Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
- (P) Participants: Subjects received endosseous implantation;
- (I) Intervention: Implants with chitosan incorporation;
- (C) Control: Implants without chitosan incorporation;
- (O) Outcome: Bone formation around the implant body.
2.2. Data Sources and Search Strategy
2.3. Inclusion and Exclusion Criteria
- In vivo studies;
- Studies where at least one layer of CS was used to coat the Ti;
- Studies where bone growth or the formation of a biological seal around the Ti implant surface coated with CS alone or in combination with other products or molecules was assessed;
- Studies on endosseous implants;
- Studies that included non-modified animals (osteoporotics, diabetics…).
- In vitro studies;
- Narrative and systematic reviews;
- Studies that did not use endosseous implants, duplicates, and informatives.
2.4. Data Extraction and Analysis
2.5. Risk of Bias (RoB) of the Selected Articles
2.6. Quality of the Reports in the Selected Articles
3. Results
3.1. Characteristics of the Studies
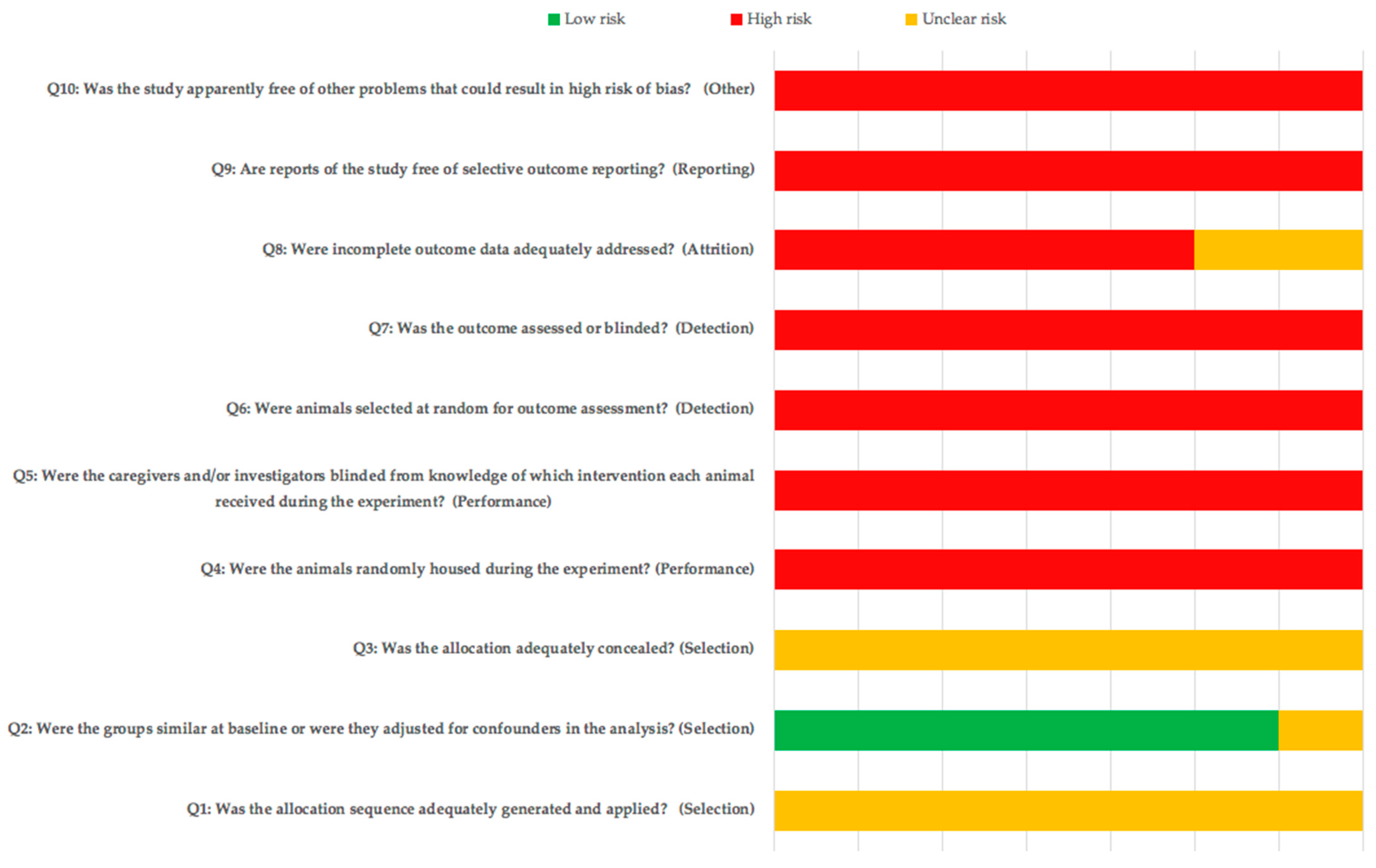
3.2. Risk of Bias and Quality Assessment of the Animal Studies Included
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ti | Titanium |
| SLA | Sandblasted, Large-Grit, Acid-Etched |
| HA | Hyaluronic Acid |
| CS | Chitosan |
| CHITLAC | Chitosan–Lactose |
| BIC | Bone-to-Implant-Contact |
| H&E | Hematoxylin and Eosin |
| GNP | Gold Nanoparticles |
| GFBP | Growth Factor Binding Protein |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| nAg | Silver Nanoparticles |
| TS | Unmodified Thermoset |
| HCL | Hydrochloric Acid |
References
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 76–184. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Kumar, V.V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo. J. Biomed. Mater. Res. A 2018, 106, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, P.W.; Pabst, A.M.; Dau, M.; Staedt, H.; Al-Nawas, B.; Heller, M. Immobilization of BMP-2, BMP-7 and alendronic acid on titanium surfaces: Adhesion, proliferation and differentiation of bone marrow-derived stem cells. J. Biomed. Mater. Res. A 2020, 108, 212–220. [Google Scholar] [CrossRef]
- Chakravorty, N.; Ivanovski, S.; Prasadam, I.; Crawford, R.; Oloyede, A.; Xiao, Y. The microRNA expression signature on modified titanium implant surfaces influences genetic mechanisms leading to osteogenic differentiation. Acta Biomater. 2012, 8, 3516–3523. [Google Scholar] [CrossRef]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs. 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.; Pereira, A.G.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug. Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef]
- Han, L.K.; Kimura, Y.; Okuda, H. Reduction in fat storage during chitin-chitosan treatment in mice fed a high-fat diet. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 174–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Three-dimensional macroporous calcium phosphate bioceramics with nested chitosan sponges for load-bearing bone implants. J. Biomed. Mater. Res. 2002, 61, 1–8. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of chitin extraction from shrimp shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Jo, G.H.; Kuk, J.H.; Kim, K.Y.; Park, R.D. Extraction of chitin from red crab Shell waste by cofermentation with Lactobacillus paracasei subsp. tolerans KCTC-3074 and Serratia marcescens FS-3. Appl. Microbiol. Biotechnol. 