A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment
Abstract
Simple Summary
Abstract
1. Introduction
2. Commercialization of GM Rapeseed
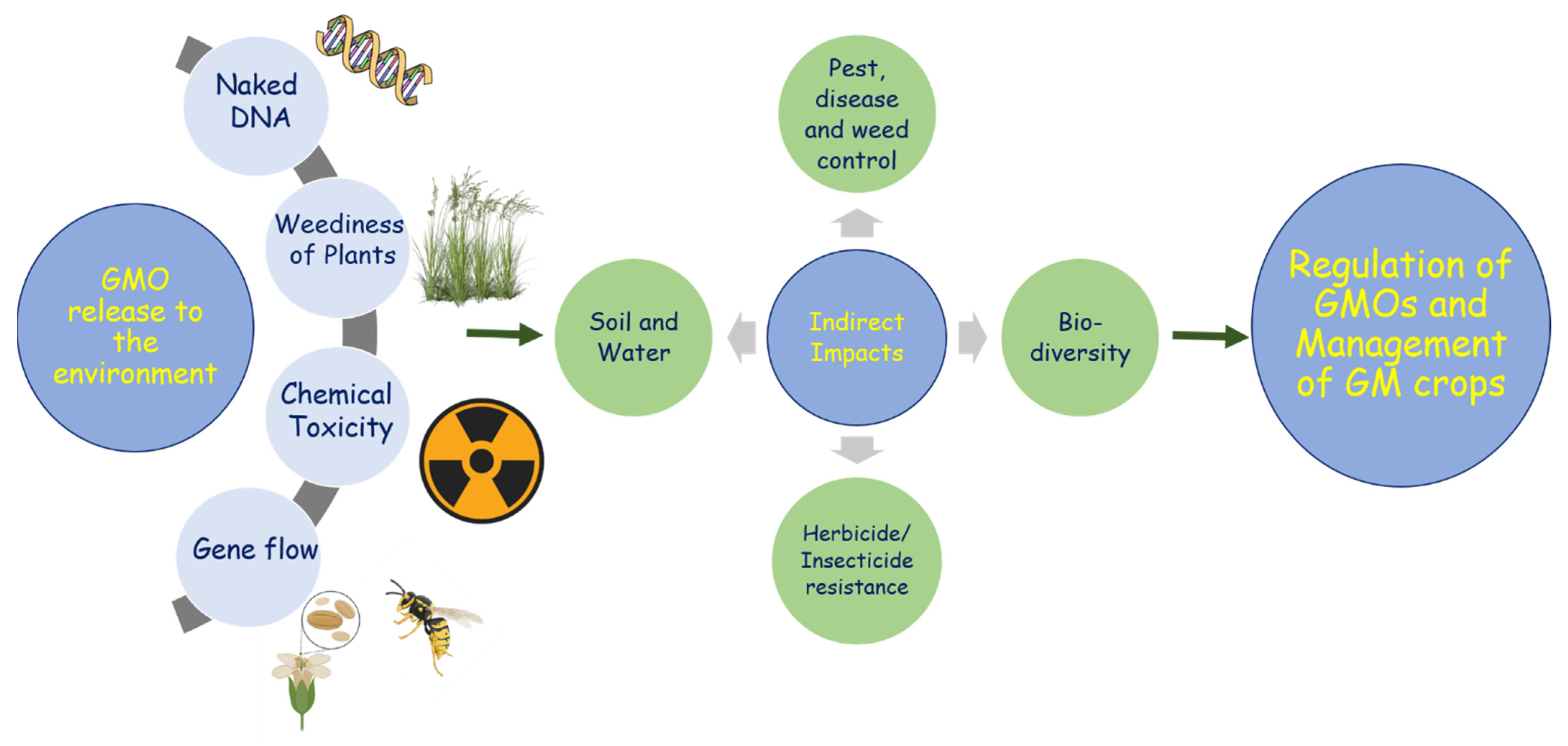
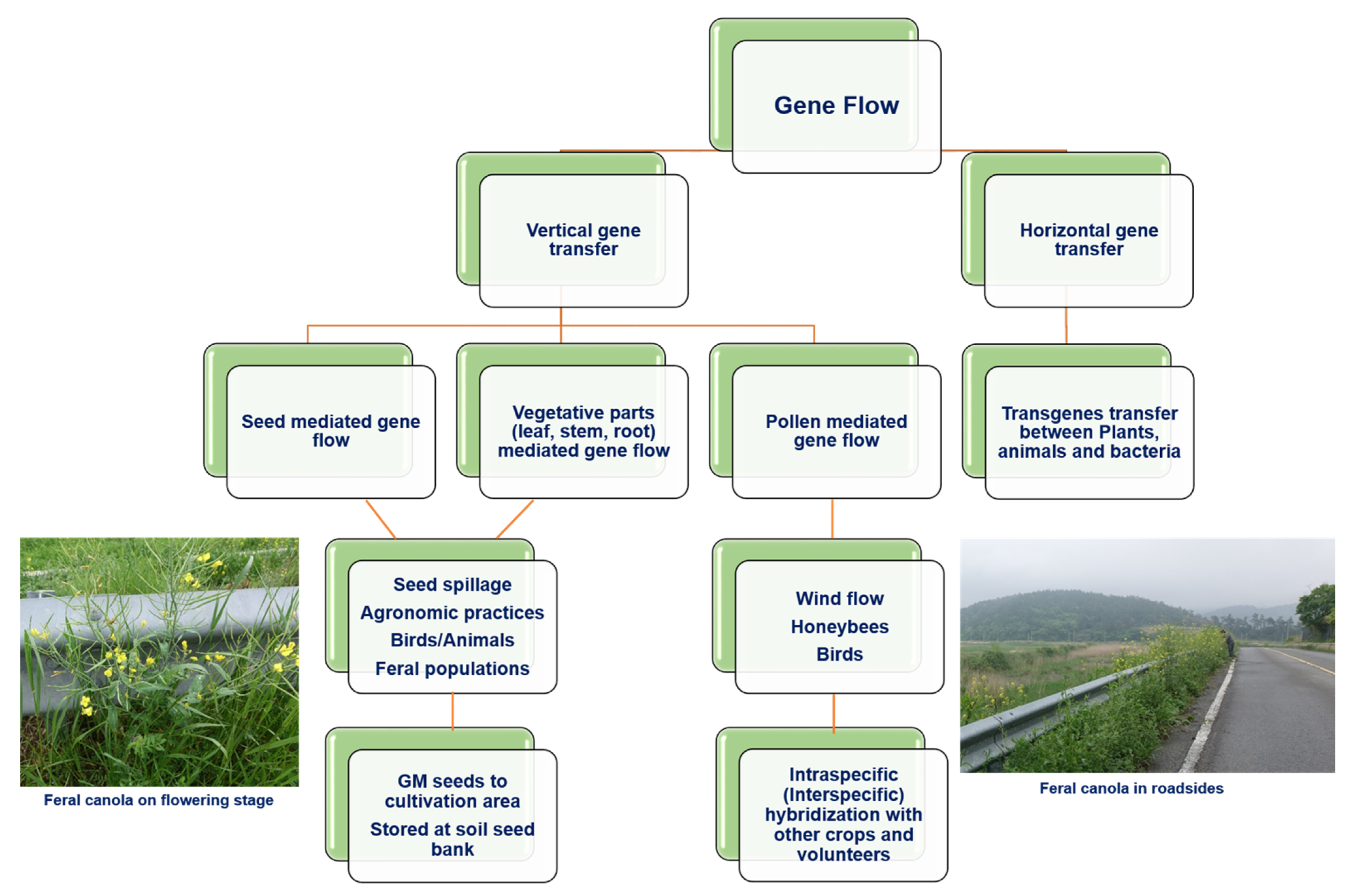
3. Cases for the Unintentional Release of GM Rapeseed in Various Countries
3.1. Japan
3.2. USA
3.3. Canada
3.4. Switzerland
3.5. Australia
3.6. Argentina
3.7. European Union
3.7.1. Austria
3.7.2. France
3.7.3. Germany
3.7.4. Sweden
4. Management Practices to Avoid the Unintentional Release of GM Rapeseed
- Controlling the seed production of Brassica crops in isolated areas in order to meet conventional purity standards for certified seed,
- Cultivating certified seed to reduce the risk of off-types with altered traits,
- Isolating fields of GM rapeseed cultivars to limit out-crossing, and
- Harvesting GM rapeseed at the right development stage of the crop with well-adjusted settings.
- To ensure the maximum germination of spilled seeds, avoid deep soil inversion for at least 3–4 weeks after harvest and use ploughing as the primary tillage method before planting the following crop.
- Applying suitable herbicide applications and planting a competitive crop following rapeseed to ensure an effective weed control in subsequent harvests,
- Rotating rapeseed in a lengthy and diversified cropping sequence to decrease the seed bank over time, and
- Preserving precise on-farm records to track a plot’s history.
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD. Consensus document on the biology of the Brassica crops (Brassica spp.). Series on Harmonisation of Regulatory oversight of Biotechnology. OECD 2012, 54, 142. [Google Scholar]
- Food and Agriculture Organization (FAO). Oilcrops, Oils and Meals, Food—Outlook, 2020. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Oilcrops/Documents/Food_outlook_oilseeds/Oilcrops_Oils_and_Meals_Food_Outlook_2020.pdf (accessed on 25 September 2021).
