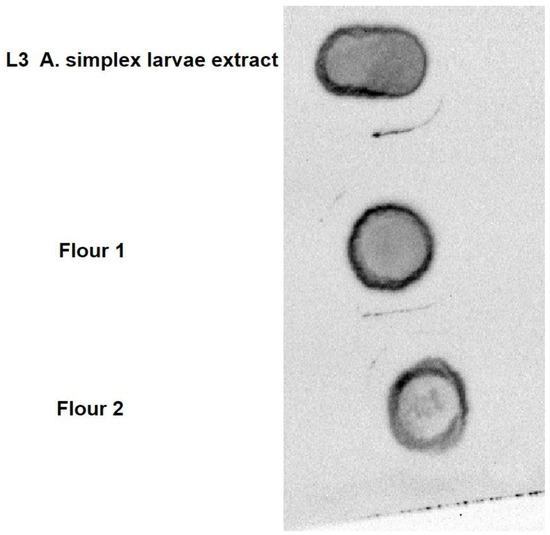
Figure 2.
Dot blot analysis of protein extracts from A. simplex L3 larvae and two commercial flours widely used for feeding fish and chicken destined for human nutrition.
4. Discussion
The results herein reported show an evident allergenicity for A. simplex allergens in the edible part of both A. simplex L3 larvae-infected and in aquacultured fish, farmed in shore cages and fed with commercial flour. It is important to underline that previous studies reported almost no risk of exposure to anisakid parasite considering the very low presence of these nematodes in farmed fish [24,25,26,27]. Similarly, no anisakid nematode was detected in the aquacultured fish by us analyzed. However, the tested flours, immunologically positive to human sera from patients with high IgE titers directed to Anisakis allergens (Figure 2), were the same used to feed the aquacultured fish.
Considering the molecular weight of the protein signal evidenced in all protein extracts (Figure 1), the detected A. simplex allergenicity seems to be determined by the presence of Ani s4, a heat and pepsin-resistant allergen.
Ani s4, the A. simplex allergen detected in 27% of allergic patients, is the first nematode protease inhibitor (cystatin) described as an allergen and has been previously shown to be heat stable (boiling for 30 min) and resistant to pepsin digestion [15]. Its resistance to autoclaving, along with pepsin resistance, suggests that Ani s4 could be clinically relevant in cases in which Ani s4–sensitized patients are again exposed to A. simplex allergens following consumption of processed parasite-contaminated fishery products [11,31].
Our data may provide an interpretation for the allergic manifestations to A. simplex reported by subjects who consumed aquacultured or previously frozen fish and, in the same patients, symptom presentation was reported coincident with an increase of specific IgE level against Anisakis allergens (4). Similarly, Armentia et al. reported allergic reactions in patients highly sensitized to A. simplex, eating chicken meat fed with fishmeal [32]. We hypothesize that, although seafood is the principal source of human infections by A. simplex, it may be possible that flours used to feed animals for human food, such as farmed fish or poultry, are contaminated with nematode allergens resistant to the treatments for their preparation. Despite the exceptions reported to the Armentia study [33,34], the problem of the possible presence of heat-resistant anisakide allergens in animal food remains. We believe that with our data, we partially clarified this topic.
The clinical and epidemiological interest in A. simplex allergens’ thermostability therefore plays a crucial role in suggesting the prophylaxis against A. simplex (fish cooked at 60 °C for 10′ or frozen at −20 °C for at least 24 h), neglecting any reference not only to the thermostable proteins of A. simplex but also to the healthiness of fishmeal widely used in aquaculture or in chicken feed.
The feeding of fish in aquaculture usually consists of flours added with fishmeal in order to increase the efficiency and growth of the animals. Fishmeal provides a balanced amount of all essential amino acids, phospholipids and fatty acids (docosahecsenic acid-DHA and eicosapentaenoic acid-EPA) [23]. Usually, aquaculture industry feeds fish using fishmeal, even if a number of carnivorous and omnivorous farmed fish species are capable of digesting poultry meals, nuts, soy, and grain on commercial scales [35,36,37], presenting the possibility that fishmeal can be eliminated as a component of fish feed. Previous studies have tested the feasibility of fishmeal-free feeds by examining how they impact different performance metrics, including growth [38,39,40], palatability [41,42], nutrition [36], fatty acid composition of the fillet [42,43] and water quality [39,44].
