Singer’s Nodules: Investigating the Etiopathogenetic Markers Progressing Their Pathogenesis and Clinical Manifestations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Material Collection
2.2. Ethical Approval
2.3. Routine Staining and Immunohistochemistry
2.4. TUNEL Reaction
2.5. Immunohistochemistry Semi-Quantification and Visualization
2.6. Statistical Analysis
3. Results
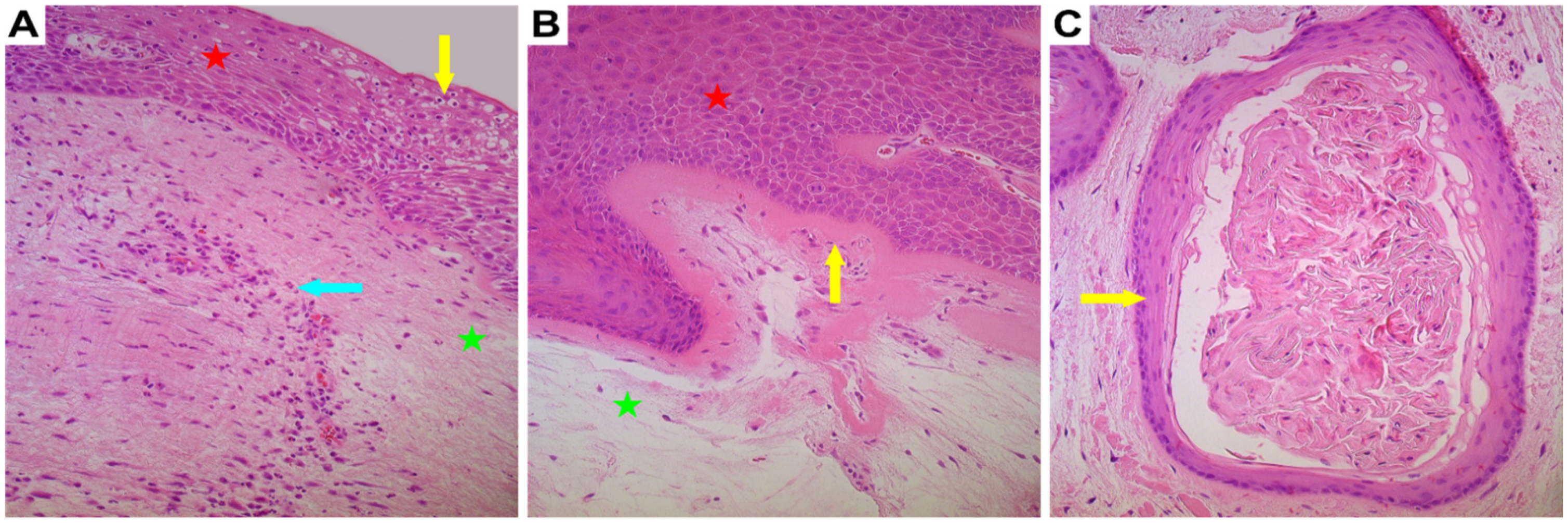
3.1. General Appearance of the Vocal Noduli Tissue
3.2. Vocal Noduli Showed Significantly Increased Epithelial Proliferation
3.3. Vocal Noduli Showed Significantly Increased Epithelial Apoptosis
3.4. Vocal Noduli Showed Significantly Increased Epithelial Cellular Growth
3.5. Vocal Noduli Tissue Showed Significant Ischaemic Compensatory Changes
3.6. Vocal Noduli Showed Presence of Pro-Inflammatory Environment
3.7. No Significant Difference in Neural Innervation Was Found in Vocal Noduli
3.8. Correlation Analysis between Different Immunohistochemical Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cipriani, N.A.; Martin, D.E.; Corey, J.P.; Portugal, L.; Caballero, N.; Lester, R.; Anthony, B.; Taxy, J.B. The clinicopathologic spectrum of benign mass lesions of the vocal fold due to vocal abuse. Int. J. Surg. Pathol. 2011, 19, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Comparative research on vocal polyps and nodules. Zhonghua Er Bi Yan Hou Ke Za Zhi 1989, 24, 53–55. (In Chinese) [Google Scholar] [PubMed]
- Shirendeb, U.; Hishikawa, Y.; Moriyama, S.; Win, N.; Thu, M.M.; Mar, K.S.; Khatanbaatar, G.; Masuzaki, H.; Koji, T. Human papillomavirus infection and its possible correlation with p63 expression in cervical cancer in Japan, Mongolia, and Myanmar. Acta Histochem. Cytochem. 2009, 42, 181–190, PMCID:PMC2808501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooghe, B.; Hulpiau, P.; van Roy, F.; De Bleser, P. ConTra: A promoter alignment analysis tool for identification of transcription factor binding sites across species. Nucleic Acids Res. 2008, 36, W128–W132, PMCID:PMC2447729. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5, PMCID:PMC6108547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, M.; Mukai, H.; Nagai, S.; Onozawa, M.; Nihei, K.; Shimada, T.; Wada, N. Retrospective analysis of risk factors for central nervous system metastases in operable breast cancer: Effects of biologic subtype and Ki67 overexpression on survival. Oncology 2013, 84, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, S.W.; Kilvaer, T.K.; Valkov, A.; Donnem, T.; Smeland, E.; Al-Shibli, K.; Bremnes, R.M.; Busund, L.T. Prognostic impact of Jab1, p16, p21, p62, Ki67 and Skp2 in soft tissue sarcomas. PLoS ONE 2012, 7, e47068, PMCID:PMC3465267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciancio, N.; Galasso, M.G.; Campisi, R.; Bivona, L.; Migliore, M.; Di Maria, G.U. Prognostic value of p53 and Ki67 expression in fiberoptic bronchial biopsies of patients with non small cell lung cancer. Multidiscip. Respir. Med. 2012, 7, 29, PMCID:PMC3537558. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, A.; Wikström, P.; Egevad, L.; Granfors, T.; Karlberg, L.; Stattin, P.; Bergh, A. Low endoglin vascular density and Ki67 index in Gleason score 6 tumours may identify prostate cancer patients suitable for surveillance. Scand. J. Urol. Nephrol. 