- Liu, Y.; Wei, W.; Ma, K.; Li, J.; Liang, Y.; Darmency, H. Consequences of gene flow between oilseed rape (Brassica napus) and its relatives. Plant Sci. 2013, 211, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, P.; Marasek-Ciolakowska, A.; Podwyszyńska, M.; Starzycki, M.; Starzycka-Korbas, E.; Nowak, K. Development and Characteristics of Interspecific Hybrids between Brassica oleracea L. and B. napus L. Agronomy 2020, 10, 1339. [Google Scholar] [CrossRef]
- Pant, U.; Joshi, S.; Lohani, P.; Dahiya, N. Pre-fertilization Barrier, Crossability and Meiotic Behavior of Interspecific Hybrids Among Brassica Species. Res. Sq. 2021. [CrossRef]
- Salisbury, P. Gene Flow between Brassica Napus and Other Brassicaceae Species; Report PAS0201; Institute of Land and Food Resources, University of Melbourne: Melbourne, Australia, 2002. [Google Scholar]
- Légère, A. Risks and consequences of gene flow from herbicide-resistant crops: Canola (Brassica napus L.) as a case study. Pest Manag. Sci. 2005, 61, 292–300. [Google Scholar] [CrossRef]
- Zhang, C.J.; Yook, M.J.; Park, H.R.; Lim, S.H.; Kim, J.W.; Song, J.S.; Nah, G.; Song, H.R.; Jo, B.H.; Roh, K.H.; et al. Evaluation of maximum potential gene flow from herbicide resistant Brassica napus to its male sterile relatives under open and wind pollination conditions. Sci. Total Environ. 2018, 634, 821–830. [Google Scholar] [CrossRef]
- Lihoreau, M.; Raine, N.E.; Reynolds, A.M.; Stelzer, R.J.; Lim, K.S.; Smith, A.D.; Osborne, J.L.; Chittka, L. Unravelling the mechanisms of trapline foraging in bees. Commun. Integr. Biol. 2013, 6, e1001392. [Google Scholar] [CrossRef]
- Ramsey, J.; Bradshaw, H.D.; Schemske, D.W. Components of reproductive isolation between the monkey flowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 2003, 57, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Paudel, B.R.; Burd, M.; Shrestha, M.; Dyer, A.G.; Li, Q.J. Reproductive isolation in alpine gingers: How do coexisting Roscoea (R. purpurea and R. tumjensis) conserve species integrity? Evolution 2018, 72, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Squire, G.R.; Breckling, B.; Dietz-Pfeilstetter, A.; Jørgensen, R.B.; Lecomte, J.; Pivard, S.; Reuter, H.; Young, M.W. Status of feral oilseed rape in Europe: Its minor role as a GM impurity and its potential as a reservoir of transgene persistence. Environ. Sci. Pollut. Res. 2011, 18, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Canola Growers Manual; Canola Council of Canada: Winnipeg, MB, Canada, 2003. [Google Scholar]
- Jursík, M.; Kolářová, M.; Soukup, J. Competition, reproduction ability, and control possibilities of conventional and Clearfield® volunteer oilseed rape in winter wheat. Crop Prot. 2019, 122, 30–34. [Google Scholar] [CrossRef]
- Pivard, S.; Adamczyk, K.; Lecomte, J.; Lavigne, C.; Bouvier, A.; Deville, A.; Gouyon, P.H.; Huet, S. Where do the feral oilseed rape populations come from? A large-scale study of their possible origin in a farmland area. J. Appl. Ecol. 2008, 45, 476–485. [Google Scholar] [CrossRef]
- Knispel, A.L.; McLachlan, S.M. Landscape-scale distribution and persistence of genetically modified oilseed rape (Brassica napus) in Manitoba, Canada. Environ. Sci. Pollut. Res. 2010, 17, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Franzaring, J.; Wedlich, K.; Fangmeier, A.; Eckert, S.; Zipperle, J.; Krah-Jentgens, I.; Hünig, C.; Züghart, W. Exploratory study on the presence of GM oilseed rape near German oil mills. Environ. Sci. Pollut. Res. 2016, 23, 23300–23307. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, D.; Ollier, S.; Lecomte, J. Long-distance dispersal of oilseed rape seeds: The role of grain trailers. bioRxiv 2020. [Google Scholar] [CrossRef]
- Simard, M.J.; Légère, A.; Pageau, D.; Lajeunnesse, J.; Warwick, S. The frequency and persistence of canola (Brassica napus) volunteers in Que´bec cropping systems. Weed Technol. 2002, 16, 433–439. [Google Scholar] [CrossRef]
- Belter, A. Long-term monitoring of field trial sites with genetically modified oilseed rape (Brassica napus L.) in Saxony-Anhalt, Germany. Fifteen years persistence to date but no spatial dispersion. Genes 2016, 7, 3. [Google Scholar] [CrossRef]