Fishmeal preparation usually follows steps which include boiling the raw material and the solid part being dried at about 80–100 °C, a temperature which must not be too high, and the process must last as short as possible in order not to destroy the proteins [34]. Moreover, as reported by Miles and Chapman, the top fishmeal producing countries are Peru, Chile, China and Thailand, and most of the fishes used to produce it are small, bony, with high content of oil and considered of little edible use (e.g., anchovies, herrings, capelin and menhaden). A small percentage of fishmeal is rendered from fish offal, trimmings or cuttings and other wastes principally from filleting and canning operations from the edible fisheries (e.g., tuna, cod, haddock, hake, pollock) [45,46].
As previously reported, the aquaculture industry has outpaced wild-capture fisheries for over two decades and currently produce nearly 50% of all seafood consumed globally. As wild-capture fisheries continue to decline, aquaculture’s role in food production will continuously grow [47,48,49].
All these lead us to consider more carefully the use of foods that are used by the farms dedicated to producing food for human consumption, and not only in the field of aquaculture, taking into consideration the possibility of allergic phenomena triggered by A. simplex. In this regard, as we previously reported, an ever-increasing number of observations report allergic phenomena in subjects who, perhaps, do not eat raw or undercooked seafood products or for whom, furthermore, fish is not part of their eating habits [4]. Our research also highlights the need for a one-health approach to increasing the awareness among stakeholders, including fish farmers, food manufacturers and fisheries authorities for change in policies and protocols for a safer seafood production [50].
5. Conclusions
In conclusion, we believe that in order to avoid the threats to human health deriving from allergic episodes caused by the ingestion of infected marine products, to preserve the possibility of being able to consume a nourishment such as fish, which rich in ω-3 lipids and proteins of high biological value, it is no longer possible to suggest the useless prophylaxis against A. simplex, which involves freezing and/or boiling the fish, but checks must be carried out on the foods used for aquacultured fish, as well as for the production of poultry, intended for human consumption, an industry which, so far, covers nearly 60% of all seafood consumed globally.
Author Contributions
Substantial contributions to the conception or design of the work: all the authors; acquisition, analysis, and interpretation of data for the work: all the authors; drafting the work and revising it critically for important intellectual content: all the authors; final approval of the version to be published: all the authors; agreement to be accountable for all aspects of the work ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in agreement with the ethical guidelines of the Declaration of Helsinki and received the approval of the Ethical Committee of the University Elbasan (INTL_ALITMKCOOP/HealthMicroPath/HMM2019_IPM).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Ferrantelli, V.; Costa, A.; Graci, S.; Buscemi, M.D.; Giangrosso, G.; Porcarello, C.; Palumbo, S.; Cammilleri, G. Anisakid Nematodes as Possible Markers to Trace Fish Products. Ital. J. Food Saf. 2015, 4, 4090. [Google Scholar] [CrossRef] [PubMed]