2006, 71, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Kafetzopoulos, D.; Martinou, A.; Bouriotis, V. Bioconversion of chitin to chitosan: Purification and characterization of chitin deacetylase from Mucor rouxii. Proc. Natl. Acad. Sci. USA 1993, 90, 2564–2568. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Meyers, S.P. Preparation and characterization of chitin and chitosan—A review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Li, B.; Hao, J.; Min, Y.; Xin, S.; Guo, L.; He, F.; Liang, C.; Wang, H.; Li, H. Biological properties of nanostructured Ti incorporated with Ca, P and Ag by electrochemical method. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 80–86. [Google Scholar] [CrossRef]
- Trampuz, A.; Widmer, A.F. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 2006, 19, 349–356. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Hutton, B.; Ferrán Catalá-López, F.; Moher, D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med. Clin. 2016, 16, 262–266. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Stadlinger, B.; Pourmand, P.; Locher, M.C.; Schulz, M.C. Systematic review of animal models for the study of implant integration, assessing the influence of material, surface and design. J. Clin. Periodontol. 2012, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, X.; Xu, X.Y.; Yin, Y.; Li, X.; Bi, C.S.; Hong, Y.L.; Chen, F.M. Surface modification via plasmid-mediated pLAMA3-CM gene transfection promotes the attachment of gingival epithelial cells to titanium sheets in vitro and improves biological sealing at the transmucosal sites of titanium implants in vivo. J. Mater. Chem. B 2019, 7, 7415–7427. [Google Scholar] [CrossRef]
- Song, Y.; Ma, A.; Ning, J.; Zhong, X.; Zhang, Q.; Zhang, X.; Hong, G.; Li, Y.; Sasaki, K.; Li, C. Loading icariin on titanium surfaces by phase-transited lysozyme priming and layer-by-layer self-assembly of hyaluronic acid/chitosan to improve surface osteogenesis ability. Int. J. Nanomed. 2018, 13, 6751–6767. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Bhattarai, G.; Lee, Y.H.; Lee, M.H.; Park, I.S.; Yi, H.K. Insulin-like growth factor binding protein-3 affects osteogenic efficacy on dental implants in rat mandible. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Lee, Y.H.; Yi, H.K. Peroxisome proliferator activated receptor gamma loaded dental implant improves osteogenesis of rat mandible. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 587–595. [Google Scholar] [CrossRef]
- Marsich, E.; Travan, A.; Donati, I.; Turco, G.; Kulkova, J.; Moritz, N.; Aro, H.T.; Crosera, M.; Paoletti, S. Biological responses of silver-coated thermosets: An in vitro and in vivo study. Acta Biomater. 2013, 9, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Foulc, M.P.; Moritz, N.; Aro, H.T.; Paoletti, S. Polysaccharide-coated thermosets for orthopedic applications: From material characterization to in vivo tests. Biomacromolecules 2012, 13, 1564–1572. [Google Scholar] [CrossRef]
- Cho, C.B.; Jung, S.Y.; Park, C.Y.; Kang, H.K.; Yeo, I.L.; Min, B.M. A Vitronectin-Derived Bioactive Peptide Improves Bone Healing Capacity of SLA Titanium Surfaces. Materials 2019, 17, 3400. [Google Scholar] [CrossRef]