2012, 46, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Novaleski, C.K.; Mizuta, M.; Rousseau, B. Evaluation of Dying Vocal Fold Epithelial Cells by Ultrastructural Features and TUNEL Method. Cells Tissues Organs 2016, 202, 355–368, PMCID:PMC5136523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdan, A.L.; Tabet, G.; Saadeddin, Z.; Btaiche, R.; Khalifeh, I. Apoptosis in Vocal Fold Polyps. J. Voice 2018, 32, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signalling by VEGF. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Austin, TX, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6482/ (accessed on 6 November 2021).
- Fang, R.; Jiang, J.J.; Smith, B.L.; Wu, D. Expression of hypoxia inducible factor-1α and vascular endothelia growth factor in vocal polyps. Laryngoscope 2013, 123, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732, PMCID:PMC3083294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branski, R.C.; Rosen, C.A.; Verdolini, K.; Hebda, P.A. Biochemical markers associated with acute vocal fold wound healing: A rabbit model. J. Voice 2005, 19, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.H.; Defaveri, J.; Domingues, M.A.C.; e Silva, R.d.a.; Fabro, A. Vocal fold nodules: Morphological and immunohistochemical investigations. J. Voice 2010, 24, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yiu, E.M.; Chan, K.M.; Li, N.Y.; Tsang, R.; Verdolini Abbott, K.; Kwong, E.; Ma, E.P.; Tse, F.W.; Lin, Z. Wound-healing effect of acupuncture for treating phonotraumatic vocal pathologies: A cytokine study. Laryngoscope 2016, 126, E18–E22, PMCID:PMC4715648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, S.N.; Guille, J.; Thibeault, S.L. Characterization of the Leukocyte Response in Acute Vocal Fold Injury. PLoS ONE 2015, 10, e0139260, PMCID:PMC4591973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, P.; Ongkasuwan, J.; Martinez, J.; Sandulache, V.; Deng, D.; Jiang, J.; Sikora, A.; Altman, K.W. Biomarkers for Malignant Potential in Vocal Fold Leukoplakia: A State-of-the-Art Review. Otolaryngol. Head Neck Surg. 2021, 164, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Albegger, K.; Hauser-Kronberger, C.E.; Saria, A.; Graf, A.H.; Bernatzky, G.; Hacker, G.W. Regulatory peptides and general neuroendocrine markers in human nasal mucosa, soft palate and larynx. Acta Otolaryngol. 1991, 111, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Atoji, Y.; Hobo, S.; Yoshihara, T.; Suzuki, Y. Morphology of the nerve endings in laryngeal mucosa of the horse. Equine Vet. J. 2001, 33, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Tanaka, S.; Tsubone, H.; Atoji, Y.; Suzuki, Y. Age-related changes in sensory and secretomotor nerve endings in the larynx of F344/N rat. Arch. Gerontol. Geriatr. 2003, 36, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59 (Suppl. S2), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Braut, T.; Krstulja, M.; Marijić, B.; Maržić, D.; Kujundžić, M.; Brumini, G.; Vučinić, D.; Oštarijaš, E. Immunohistochemical analysis of vocal cord polyps applying markers of squamous cell carcinogenesis. Pathol. Res. Pract. 2019, 215, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Nunes, R.B.; Behlau, M.; Nunes, M.B.; Paulino, J.G. Clinical diagnosis and histological analysis of vocal nodules and polyps. Braz. J. Otorhinolaryngol. 2013, 79, 434–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negoescu, A.; Guillermet, C.; Lorimier, P.; Brambilla, E.; Labat-Moleur, F. Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed. Pharmacother. 1998, 52, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Pilmane, M.; Jain, N.; Vitenberga-Verza, Z. Expression Analysis of FGF/FGFR and FOX Family Proteins in Mucosal Tissue Obtained from Orofacial Cleft-Affected Children. Biology 2021, 10, 423, PMCID:PMC8151933. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Zhang, Y.Y.; Kang, J.; Dou, Q.H.; Zhu, X.F. A bispecific decoy receptor VEGFR-EGFR/Fc binding EGF-like ligands and VEGF shows potent antitumor efficacy. J. Drug Target. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hogquist, K.A.; Nett, M.A.; Unanue, E.R.; Chaplin, D.D. Interleukin 1 is processed and released during apoptosis. Proc. Natl. Acad. Sci. USA 1991, 88, 8485–8489, PMCID:PMC52533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leydon, C.; Imaizumi, M.; Bartlett, R.S.; Wang, S.F.; Thibeault, S.L. Epithelial cells are active participants in vocal fold wound healing: An in vivo animal model of injury. PLoS ONE 2014, 9, e115389, PMCID:PMC4267843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, W.X.; Xu, W.; Yang, Q.W.; Li, Y.R.; Hu, R.; Cheng, L.Y. The expression characteristics and clinical significance of candidate molecular markers in vocal cord leukoplakia. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2018, 53, 592–596. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.; Quinchia Rios, B.; Bartlett, R.; Berchtold, C.; Thibeault, S.L. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS ONE 2012, 7, e30965, PMCID:PMC3281043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhalgh, D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low. Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Novaleski, C.K.; Carter, B.D.; Sivasankar, M.P.; Ridner, S.H.; Dietrich, M.S.; Rousseau, B. Apoptosis and Vocal Fold Disease: Clinically Relevant Implications of Epithelial Cell Death. J. Speech Lang. Hear. Res. 2017, 60, 1264–1272, PMCID:PMC5755547. [Google Scholar] [CrossRef] [PubMed]
- Bick, E.; Dumberger, L.D.; Farquhar, D.R.; Davis, H.; Ramsey, E.; Buckmire, R.A.; Shah, R.N. Does Voice Therapy Improve Vocal Outcomes in Vocal Fold Atrophy? Ann. Otol. Rhinol. Laryngol. 2021, 130, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.K.; Menier, J.; Grant, N. Voice Changes in the Elderly. Otolaryngol. Clin. North Am. 2018, 51, 759–768. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibody * | Antibody Characteristics ** | Clone | Dilution | Catalogue No. | Manufacturer |
|---|---|---|---|---|---|
| Ki-67 | Monoclonal rabbit AB against human AG | SP6 | 1:100 | 1325506A | Cell Marque (USA) |
| IL-1α | Polyclonal rabbit AB against human AG | - | 1:100 | orb308737 | Biorbyt (UK) |
| IL-10 | Polyclonal rabbit AB against human AG | - | 1:100 | 250713 | Abbiotec (USA) |
| VEGF | Polyclonal rabbit AB against human AG | - | 1:100 | orb191500 | Biorbyt (UK) |
| PGP 9.5 | Polyclonal rabbit AB against human AG | - | 1:100 | 439273A | Invitrogen (USA) |
| EGFR | Monoclonal mouse AB against human AG | 0.N.26 | 1:100 | sc-71034 | Santa Cruz (USA) |
| Grade Assigned | Interpretation |
|---|---|
| 0 | No positive structures |
| 0/+ | Occasionally positive structures |
| + | Few positive structures |
| +/++ | Few to moderate positive structures |
| ++ | Moderate positive structures |
| ++/+++ | Moderate to numerous positive structures |
| +++ | Numerous positive structures |