- Warwick, S.I.; Simard, M.J.; Legere, A.; Beckie, H.J.; Braun, L.; Zhu, B.; Mason, P.; Seguin-Swartz, G.; Stewart, C.N., Jr. Hybridization between transgenic Brassica napus L. and its wild relatives: B. rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O.E. Schulz. Theor. Appl. Genet. 2003, 107, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gruber, S.; Claupein, W. Timing and depth of post-harvest soil disturbance can reduce seedbank and volunteers of oilseed rape. Soil Tillage Res. 2018, 175, 187–193. [Google Scholar] [CrossRef]
- Brown, C.H. Secondary Dormancy of a Diverse Collection of Annual Brassica napus L. Genotypes and the Relationship with Seed Germination, Vigour and Quality Traits. Ph.D. Thesis, University of Saskatchewan, Saskatoon, SA, Canada, 2019. [Google Scholar]
- Hilhorst, H.W.; Toorop, P.E. Review on dormancy, germinability, and germination in crop and weed seeds. Adv. Agron. 1997, 61, 112–165. [Google Scholar]
- Soltani, E.; Baskin, J.M.; Baskin, C.C. A review of the relationship between primary and secondary dormancy, with reference to the volunteer crop weed oilseed rape (Brassica napus). Weed Res. 2019, 59, 5–14. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Gulden, R.H.; Shirtliffe, S.J.; Thomas, A.G. Secondary seed dormancy prolongs persistence of volunteer canola in western Canada. Weed Sci. 2003, 51, 904–913. [Google Scholar] [CrossRef]
- ISAAA. Global Status of Commercialized Biotech/GM Crops. 2019. Available online: https://www.isaaa.org/resources/publications/briefs/55/default.asp (accessed on 1 July 2021).
- Yoshimura, Y.; Beckie, H.J.; Matsuo, K. Transgenic oilseed rape along transportation routes and port of Vancouver in western Canada. Environ. Biosafety Res. 2007, 5, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Devos, Y.; Hails, R.S.; Messéan, A.; Perry, J.N.; Squire, G.R. Feral genetically modified herbicide tolerant oilseed rape from seed import spills: Are concerns scientifically justified? Trans. Res. 2012, 21, 1–21. [Google Scholar] [CrossRef]
- Frieß, J.L.; Breckling, B.; Pascher, K.; Schröder, W. Case Study 2: Oilseed Rape (Brassica napus L.). In Gene Drives at Tipping Point; Springer: Cham, Switzerland, 2020; pp. 103–145. [Google Scholar]
- Beckie, H.J.; Se´guin-Swartz, G.; Nair, H.; Warwick, S.I.; Johnson, E. Multiple herbicide-resistant canola (Brassica napus) can be controlled by alternative herbicides. Weed Sci. 2004, 52, 152–157. [Google Scholar] [CrossRef]
- Rostoks, N.; Grantiņa-Ieviņa, L.; Ieviņa, B.; Evelone, V.; Valciņa, O.; Aleksejeva, I. Genetically modified seeds and plant propagating material in Europe: Potential routes of entrance and current status. Heliyon 2019, 5, 01242. [Google Scholar] [CrossRef]
- Padgette, S.R.; Kolacz, K.H.; Delannay, X.; Re, D.B.; LaVallee, B.J.; Tinius, C.N.; Rhodes, W.K.; Otero, Y.I.; Barry, G.F.; Eichholtz, D.A.; et al. Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Sci. 1995, 35, 1451–1461. [Google Scholar] [CrossRef]
- Barry, G.F.; Kishore, G.M. Glyphosate Tolerance Crop Plants. U.S. Patent 5,463,175, 31 October 1995. [Google Scholar]
- Thompson, C.J.; Movva, N.R.; Tizard, R.; Crameri, R.; Davies, J.E.; Lauwereys, M.; Botterman, J. Characterization of the herbicide-resistance gene bar from Streptomyces hygroscopicus. EMBO J. 1987, 6, 2519–2523. [Google Scholar] [CrossRef]
- Paddon, C.J.; Hartley, R.W. Cloning, sequencing and transcription of an inactivated copy of Bacillus amyloliquefaciens extracellular ribonuclease (barnase). Gene 1985, 40, 231–239. [Google Scholar] [CrossRef]
- Mariani, C.; De Beuckeleer, M.; Truettner, J.; Leemans, J.; Goldberg, R.B. Induction of male sterility in plants by a chimeric ribonuclease gene. Nature 1990, 347, 737–741. [Google Scholar] [CrossRef]
- Katsuta, K.; Matsuo, K.; Yoshimura, Y.; Ohsawa, R. Long-term monitoring of feral genetically modified herbicide-tolerant Brassica napus populations around unloading Japanese ports. Breed. Sci. 2015, 65, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Hoshikawa, K.; Shimono, A.; Ohsawa, R. Transportability of confined field trial data from cultiva-tion to import countries for environmental risk assessment of genetically modified crops. Transgenic Res. 2015, 24, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Yook, M.J.; Park, H.R.; Lim, S.H.; Kim, J.W.; Nah, G.; Song, H.R.; Jo, B.H.; Roh, K.H.; Park, S.; et al. Assessment of potential environmental risks of transgene flow in smallholder farming systems in Asia: Brassica napus as a case study in Korea. Sci. Total Environ. 2018, 640, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Pessel, F.D.; Lecomte, J.; Emeriau, V.; Krouti, M.; Messe´an, A.; Gouyon, P.H. Persistence of oilseed rape (Brassica napus L.) outside of cultivated fields. Theor. Appl. Genet. 2001, 102, 841–846. [Google Scholar] [CrossRef]