- Mattiucci, S.; Nascetti, G. Molecular systematics, phylogeny and ecology of anisakid nematodes of the genus Anisakis Dujardin, 1845: An update. Parasite 2006, 13, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Carballeda-Sangiao, N.; Rodríguez-Mahillo, A.I.; Careche, M.; Nava, S.A.; Moneo, I.; González-Muñoz, M. Changes over Time in IgE Sensitization to Allergens of the Fish Parasite Anisakis spp. PLoS Negletted Trop. Dis. 2016, 10, e0004864. [Google Scholar] [CrossRef] [PubMed]
- Cavallero, S.; Magnabosco, C.; Civettini, M.; Boffo, L.; Mingarelli, G.; Buratti, P.; Giovanardi, O.; Fortuna, C.M.; Arcangeli, G. Survey of Anisakis sp. and Hysterothylacium sp. in sardines and anchovies from the North Adriatic Sea. Int. J. Food Microbiol. 2015, 200, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.R.; Kiani, B.; Afshari, A.; Moghaddas, E.; Williams, M.; Shamsi, S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: A systematic review. Parasitol. Res. 2020, 119, 3585–3594. [Google Scholar] [CrossRef]
- López-Serrano, M.C.; Gomez, A.A.; Daschner, A.; Moreno-Ancillo, A.; de Parga, J.M.; Caballero, M.T.; Barranco, P.; Cabañas, R. Gastroallergic anisakiasis: Findings in 22 patients. J. Gastroenterol. Hepatol. 2000, 15, 503–506. [Google Scholar] [CrossRef]
- Arthur, J.R.; Te, B.Q. Checklist of the Parasites of Fishes of Viet Nam; FAO Fisheries Technical Paper, No. 369/2; FAO: Rome, Italy, 2006; 133p. [Google Scholar]
- Shamsi, S.; Briand, M.J.; Justine, J.L. Occurrence of Anisakis (Nematoda: Anisakidae) larvae in unusual hosts in Southern hemisphere. Parasitol. Int. 2017, 66, 837–840. [Google Scholar] [CrossRef]
- Shamsi, S.; Gasser, R.; Beveridge, I. Genetic characterisation and taxonomy of species of Anisakis (Nematoda: Anisakidae) parasitic in Australian marine mammals. Invertebr. Syst. 2012, 26, 204–212. [Google Scholar] [CrossRef]
- Rodríguez-Mahillo, A.I.; González-Muñoz, M.; de las Heras, C.; Tejada, M.; Moneo, I. Quantification of Anisakis simplex allergens in fresh, long-term frozen, and cooked fish muscle. Foodborne Pathog. Dis. 2010, 7, 967–973. [Google Scholar] [CrossRef]
- Sastre, J.; Lluch-Bernal, M.; Quirce, S.; Arrieta, I.; Lahoz, C.; Del Amo, A.; Fernandez-Caldas, E.; Maranòn, F. A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy 2000, 55, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.T.; Tummolo, R.A.; Di Leo, E.; D’Erasmo, M.; Arsieni, A. Immediate and cell-mediated reactions in parasitic in-fections by Anisakis simplex. J. Investig. Allergol. Clin. Immunol. 2008, 18, 253–259. [Google Scholar] [PubMed]
- Shimakura, K.; Miura, H.; Ikeda, K.; Ishizaki, S.; Nagashima, Y.; Shirai, T.; Kasuya, S.; Shiomi, K. Purification and molecular cloning of a major allergen from Anisakis simplex. Mol. Biochem. Parasitol. 2004, 135, 69–75. [Google Scholar] [CrossRef]
- Rodriguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Gomez-Aguado, F.; Rodriguez-Perez, R.; Corcuera, M.T.; Caballero, M.L.; Moneo, I. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 2007, 37, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Ishizaki, S.; Shimakura, K.; Nagashima, Y.; Shiomi, K. Molecular cloning and expression of two new al-lergens from Anisakis simplex. Parasitol. Res. 2007, 100, 1233–1241. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimakura, K.; Ishizaki, S.; Nagashima, Y.; Shiomi, K. Purification and cDNA cloning of a new heat-stable allergen from Anisakis simplex. Mol. Biochem. Parasitol. 2007, 155, 138–145. [Google Scholar] [CrossRef]
