Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Irradiation
2.2. Global DNA Methylation Assessment
2.3. Measurement of mRNA Levels by Quantitative RT-PCR
2.4. Statistics
3. Results
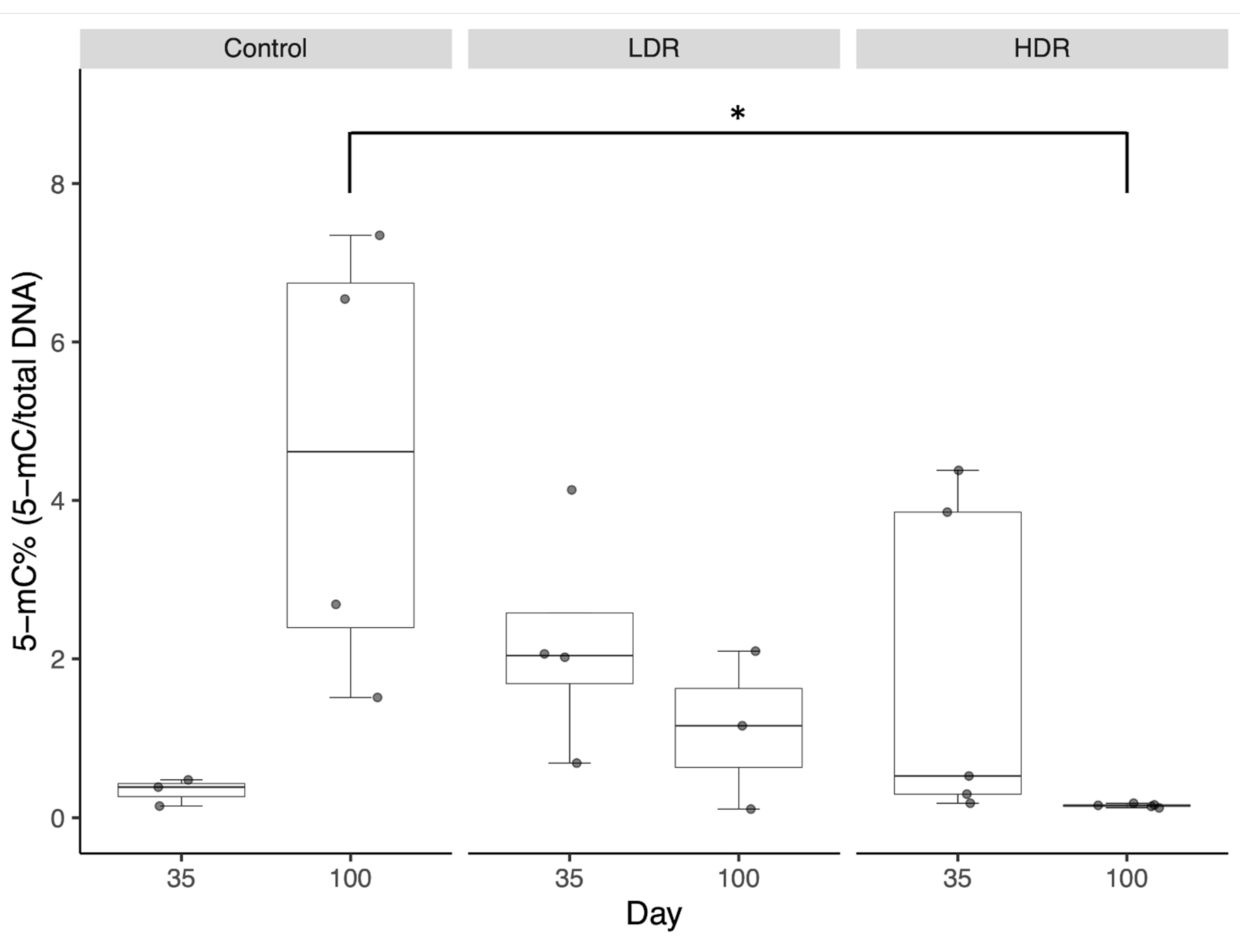
3.1. Global DNA Methylation in the Testis
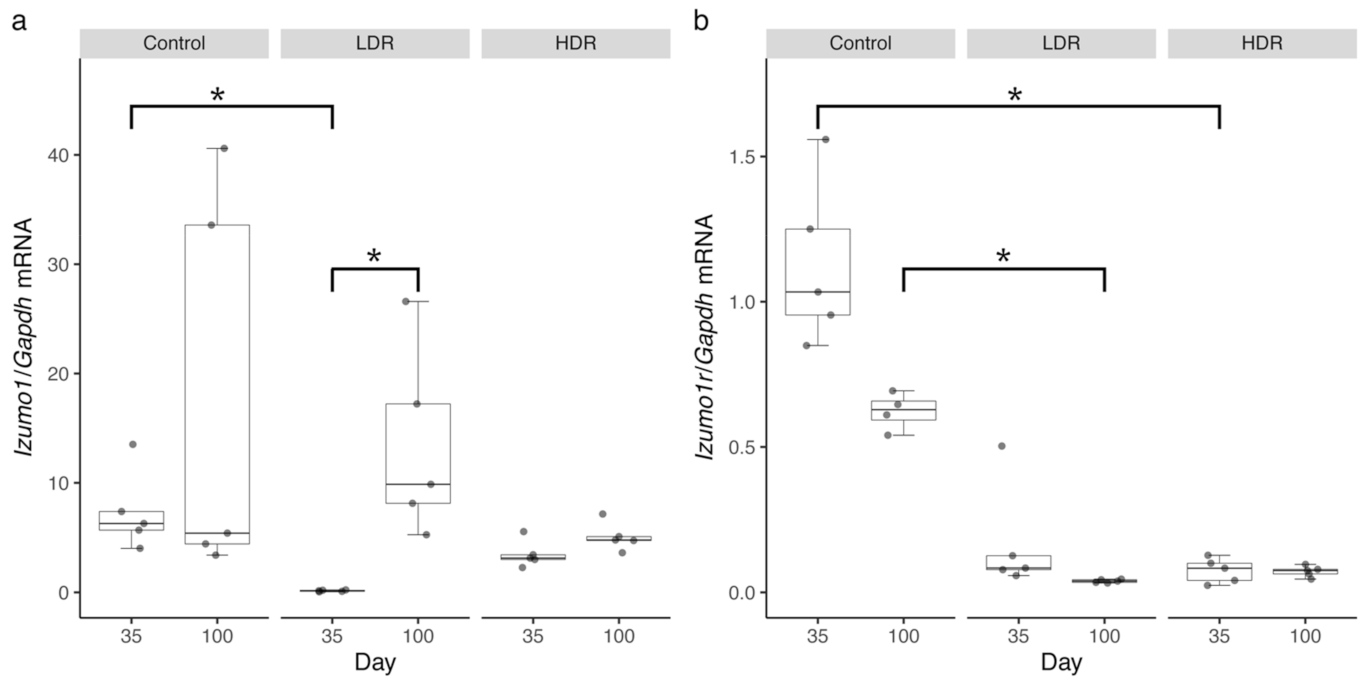
3.2. The Gene Expression Profiles of Izumo1 and Izumo1r in the Gonads
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruce, W.R.; Furrer, R.; Wyrobek, A.J. Abnormalities in the shape of murine sperm after acute testicular x-irradiation. Mutat. Res. 1974, 23, 381–386. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Jeon, B.; Jang, W.; Moon, C.; Kim, S. Protection of spermatogenesis against gamma ray-induced damage by granulocyte colony-stimulating factor in mice. Andrologia 2011, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Heo, K.; Yi, J.M.; Gong, E.J.; Yang, K.; Moon, C.; Kim, S.H. Genistein mitigates radiation-induced testicular injury. Phytother. Res. 2012, 26, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Kimler, B.F.; Briley, S.M.; Johnson, B.W.; Armstrong, A.G.; Jasti, S.; Duncan, F.E. Radiation-induced ovarian follicle loss occurs without overt stromal changes. Reproduction 2018, 155, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Selby, P.B.; Russell, W.L. First-generation litter-size reduction following irradiation of spermatogonial stem cells in mice and its use in risk estimation. Environ. Mutagen. 1985, 7, 451–469. [Google Scholar] [CrossRef]
- Ingram, D.L. Fertility and oocyte numbers after x-irradiation of the ovary. J. Endocrinol. 1958, 17, 81–90. [Google Scholar] [CrossRef]
- Ward, J.F. Nature of Lesions Formed by Ionizing Radiation. In DNA Damage and Repair; Nickoloff, J.A., Hoekstra, M.F., Eds.; Contemporary Cancer Research; Humana Press: Totowa, NJ, USA, 1998; pp. 65–84. [Google Scholar]
- Olive, P.L. The role of DNA single- and double-strand breaks in cell killing by ionizing radiation. Radiat. Res. 1998, 150, S42–S51. [Google Scholar] [CrossRef]
- Nakano, T.; Xu, X.; Salem, A.M.H.; Shoulkamy, M.I.; Ide, H. Radiation-induced DNA-protein cross-links: Mechanisms and biological significance. Free Radic. Biol. Med. 2017, 107, 136–145. [Google Scholar] [CrossRef]
- Dextraze, M.E.; Gantchev, T.; Girouard, S.; Hunting, D. DNA interstrand cross-links induced by ionizing radiation: An unsung lesion. Mutat. Res. 2010, 704, 101–107. [Google Scholar] [CrossRef]