- Pivard, S.; Demšar, D.; Lecomte, J.; Debeljak, M.; Džeroski, S. Characterizing the presence of oilseed rape feral populations on field margins using machine learning. Ecol. Model. 2008, 212, 147–154. [Google Scholar] [CrossRef]
- Garnier, A.; Pivard, S.; Lecomte, J. Measuring and modelling anthropogenic secondary seed dispersal along roadverges for feral oilseed rape. Basic Appl. Ecol. 2008, 9, 533–541. [Google Scholar] [CrossRef]
- Saji, H.; Nakajima, N.; Aono, M.; Tamaoki, M.; Kubo, A.; Wakiyama, S.; Hatase, Y.; Nagatsu, M. Monitoring the escape of transgenic oilseed rape around Japanese ports and roadsides. Environ. Biosafety Res. 2005, 4, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Aono, M.; Wakiyama, S.; Nagatsu, M.; Nakajima, N.; Tamaoki, M.; Kubo, A.; Saji, H. Detection of feral transgenic oilseed rape with multiple-herbicide resistance in Japan. Environ. Biosaf. Res. 2006, 5, 77–87. [Google Scholar] [CrossRef]
- Kawata, M.; Murakami, K.; Ishikawa, T. Dispersal and persistence of genetically modified oilseed rape around Japanese harbors. Environ. Sci. Pollut. Res. 2009, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Tamaoki, M.; Aono, M.; Kubo, A.; Saji, H.; Nakajima, N. Rapeseed species and environmental concerns related to loss of seeds of genetically modified oilseed rape in Japan. GM Crops. 2010, 1, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Mizuguti, A.; Yoshimura, Y.; Shibaike, H.; Matsuo, K. Persistence of feral populations of Brassica napus originated from spilled seeds around the Kashima seaport in Japan. Jpn. Agric. Res. 2011, 45, 181–185. [Google Scholar] [CrossRef][Green Version]
- Aono, M.; Wakiyama, S.; Nagatsu, M.; Kaneko, Y.; Nishizawa, T.; Nakajima, N.; Tamaoki, M.; Kubo, A.; Saji, H. Seeds of a possible natural hybrid between herbicide-resistant Brassica napus and Brassica rapa detected on a riverbank in Japan. GM Crop. 2011, 2, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Nishizawa, T.; Aono, M.; Tamaoki, M.; Saji, H. Occurrence of spilled genetically modified oilseed rape growing along a Japanese roadside over 10 years. Weed Biol. Manag. 2020, 20, 139–146. [Google Scholar] [CrossRef]
- Hall, L.; Topinka, K.; Huffman, J.; Davis, L.; Good, A. Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci. 2000, 48, 688–694. [Google Scholar] [CrossRef]
- Friesen, L.F.; Nelson, A.G.; Van Acker, R.C. Evidence of contamination of pedigreed canola (Brassica napus) seedlots in western Canada with genetically modified herbicide resistance traits. Agron. J. 2003, 95, 1342–1347. [Google Scholar] [CrossRef]
- Warwick, S.I.; Legere, A.; Simard, M.-J.; James, T. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Mol. Ecol. 2008, 17, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Knispel, A.L.; McLachlan, S.M.; Van Acker, R.C.; Friesen, L.F. Gene flow and multiple herbicide resistance in escaped canola populations. Weed Sci. 2008, 56, 72–80. [Google Scholar] [CrossRef]
- Schafer, M.G.; Ross, A.A.; Londo, J.P.; Burdick, C.A.; Lee, E.H.; Travers, S.E.; Van de Water, P.K.; Sagers, C.L. The establishment of genetically engineered canola populations in the US. PLoS ONE 2011, 6, e25736. [Google Scholar] [CrossRef] [PubMed]
- Munier, D.J.; Brittan, K.L.; Lanini, W.T. Seed bank persistence of genetically modified canola in California. Environ. Sci. Pollut. Res. 2012, 19, 2281–2284. [Google Scholar] [CrossRef]
- Schoenenberger, N.; D’Andrea, L. Surveying the occurrence of subspontaneous glyphosate-tolerant genetically engineered Brassica napus L.(Brassicaceae) along Swiss railways. Environ. Sci. Eur. 2012, 24, 1–8. [Google Scholar] [CrossRef]
- Schulze, J.; Frauenknecht, T.; Brodmann, P.; Bagutti, C. Unexpected diversity of feral genetically modified oilseed rape (Brassica napus L.) despite a cultivation and import ban in Switzerland. PLoS ONE 2014, 9, e114477. [Google Scholar] [CrossRef]