| +++/++++ | Numerous to abundant positive structures |
| ++++ | Abundant positive structures |
| Patient No. | Sex | Age | Ki-67 | TUNEL | EGFR | VEGF | IL-1α | IL-10 | PGP 9.5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epi | Endo | Epi | C.T. | ||||||||
| 1 | F | 7 | + | +++ | ++/+++ | ++ | +++ | +++ | ++ | 0 | 0/+ |
| 2 | F | 18 | + | ++ | ++ | ++/+++ | +++ | +++ | ++ | 0 | +/++ |
| 3 | F | 28 | ++ | ++/+++ | +++ | +++ | ++ | +++ | +++ | + | +/++ |
| 4 | F | 32 | ++ | ++++ | ++/+++ | +++ | ++ | +++ | +++ | ++ | ++ |
| 5 | F | 32 | +/++ | ++/+++ | ++ | +++ | +++ | +++/++++ | +++ | 0/+ | +/++ |
| 6 | F | 42 | +++ | ++++ | ++++ | +++ | +++ | +++ | +++ | ++ | 0/+ |
| 7 | F | 43 | +/++ | +++ | ++ | ++ | ++ | +++ | +++/++++ | 0 | 0/+ |
| 8 | F | 45 | +/++ | +++ | +++/++++ | +++ | +++ | +++ | ++/+++ | 0 | + |
| 9 | F | 55 | ++ | +++ | +++ | ++ | ++ | +++ | +++ | + | + |
| 10 | F | 56 | ++ | +++ | +++ | +++ | +++ | +++ | +++ | + | ++ |
| Avg. Vocal Noduli Tissue | ++ | +++ | +++ | +++ | ++/+++ | +++ | +++ | Var 0 to ++ | Var 0/+ to ++ | ||
| Avg. Control Tissue | 0/+ | +/++ | ++ | 0 | 0/+ | 0 | 0/++ | +/++ | + | ||
| Difference (p value) | 0.0003 ** | 0.0006 ** | 0.005 ** | 0.0003 ** | 0.0003 ** | 0.0003 ** | 0.0003 ** | 0.050 | 0.091 | ||
| Strength of Correlation | Factor 1 ** | Factor 2 ** | Rho (ρ) | p Value |
|---|---|---|---|---|
| Strong positive correlation (ρ = 0.7–0.9) | Ki-67 | TUNEL | 0.888 | <0.001 |
| Ki-67 | VEGF | 0.884 | <0.001 | |
| Ki-67 | EGFR | 0.839 | <0.001 | |
| Ki-67 | IL-1α (Epithelium) | 0.834 | <0.001 | |
| Ki-67 | IL-1α (Connective tissue) | 0.899 | <0.001 | |
| TUNEL | VEGF | 0.802 | <0.001 | |
| TUNEL | IL-1α (Epithelium) | 0.786 | <0.001 | |
| TUNEL | IL-1α (Connective tissue) | 0.820 | <0.001 | |
| TUNEL | EGFR | 0.753 | <0.001 | |
| EGFR | VEGF | 0.750 | <0.001 | |
| IL-1α (Epithelium) | IL-1α (Connective tissue) | 0.868 | <0.001 | |
| VEGF | IL-1α (Epithelium) | 0.889 | <0.001 | |
| VEGF | IL-1α (Connective tissue) | 0.745 | <0.001 | |
| Moderate positive correlation (ρ = 0.5–0.7) | Ki-67 | PGP 9.5 | 0.504 | 0.032 |
| EGFR | IL-1α (Epithelium) | 0.625 | 0.005 | |
| EGFR | IL-1α (Connective tissue) | 0.611 | 0.007 | |
| VEGF | PGP 9.5 | 0.614 | 0.006 | |
| Weak positive correlation (ρ = 0.3–0.5) | IL-1α (Epithelium) | PGP 9.5 | 0.469 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilmane, M.; Sumerags, D.; Jain, N.; Jain, S.; Sumeraga, G. Singer’s Nodules: Investigating the Etiopathogenetic Markers Progressing Their Pathogenesis and Clinical Manifestations. Biology 2021, 10, 1268. https://doi.org/10.3390/biology10121268
Pilmane M, Sumerags D, Jain N, Jain S, Sumeraga G. Singer’s Nodules: Investigating the Etiopathogenetic Markers Progressing Their Pathogenesis and Clinical Manifestations. Biology. 2021; 10(12):1268. https://doi.org/10.3390/biology10121268
Chicago/Turabian StylePilmane, Mara, Dins Sumerags, Nityanand Jain, Shivani Jain, and Gunta Sumeraga. 2021. "Singer’s Nodules: Investigating the Etiopathogenetic Markers Progressing Their Pathogenesis and Clinical Manifestations" Biology 10, no. 12: 1268. https://doi.org/10.3390/biology10121268
APA StylePilmane, M., Sumerags, D., Jain, N., Jain, S., & Sumeraga, G. (2021). Singer’s Nodules: Investigating the Etiopathogenetic Markers Progressing Their Pathogenesis and Clinical Manifestations. Biology, 10(12), 1268. https://doi.org/10.3390/biology10121268