- Miousse, I.R.; Kutanzi, K.R.; Koturbash, I. Effects of ionizing radiation on DNA methylation: From experimental biology to clinical applications. Int. J. Radiat. Biol. 2017, 93, 457–469. [Google Scholar] [CrossRef]
- Brabson, J.P.; Leesang, T.; Mohammad, S.; Cimmino, L. Epigenetic Regulation of Genomic Stability by Vitamin C. Front. Genet. 2021, 12, 675780. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Hashimshony, T.; Zhang, J.; Keshet, I.; Bustin, M.; Cedar, H. The role of DNA methylation in setting up chromatin structure during development. Nat. Genet. 2003, 34, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Mansuy, I.M. Epigenetic inheritance in mammals: Evidence for the impact of adverse environmental effects. Neurobiol. Dis. 2010, 39, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef]
- Layman, L.C. The genetic basis of female reproductive disorders: Etiology and clinical testing. Mol. Cell Endocrinol. 2013, 370, 138–148. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of male infertility. Curr. Opin. Genet. Dev. 2014, 26, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Cortessis, V.K.; Siegmund, K.; Yang, A.; Laird, P.W.; Sokol, R.Z. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE 2007, 2, e1289. [Google Scholar] [CrossRef]
- Marques, C.J.; Costa, P.; Vaz, B.; Carvalho, F.; Fernandes, S.; Barros, A.; Sousa, M. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol. Hum. Reprod. 2008, 14, 67–74. [Google Scholar] [CrossRef]
- Rajender, S.; Avery, K.; Agarwal, A. Epigenetics, spermatogenesis and male infertility. Mutat. Res. 2011, 727, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, A.G.; Peters, C.J.; Bowdin, S.; Temple, K.; Reardon, W.; Wilson, L.; Clayton-Smith, J.; Brueton, L.A.; Bannister, W.; Maher, E.R. Assisted reproductive therapies and imprinting disorders--a preliminary British survey. Hum. Reprod. 2006, 21, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Moll, A.C.; Imhof, S.M.; Cruysberg, J.R.; Schouten-van Meeteren, A.Y.; Boers, M.; van Leeuwen, F.E. Incidence of retinoblastoma in children born after in-vitro fertilisation. Lancet 2003, 361, 309–310. [Google Scholar] [CrossRef]
- Manipalviratn, S.; DeCherney, A.; Segars, J. Imprinting disorders and assisted reproductive technology. Fertil. Steril. 2009, 91, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Jangiam, W.; Udomtanakunchai, C.; Reungpatthanaphong, P.; Tungjai, M.; Honikel, L.; Gordon, C.R.; Rithidech, K.N. Late Effects of Low-Dose Radiation on the Bone Marrow, Lung, and Testis Collected From the Same Exposed BALB/cJ Mice. Dose Response 2018, 16, 1559325818815031. [Google Scholar] [CrossRef] [PubMed]