- Hecht, M.; Oehen, B.; Schulze, J.; Brodmann, P.; Bagutti, C. Detection of feral GT73 transgenic oilseed rape (Brassica napus) along railway lines on entry routes to oilseed factories in Switzerland. Environ. Sci. Pollut. Res. 2014, 21, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, C.E.; Presotto, A.; Carbonell, F.T.; Ureta, S.; Poverene, M.; Cantamutto, M. Transgenic glyphosate-resistant oilseed rape (Brassica napus) as an invasive weed in Argentina: Detection, characterization, and control alternatives. Environ. Sci. Pollut. Res. 2016, 23, 24081–24091. [Google Scholar] [CrossRef] [PubMed]
- Berben, G.Y. Yat-il des colzas transgéniques dans l’environnement Wallon. CRAW-Info 2008, 18, 3. [Google Scholar]
- Berben, G. L’environnement de la re´gion Wallonne comprend du colza transge´nique. CRAW-Info 2009, 24, 3. [Google Scholar]
- Mbongolo Mbella, G.; Vandermassen, E.; Van Geel, D.; Sneyers, M.; Broeders, S.; Roosens, S. Federal Public Service of Health, Food Chain Safety and Environment/Contract Fp2010-1: Report from the Gmo Laboratory of the Scientific Institute of Public Health; Gmo Laboratory of the Scientific Institute of Public Health: Brussels, Belgium, 2010. [Google Scholar]
- Pascher, K.; Narendja, F.; Rau, D. Feral Oilseed Rape—Investigations on Its Potential for Hybridisation; The Federal Ministry of Health and Women’s Affairs: Vienna, Austria, 2006. [Google Scholar]
- Pascher, K.; Macalka, S.; Rau, D.; Gollmann, G.; Reiner, H.; Glössl, J.; Grabherr, G. Molecular differentiation of commercial varieties and feral populations of oilseed rape (Brassica napus L.). BMC Evol. Biol. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Pascher, K.; Hainz-Renetzeder, C.; Gollmann, G.; Schneeweiss, G.M. Spillage of viable seeds of oilseed rape along transportation routes: Ecological risk assessment and perspectives on management efforts. Front. Ecol. Evol. 2017, 5, 104. [Google Scholar] [CrossRef]
- Deville, A. Suivi de Terrain, Expérimentations et Mode´Lisation: Des Approches Complémentaires Pour Létude de L’impact des Populations de Colza Hors-Champ Sur Les Flux de Geènes au Sein des Agro-éCosystèmes. Ph.D. Thesis, Universite’ Paris XI, Paris, French, 2004. [Google Scholar]
- Bailleul, D.; Ollier, S.; Huet, S.; Gardarin, A.; Lecomte, J. Seed spillage from grain trailers on road verges during oilseed rape harvest: An experimental survey. PLoS ONE 2012, 7, 32752. [Google Scholar] [CrossRef] [PubMed]
- Bailleul, D.; Ollier, S.; Lecomte, J. Genetic diversity of oilseed rape fields and feral populations in the context of coexistence with GM crops. PLoS ONE 2016, 11, e0158403. [Google Scholar] [CrossRef]
- Menzel, G. Verbreitungsdynamik und Auskreuzungspotential von Brassica napus L. (Raps) im Großraum Bremen; GCA-Verlag: Waabs, Germany, 2006; ISBN 3-89863-213-X. [Google Scholar]
- Reuter, H.; Menzel, G.; Pehlke, H.; Breckling, B. Hazard mitigation or mitigation hazard? Would genetically modified dwarfed oilseed rape (Brassica napus) increase feral survival? Environ. Sci. Poll. Res. 2008, 15, 529–535. [Google Scholar] [CrossRef]
- Dietz-Pfeilstetter, A.; Metge, K.; Schonfeld, J.; Zwerger, P. Assessment of transgene spread from oilseed rape by population dynamic and molecular analyses of feral oilseed rape. J. Plant. Dis. Protect. 2006, 1, 39–47. [Google Scholar]
- Dietz-Pfeilstetter, A.; Zwerger, P. In-field frequencies and characteristics of oilseed rape with double herbicide resistance. Environ. Biosafety Res. 2009, 8, 101–111. [Google Scholar] [CrossRef]
- Elling, B.; Neuffer, B.; Bleeker, W. Sources of genetic diversity in feral oilseed rape (Brassica napus) populations. Basic App. Ecol. 2009, 10, 544–553. [Google Scholar] [CrossRef]
- Crawley, M.J.; Brown, S.L. Seed limitation and the dynamics of feral oilseed rape on the M25 motorway. Proc. R. Soc. B Biol. Sci. 1995, 259, 49–54. [Google Scholar]
- Crawley, M.J.; Brown, S.L. Spatially structured population dynamics in feral oilseed rape. Proc. R. Soc. B Biol. Sci. 2004, 271, 1909–1916. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Timmons, A.M.; Charters, Y.; Dubbels, S.; Robertson, A.; Wilson, N.; Scott, S.; O’Brien, E.; Lawson, H.M. Problems of risk assessment with genetically modified oilseed rape. In Proceedings of the Brighton Crop Protection Conference Weeds, Brighton, UK, 20–23 November 1995; Volume 3, pp. 1035–1044. [Google Scholar]
- Charters, Y.M.; Robertson, A.; Squire, G.R. Investigation of feral oilseed rape populations, genetically modified organisms research report (No.12). Dep. Environ. Transp. Reg. 1999. Available online: http://www.defra.gov.uk/environment/gm/research/reports.htm (accessed on 25 September 2021).