- Fischer, N.G.; Münchow, E.A.; Tamerler, C.; Bottino, M.C.; Aparicio, C. Harnessing biomolecules for bioinspired dental biomaterials. J. Mater. Chem. B 2020, 14, 8713–8747. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, E.; Mattiuzzo, E.; Belluzzi, E.; Elia, R.; Benetti, A.; Venerando, R.; Vindigni, V.; Ruggieri, P.; Brun, P. Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 2020, 9, 1328. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Chesnutt, B.M.; Yuan, Y.; Yang, Y.; Appleford, M.; Oh, S.; McLaughlin, R.; Elder, S.H.; Ong, J.L. The integration of chitosan-coated titanium in bone: An in vivo study in rabbits. Implant. Dent. 2007, 16, 66–79. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, G.Y.; Pham, H.N.; Byun, H.G.; Kim, S.K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Abdallah, M.N.; Badran, Z.; Ciobanu, O.; Hamdan, N.; Tamimi, F. Strategies for Optimizing the Soft Tissue Seal around Osseointegrated Implants. Adv. Healthc. Mater. 2017, 6, 1700549. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.; Papadogiannis, F.; Batsali, A.; Marakis, J.; Alpantaki, K.; Eliopoulos, A.G.; Pontikoglou, C.; Chatzinikolaidou, M. Chitosan/gelatin scaffolds support bone regeneration. J. Mater. Sci. Mater. Med. 2018, 29, 59. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A.; Kamali, A. Effectiveness of tissue engineered chitosan-gelatin composite scaffold loaded with human platelet gel in regeneration of critical sized radial bone defect in rat. J. Control. Release 2017, 254, 65–74. [Google Scholar] [CrossRef]
- Park, K. Chitosan-gelatin-platelet gel composite scaffold for bone regeneration. J. Control. Release 2017, 254, 137. [Google Scholar] [CrossRef]
- Li, X.; Xu, P.; Cheng, Y.; Zhang, W.; Zheng, B.; Wang, Q. Nano-pearl powder/chitosan-hyaluronic acid porous composite scaffold and preliminary study of its osteogenesis mechanism. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110749. [Google Scholar] [CrossRef] [PubMed]
- Min, Q.; Yu, X.; Liu, J.; Wu, J.; Wan, Y. Chitosan-Based Hydrogels Embedded with Hyaluronic Acid Complex Nanoparticles for Controlled Delivery of Bone Morphogenetic Protein-2. Pharmaceutics 2019, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Drug. Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Blanc, V.; Isabal, S.; Sánchez, M.C.; Llama-Palacios, A.; Herrera, D.; Sanz, M.; León, R. Characterization and application of a flow system for in vitro multispecies oral biofilm formation. J. Periodontal. Res. 2014, 49, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Blanc, V.; León, R.; Herrera, D.; Sanz, M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J. Periodontal. Res. 2011, 46, 252–260. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Tangl, S.; Barnewitz, D.; Genzel, A.; Heimel, P.; Hruschka, V.; Redl, H.; Nau, T. A large animal model for standardized testing of bone regeneration strategies. BMC Vet. Res. 2018, 14, 330. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Translat. 2015, 3, 95–104. [Google Scholar] [CrossRef]


| Studies | Wang et al. 2019 [23] | Song et al. 2018 [24] | Chen et al. 2017 [25] | Bhattarai et al. 2015 (a) [26] | Bhattarai et al. 2015 (b) [27] | Marsich et al. 2013 [28] | Travan et al. 2012 [29] |
|---|---|---|---|---|---|---|---|
| 1. Title | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Abstract | |||||||
| 2. Species | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Key finding | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Introduction | |||||||