- Tawa, R.; Kimura, Y.; Komura, J.; Miyamura, Y.; Kurishita, A.; Sasaki, M.S.; Sakurai, H.; Ono, T. Effects of X-ray irradiation on genomic DNA methylation levels in mouse tissues. J. Radiat. Res. 1998, 39, 271–278. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Burke, P.; Besplug, J.; Slovack, M.; Filkowski, J.; Pogribny, I. Methylation changes in muscle and liver tissues of male and female mice exposed to acute and chronic low-dose X-ray-irradiation. Mutat. Res. 2004, 548, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Koturbash, I.; Pogribny, I.; Kovalchuk, O. Stable loss of global DNA methylation in the radiation-target tissue--a possible mechanism contributing to radiation carcinogenesis? Biochem. Biophys. Res. Commun. 2005, 337, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.; Raiche, J.; Slovack, M.; Kovalchuk, O. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem. Biophys. Res. Commun. 2004, 320, 1253–1261. [Google Scholar] [CrossRef]
- Gong, E.J.; Shin, I.S.; Son, T.G.; Yang, K.; Heo, K.; Kim, J.S. Low-dose-rate radiation exposure leads to testicular damage with decreases in DNMT1 and HDAC1 in the murine testis. J. Radiat. Res. 2014, 55, 54–60. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Ikawa, M.; Okabe, M. The mechanism of sperm-egg interaction and the involvement of IZUMO1 in fusion. Asian J. Androl. 2011, 13, 81–87. [Google Scholar] [CrossRef]
- Shang, Y.; Kakinuma, S.; Yamauchi, K.; Morioka, T.; Kokubo, T.; Tani, S.; Takabatake, T.; Kataoka, Y.; Shimada, Y. Cancer prevention by adult-onset calorie restriction after infant exposure to ionizing radiation in B6C3F1 male mice. Int. J. Cancer 2014, 135, 1038–1047. [Google Scholar] [CrossRef]
- Tani, S.; Blyth, B.J.; Shang, Y.; Morioka, T.; Kakinuma, S.; Shimada, Y. A Multi-stage Carcinogenesis Model to Investigate Caloric Restriction as a Potential Tool for Post-irradiation Mitigation of Cancer Risk. J. Cancer Prev. 2016, 21, 115–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tachibana, H.; Morioka, T.; Daino, K.; Shang, Y.; Ogawa, M.; Fujita, M.; Matsuura, A.; Nogawa, H.; Shimada, Y.; Kakinuma, S. Early induction and increased risk of precursor B-cell neoplasms after exposure of infant or young-adult mice to ionizing radiation. J. Radiat. Res. 2020, 61, 648–656. [Google Scholar] [CrossRef]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Yamashiro, H.; Abe, Y.; Fukuda, T.; Kino, Y.; Kawaguchi, I.; Kuwahara, Y.; Fukumoto, M.; Takahashi, S.; Suzuki, M.; Kobayashi, J.; et al. Effects of radioactive caesium on bull testes after the Fukushima nuclear plant accident. Sci. Rep. 2013, 3, 2850. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Ishiniwa, H.; Onuma, M.; Shindo, J.; Yokohata, Y.; Tamaoki, M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016, 6, 23601. [Google Scholar] [CrossRef]