- Bond, J.M.; Mogg, R.J.; Squire, G.R.; Johnstone, C. Microsatellite amplification in Brassica napus cultivars: Cultivar variability and relationship to a long-term feral population. Euphytica 2004, 139, 173–178. [Google Scholar] [CrossRef]
- Norris, C.; Sweet, J. Monitoring Large Scale Releases of Genetically Modified Crops (EPG1/5/84) Incorporating Report on Project EPG 1/5/30: Monitoring Releases of Genetically Modified Crop Plants. DEFRA Report, 2002, EPG 1/5/84. Available online: http://www.defra.gov.uk/environment/gm/research/pdf/epg_1-5-84_screen.pdf (accessed on 25 September 2021).
- McCauley, R.; Davies, M.; Wyntje, A. The step-wise approach to adoption of genetically modified (GM) canola in Western Australia. AgBioForum 2012, 15, 61–69. [Google Scholar]
- Busi, R.; Powles, S.B. Transgenic glyphosate-resistant canola (Brassica napus) can persist outside agricultural fields in Australia. Agric. Ecosys. Environ. 2016, 220, 28–34. [Google Scholar] [CrossRef]
- Tamis, W.L.M.; de Jong, T.J. Transport Chains and Seed Spillage of Potential GM Crops with Wild Relatives in The Netherlands. COGEM Report: CGM 2010-02. 2010. Available online: http://www.cogem.net/ContentFiles/2010-02%20Transport_chains2.Pdf (accessed on 25 September 2021).
- Heenan, P.B.; FitzJohn, R.G.; Dawson, M.I. Diversity of Brassica (Brassicaceae) species naturalised in Canterbury, New Zealand. N. Z. J. Bot. 2004, 42, 815–832. [Google Scholar] [CrossRef]
- Peltzer, D.A.; Ferriss, S.; FitzJohn, R.G. Predicting weed distribution at the landscape scale: Using naturalized Brassica as a model system. J. Appl. Ecol. 2008, 45, 467–475. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Oilseeds: World Markets and Trade. 2020. Available online: https://www.fas.usda.gov/data/oilseeds-world-markets-and-trade (accessed on 18 August 2021).
- Shiga, T. Rape breeding by interspecific crossing between Brassica napas and Brassica campestris in Japan. Jpn. Agric. Res. Q. 1970, 5, 5–10. [Google Scholar]
- Statistics of Japan. Crop Statistics Survey/Crop Survey (Upland Rice, Wheat, Beans, Kansho, Forage Crop, Industrial Crop)/Confirmation/Reiwa 2nd Year Crop Statistics (Ordinary Crop/Forage Crop/Industrial Crop). 2020. Available online: http://www.stat.go.jp/index.html (accessed on 25 August 2021).