- Rodriguez-Perez, R.; Moneo, I.; Rodriguez-Mahillo, A.; Caballero, M.L. Cloning and expression of Ani s9, a new Anisakis simplex allergen. Mol. Biochem. Parasitol. 2008, 159, 92–97. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef]
- Olivares, F.; González-Muñoz, M.; Carballeda-Sangiao, N.; Rodríguez-Mahillo, A.; Careche, M.; de Las Heras, C.; Alfonso Navas, A.; Tejada, M. Removal of Anisakis simplex allergens from infected fish during the washing step of surimi production. J. Sci. Food Agric. 2015, 95, 2626–2631. [Google Scholar] [CrossRef]
- Fæste, C.K.; Levsen, A.; Lin, A.H.; Larsen, N.; Plassen, C.; Moen, A.; Van Do, T.; Egaas, E. Fish feed as source of potentially allergenic peptides from the fish parasite Anisakis simplex (s.l.). Anim. Feed Sci. Technol. 2015, 202, 52–61. [Google Scholar] [CrossRef]
- Lovell, R.T. Nutrition of aquaculture species. J. Anim. Sci. 1991, 69, 4193–4200. [Google Scholar] [CrossRef] [PubMed]
- Cammilleri, G.; Costa, A.; Graci, S.; Buscemi, M.D.; Collura, R.; Vella, A.; Pulvirenti, A.; Cicero, A.; Giangrosso, G.; Schembri, P.; et al. Presence of Anisakis pegreffii in farmed sea bass (Dicentrarchus labrax L.) commercialized in Southern Italy: A first report. Vet. Parasitol. 2018, 259, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Lunestad, B.T. Absence of nematodes in farmed Atlantic salmon (Salmo salar L.) in Norway. J. Food Prot. 2003, 66, 122–124. [Google Scholar] [CrossRef]
- Marty, G.D. Anisakid larva in the viscera of a farmed Atlantic salmon (Salmo salar). Aquaculture 2008, 279, 209–210. [Google Scholar] [CrossRef]
- Peñalver, J.; Dolores, M.E.; Muñoz, P. Absence of anisakid larvae in farmed European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.) in Southeast Spain. J. Food Prot. 2010, 73, 1332–1334. [Google Scholar]
- Roca-Geronès, X.; Montoliu, I.; Godínez-González, C.; Fisa, R.; Shamsi, S. Morphological and genetic characterization of Hysterothylacium Ward & Magath, 1917 (Nematoda: Raphidascarididae) larvae in horse mackerel, blue whiting and anchovy from Spanish Atlantic and Mediterranean waters. J. Fish. Dis. 2018, 41, 1463–1475. [Google Scholar]
- Roca-Geronès, X.; Segovia, M.; Godínez-González, C.; Fisa, R.; Montoliu, I. Anisakis and Hysterothylacium species in Mediterranean and North-East Atlantic fishes commonly consumed in Spain: Epidemiological, molecular and morphometric discriminant analysis. Int. J. Food Microbiol. 2020, 325, 108642. [Google Scholar] [CrossRef]
- Polimeno, L.; Pesetti, B.; De Santis, F.; Resta, L.; Rossi, R.; De Palma, A.; Girardi, B.; Amoruso, A.; Francavilla, A. Decreased expression of the augmenter of liver regeneration results in increased apoptosis and oxidative damage in human-derived glioma cells. Cell Death Dis. 2012, 3, e289. [Google Scholar] [CrossRef]
- Moneo, I.; Caballero, M.L.; Gonzalez-Muñoz, M.; Rodrıguez-Mahillo, A.I.; Rodrıguez-Perez, R.; Silva, A. Isolation of a heat resistant allergen from the fish parasite Anisakis simplex. Parasitol. Res. 2005, 96, 285–289. [Google Scholar] [CrossRef]
- Armentia, A.; Martin-Gil, F.J.; Pascual, C.; Martín-Esteban, M.; Callejo, A.; Martínez, C. Anisakis simplex allergy after eating chicken meat. J. Investig. Allergol. Clin. Immunol. 2006, 16, 258–263. [Google Scholar] [PubMed]
- Sastre, J. Allergy to chicken in patients sensitized to Anisakis species. J. Investig. Allergol. Clin. Immunol. 2007, 17, 129. [Google Scholar] [PubMed]
- Armentia, A. Allergy to chicken in patients sensitized to Anisakis species—Author’s reply. J. Investig. Allergol. Clin. Immunol. 2007, 17, 129–130. [Google Scholar]