- Takino, S.; Yamashiro, H.; Sugano, Y.; Fujishima, Y.; Nakata, A.; Kasai, K.; Hayashi, G.; Urushihara, Y.; Suzuki, M.; Shinoda, H.; et al. Analysis of the Effect of Chronic and Low-Dose Radiation Exposure on Spermatogenic Cells of Male Large Japanese Field Mice (Apodemus speciosus) after the Fukushima Daiichi Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Hermosell, I.G.; Laskemoen, T.; Rowe, M.; Moller, A.P.; Mousseau, T.A.; Albrecht, T.; Lifjeld, J.T. Patterns of sperm damage in Chernobyl passerine birds suggest a trade-off between sperm length and integrity. Biol. Lett. 2013, 9, 20130530. [Google Scholar] [CrossRef] [PubMed]
- Pomerantseva, M.D.; Ramaiya, L.K.; Chekhovich, A.V. Genetic disorders in house mouse germ cells after the Chernobyl catastrophe. Mutat. Res. 1997, 381, 97–103. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L.; Wei, Z.Q.; Li, W.J.; Gao, Q.X.; Chen, W.Q.; Wang, Z.H.; He, J.; Liang, J.P.; Han, G.W.; et al. Effects of pre-exposure of mouse testis with low-dose (16)O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int. J. Radiat. Biol. 1998, 73, 163–167. [Google Scholar] [CrossRef]
- Forand, A.; Messiaen, S.; Habert, R.; Bernardino-Sgherri, J. Exposure of the mouse perinatal testis to radiation leads to hypospermia at sexual maturity. Reproduction 2009, 137, 487–495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jahnukainen, K.; Ehmcke, J.; Quader, M.A.; Saiful Huq, M.; Epperly, M.W.; Hergenrother, S.; Nurmio, M.; Schlatt, S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum. Reprod. 2011, 26, 1945–1954. [Google Scholar] [CrossRef]
- Shin, S.C.; Kang, Y.M.; Jin, Y.W.; Kim, H.S. Relative morphological abnormalities of sperm in the caudal epididymis of high- and low-dose-rate gamma-irradiated ICR mice. J. Radiat. Res. 2009, 50, 261–266. [Google Scholar] [CrossRef]
- Komatsu, K.; Iwasaki, T.; Murata, K.; Yamashiro, H.; Goh, V.S.T.; Nakayama, R.; Fujishima, Y.; Ono, T.; Kino, Y.; Simizu, Y.; et al. Morphological reproductive characteristics of testes and fertilization capacity of cryopreserved sperm after the Fukushima accident in raccoon (Procyon lotor). Reprod. Domest. Anim. 2021, 56, 484–497. [Google Scholar] [CrossRef]