| 4. Background | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5. Reasons for animal models | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6. Objectives | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Methods | |||||||
| 7. Ethical statement | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8. Study design | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9. Experimental procedures | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10. Experimental animals | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11. Accommodation and handling of animals | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| 12. Sample size | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13. Assignment of animals to experimental groups | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14. Anesthesia | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15. Statistical methods | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Results | |||||||
| 16. Experimental results | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17. Results and estimation | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Discussion | |||||||
| 18. Interpretation and scientific implications | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19. 3Rs reported | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20. Adverse events | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21. Study limitations | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| 22. Generalization/applicability | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| 23. Funding | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| TOTAL, SCORE | 18 | 16 | 17 | 17 | 18 | 17 | 17 |
| Studies | Animal Model (n) | Location of Implant Placement | Follow-Up | Analysis Methods | Conclusions |
|---|---|---|---|---|---|
| Wang et al. 2019 [23] | Rat model (60) | Mesial (root area of upper right first molar) | 4 weeks |
| The plasmid pLAMA3-CM released from a chitosan/collagen coating was used for adhesion and peri-implant tissue attachment to titanium implants by functioning as a transmucosal barrier. |
| Song et al. 2018 [24] | Rat model (20) | Femur (midshafts) | 2 weeks |
| The HA/CS multilayer alone improved surface hydrophilicity. Phase-transited lysozyme nanofilm modulated materials and was applied for surface modification of implants. |
| Chen et al. 2017 [25] | New Zealand white rabbits (4) | Femora condyles | 4 and 12 weeks |
| The multilayer coated Ti implants were capable of promoting the proliferation, osteogenesis differentiation, and osteogenesis-related gene expression of osteoblasts and had great potential for clinical implementation in vivo with enhanced osteogenesis at the interface of the bone and implant. |
| Bhattarai et al. (a) 2015 [26] | Rat model (10) | Mandibles (lower first molar area) | 4 weeks |
| The application of CS-GNP/GFBP-3 enhanced bone remodeling around Ti implant surfaces by down-regulating osteoclastogenesis and up-regulating osteogenesis. |
| Bhattarai et al. (b) 2015 [27] | Rat model (24) | Mandibles (lower first molar area) | 1, 2, 3, and 6 weeks |
| Local administration of CS-GNP/PPAR decreases implant-induced inflammation and enhances the expression levels of osteogenic molecules around the implantation site and helps to accelerate bone formation and bone–implant integration. |
| Marsich et al. 2013 [28] | Minipig model | Femur | 8 weeks |