- Caballero, M.; Obach Rosenlund, G.; Montero, D.; Gisvold, M.; Izquierdo, M. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Allan, G.L.; Parkinson, S.; Booth, M.A.; Stone, D.A.J.; Rowland, S.J.; Frances, J.; Warner-Smith, R. Replacement of fish meal in diets for Australian silver perch, Bidyanus bidyanus: Digestibility of alternative ingredients. Aquaculture 2000, 186, 293–310. [Google Scholar] [CrossRef]
- Gomes, E.F.; Rema, P.; Kaushik, S.J. Replacement of fish meal by plant proteins in the diet of rainbow trout (Oncorhynchus mykiss): Digestibility and growth performance. Aquaculture 1995, 130, 177–186. [Google Scholar] [CrossRef]
- Carter, C.G.; Hauler, R.C. Fish meal replacement by plant meals in extruded feeds for Atlantic salmon, Salmo salar. Aquaculture 2000, 185, 299–311. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Barrows, F.T.; Welsh, C.; Kenney, P.B.; Summerfelt, S.T. Comparing the effects of feeding a grain- or a fish meal-based diet on water quality, waste production, and rainbow trout Oncorhynchus mykiss performance within low exchange water recirculating aquaculture systems. Aquac. Eng. 2013, 52, 45–57. [Google Scholar] [CrossRef]
- Barrows, F.T.; Gaylord, T.G.; Stone, D.A.J.; Smith, C.E. Effect of protein source and nutrient density on growth efficiency, histology and plasma amino acid concentration of rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2007, 38, 1747–1758. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Covès, D.; Dutto, G.; Blanc, D. Almost total replacement of fish meal by plant protein sources in the diet of a marine teleost, the European seabass, Dicentrarchus labrax. Aquaculture 2004, 230, 391–404. [Google Scholar] [CrossRef]
- Stickney, R.R.; Hardy, R.W.; Koch, K.; Harrold, R.; Seawright, D.; Massee, K.C. The effects of substituting selected oilseed protein concentrates for fish meal in rainbow trout Oncorhynchus mykiss diets. J. World Aquac. Soc. 1996, 27, 57–63. [Google Scholar] [CrossRef]
- Bell, J.G.; McGhee, F.; Campbell, P.J.; Sargent, J.R. Rapeseed oil as an alternative to marine fish oil in diets of postsmolt Atlantic salmon (Salmo salar): Changes in flesh fatty acid composition and effectiveness of subsequent fish oil “wash out”. Aquaculture 2003, 218, 515–528. [Google Scholar] [CrossRef]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Pallab, K.; Sarker Kapuscinski, A.R.; Bae, A.Y.; Donaldson, E.; Sitek, A.J.; Fitzgerald, D.S.; Edelson, O.F. Towards sustainable aquafeeds: Evaluating substitution of fishmeal with lipid-extracted microalgal co-product (Nannochloropsis oculata) in diets of juvenile Nile tilapia (Oreochromis niloticus). PLoS ONE 2018, 13, e0201315. [Google Scholar]
- Miles, R.D.; Chapman, F.A. The Benefits of Fish Meal in Aquaculture Diets; FA122; IFAS Extension, University of Florida: Gainesville, FL, USA, 2006; pp. 1–6. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Zhao, L.; He, K.; Luo, J.; Sun, J.; Liao, L.; Tang, X.; Liu, Q.; Yang, S. Co-modulation of Liver Genes and Intestinal Microbiome of Largemouth Bass Larvae (Micropterus salmoides) During Weaning. Front. Microbiol. 2020, 11, 1332. [Google Scholar] [CrossRef]
- Charitos, I.A.; Castellaneta, F.; Santacroce, L.; Bottalico, L. Historical anecdotes and breakthroughs of histamine: From discovery to date. Endocr. Metab. Immune Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- Shamsi, S. Seafood-Borne Parasitic Diseases: A “One-Health” Approach Is Needed. Fishes 2019, 4, 9. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).