- Giotopoulos, G.; McCormick, C.; Cole, C.; Zanker, A.; Jawad, M.; Brown, R.; Plumb, M. DNA methylation during mouse hemopoietic differentiation and radiation-induced leukemia. Exp. Hematol. 2006, 34, 1462–1470. [Google Scholar] [CrossRef]
- Loree, J.; Koturbash, I.; Kutanzi, K.; Baker, M.; Pogribny, I.; Kovalchuk, O. Radiation-induced molecular changes in rat mammary tissue: Possible implications for radiation-induced carcinogenesis. Int. J. Radiat. Biol. 2006, 82, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Suelves, M.; Carrio, E.; Nunez-Alvarez, Y.; Peinado, M.A. DNA methylation dynamics in cellular commitment and differentiation. Brief. Funct. Genomics. 2016, 15, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Hacker-Klom, U.B. Age dependence of murine spermatogenesis. Z. Nat. C J. Biosci. 1995, 50, 303–310. [Google Scholar] [CrossRef]
- Borum, K. Oogenesis in the mouse. A study of the meiotic prophase. Exp. Cell Res. 1961, 24, 495–507. [Google Scholar] [CrossRef]
- Faddy, M.J. Follicle dynamics during ovarian ageing. Mol. Cell Endocrinol. 2000, 163, 43–48. [Google Scholar] [CrossRef]
- Kerr, J.B.; Brogan, L.; Myers, M.; Hutt, K.J.; Mladenovska, T.; Ricardo, S.; Hamza, K.; Scott, C.L.; Strasser, A.; Findlay, J.K. The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction 2012, 143, 469–476. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakata, A.; Sato, K.; Fujishima, Y.; Ting, V.G.S.; Nakayama, K.; Ariyoshi, K.; Tsuruoka, C.; Shang, Y.; Iizuka, D.; Kakinuma, S.; et al. Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice. Biology 2021, 10, 1270. https://doi.org/10.3390/biology10121270
Nakata A, Sato K, Fujishima Y, Ting VGS, Nakayama K, Ariyoshi K, Tsuruoka C, Shang Y, Iizuka D, Kakinuma S, et al. Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice. Biology. 2021; 10(12):1270. https://doi.org/10.3390/biology10121270
Chicago/Turabian StyleNakata, Akifumi, Keisuke Sato, Yohei Fujishima, Valerie Goh Swee Ting, Kanade Nakayama, Kentaro Ariyoshi, Chizuru Tsuruoka, Yi Shang, Daisuke Iizuka, Shizuko Kakinuma, and et al. 2021. "Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice" Biology 10, no. 12: 1270. https://doi.org/10.3390/biology10121270
APA StyleNakata, A., Sato, K., Fujishima, Y., Ting, V. G. S., Nakayama, K., Ariyoshi, K., Tsuruoka, C., Shang, Y., Iizuka, D., Kakinuma, S., Yamashiro, H., & Miura, T. (2021). Evaluation of Global DNA Methylation and Gene Expression of Izumo1 and Izumo1r in Gonads after High- and Low-Dose Radiation in Neonatal Mice. Biology, 10(12), 1270. https://doi.org/10.3390/biology10121270