| It is assumed that the addition of nAg to the Chitlac coating may have influenced the peri-implant bone response, which was manifested in the absence of lamellar peri-implant bone. The mechanisms are not clear and need further investigation. |
| Travan et al. [29] 2012 | Minipig model | Femur | 8 weeks |
| For the Chitlac implants, the total BIC was 72% (min 59%, max 80%). Histomorphometric analysis: Chitlac-TS (nonroughened surface), 72% of the implant interface was in close contact with the cortical bone. |
| Studies | Implants (n) | Implant Dimensions, D(Ø) × L (mm) | Implant Shape | Chitosan Incorporation (See Figure 2) | CS-Modified Implant Surface Characteristics |
|---|---|---|---|---|---|
| Wang et al. [23] | 16 | 2 Ø × L 4 | Screw | NR | A CS coating was designed to release plasmid DNA and the codeposition of type IV collagen was applied with the purpose of synergistically promoting cellular adhesion and new tissue attachment to the titanium implants. |
| Song et al. [24] | 20 | 2 Ø × L 2 | Ti rods | By immersion in CS solution dissolving 0.1% CS in a 1% acetic acid solution. | Nanofilm coated with multilayer of HA-CS. |
| Chen et al. [25] | 16 | 3 Ø × L 13 | Ti rods | CS solution (3 mg mL−1) was prepared with HCl solution (pH 5.0). First, a thin layer of CS was deposited on the Ti surface, followed by three gel–CS bilayers and one HA layer. | Three gel–CS bilayers. |
| Bhattarai et al. (a) [26] | 10 | 0.85 Ø × 4.5 | Screw | For coating with CS-GNP–IGFBP-3 the implants were immersed 10 times in a nanoparticle–DNA solution and frozen at −40 °C. | NR |
| Bhattarai et al. (b) [27] | 24 | 0.85 Ø × 4.5 | Screw | The CS-GNP–PPAR-coated implants were immersed in a nanoparticle–DNA solution and frozen at −240 °C. | NR |
| Marsich et al. [28] | 6 | 3.6–5 Ø × 8 | Truncated cone | Coated with Chitlac or Chitlac–nAg. | NR |
| Travan et al. [29] | 3.6–5 Ø × 8 | Truncated cone | Coated with Chitlac or Chitlac–TS. | NR |
| Studies, Year | Soft Tissue | Bone Formation |
|---|---|---|
| Wang et al. 2019 [23] | Inform through images | Inform through images |
| Song et al. 2018 [24] | NR |
|
| Chen et al. 2017 [25] | NR |
|
| Bhattarai et al. 2015 (a) [26] | NR |
|
| Bhattarai et al. 2015 (b) [27] | NR | Bone formation around the implant body (inform through images). |
| Marsich et al. 2013 [28] | NR | BIC for Chitlac–nAg 26% (minimum 22%, maximum 27%) |
| Travan et al. 2012 [29] | NR | Chitlac-TS implants showed direct bone–implant contact with a minimal soft tissue interlayer, indicating good biological compatibility of the material. For the Chitlac-TS implants, the total BIC was 72% (minimum 59%, maximum 80%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Valverde, N.; López-Valverde, A.; Ramírez, J.M. Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants. Biology 2021, 10, 102. https://doi.org/10.3390/biology10020102
López-Valverde N, López-Valverde A, Ramírez JM. Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants. Biology. 2021; 10(2):102. https://doi.org/10.3390/biology10020102
Chicago/Turabian StyleLópez-Valverde, Nansi, Antonio López-Valverde, and Juan Manuel Ramírez. 2021. "Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants" Biology 10, no. 2: 102. https://doi.org/10.3390/biology10020102
APA StyleLópez-Valverde, N., López-Valverde, A., & Ramírez, J. M. (2021). Systematic Review of Effectiveness of Chitosan as a Biofunctionalizer of Titanium Implants. Biology, 10(2), 102. https://doi.org/10.3390/biology10020102