- Warwick, S.; Beckie, H.J.; Simard, M.J.; Légère, A.; Nair, H.; SéguinSwartz, G. Environmental and agronomic consequences of herbicide-resistant (HR) canola in Canada. In Introgression from Genetically Modified Plants into Wild Relatives; den Nijs, H.C.M., Bartsch, D., Sweet, J., Eds.; CABI Publishing: Wallingford, UK, 2004; pp. 323–337. [Google Scholar]
- Chen, R.; Shimono, A.; Aono, M.; Nakajima, N.; Ohsawa, R.; Yoshioka, Y. Genetic diversity and population structure of feral rapeseed (Brassica napus L.) in Japan. PLoS ONE 2020, 15, e0227990. [Google Scholar] [CrossRef]
- Fernandez-Cornejo, J.; Wechsler, S.; Livingston, M.; Mitchell, L. Genetically engineered crops in the United States. USDA-ERS Econ. Res. Rep. 2014, 162, 1–60. [Google Scholar] [CrossRef]
- Mallory-Smith, C.A.; Zapiola, M.L. Gene flow from glyphosate-resistant crops. Pest. Manag. Sci. 2008, 64, 428–440. [Google Scholar] [CrossRef]
- Gruber, S.; Pekrun, C.; Claupein, W. Seed persistence of oilseed rape (Brassica napus): Variation in transgenic and conventionally bred cultivars. J. Agric. Sci. 2004, 142, 29–40. [Google Scholar] [CrossRef]
- Murdoch, A.J.; Froud Williams, R.J. Weed seedbanks: Determination, dynamics and manipulation. In Proceedings of the Weed Seedbanks: Determination, Dynamics and Manipulation, Oxford, UK, 23–24 March 1998; Volume 51, pp. 119–126. [Google Scholar]
- Mikkelsen, T.R.; Andersen, B.; Jørgensen, R.B. The risk of crop transgene spread. Nature 1996, 380, 31. [Google Scholar] [CrossRef]
- Beckie, H.J.; Harker, K.N.; Hall, L.M.; Warwick, S.I.; Legere, A.; Sikkema, P.H.; Clayton, G.W.; Thomas, A.G.; Leeson, J.Y.; Seguin-Swartz, G.; et al. A decade of herbicide-resistant crops in Canada. Can. J. Plant. Sci. 2006, 86, 1243–1264. [Google Scholar] [CrossRef]
- Mauro, I.J.; McLachlan, S.M.; Van Acker, R.C. Farmer knowledge and a priori risk analysis: Pre-release evaluation of genetically modified Roundup Ready wheat across the Canadian prairies. Environ. Sci. Pollut. Res. 2009, 16, 689–701. [Google Scholar] [CrossRef]
- Demeke, T.; Perry, D.J.; Scowcroft, W.R. Adventitious presence of GMOs: Scientific overview for Canadian grains. Can. J. Plant. Sci. 2006, 86, 1–23. [Google Scholar] [CrossRef]
- Crawley, M.J.; Hails, R.S.; Rees, M.; Kohn, D.; Buxton, J. Ecology of transgenic oilseed rape in natural habitats. Nature 1993, 363, 620–623. [Google Scholar] [CrossRef]
- Instituto Nacional de Semillas (INASE) Catálogo Nacional de Cultivares. 2016. Available online: http://www.inase.gov.ar (accessed on 25 September 2021).
- Middelhoff, U.; Reuter, H.; Breckling, B. GeneTraMP, a spatio-temporal model of the dispersal and persistence of transgenes in feral, volunteer and crop plants of oilseed rape and related species. Ecol. Indic. 2011, 11, 974–988. [Google Scholar] [CrossRef]
- Moser, D.; Eckerstorfer, M.; Pascher, K.; Essl, F.; Zulka, K.P. Potential of genetically modified oilseed rape for biofuels in Austria: Land use patterns and coexistence constraints could decrease domestic feedstock production. Biomass Bioenergy 2013, 50, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Lecomte, J. Using a spatial and stage-structured invasion model to assess the spread of feral populations of transgenic oilseed rape. Ecol. Model. 2006, 194, 141–149. [Google Scholar] [CrossRef]
- EC Regulation (EC) 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Off. J. Eur. Comm. 2003, L268, 1–23.
- Wurbs, A.; Glemnitz, M.; Graef, F.; Funke, B.; Ehlert, S. Regionalisation of flora elements in field boundaries sensitive to hybridisation with genetically modified oilseed rape. Environ. Sci. Eur. 2010, 22, 252–263. [Google Scholar] [CrossRef]
- Breckling, B.; Reuter, H.; Middelhoff, U.; Glemnitz, M.; Wurbs, A.; Schmidt, G.; Schröder, W.; Windhorst, W. Risk indication of genetically modified organisms (GMO): Modelling environmental exposure and dispersal across different scales: Oilseed rape in Northern Germany as an integrated case study. Ecol. Indic. 2011, 11, 936–941. [Google Scholar] [CrossRef]
- Reuter, H.; Schmidt, G.; Schröder, W.; Middelhoff, U.; Pehlke, H.; Breckling, B. Regional distribution of genetically modified organisms (GMOs)—Up-scaling the dispersal and persistence potential of herbicide resistant oilseed rape (Brassisca napus). Ecol. Indic. 2011, 11, 989–999. [Google Scholar] [CrossRef]
- D’Hertefeldt, T.; Jørgensen, R.B.; Pettersson, L.B. Long-term persistence of GM oilseed rape in the seedbank. Biol. Lett. 2008, 4, 314–317. [Google Scholar] [CrossRef]
- Devos, Y.; De Schrijver, A.; Reheul, D. Quantifying the introgressive hybridisation propensity between transgenic oilseed rape and its wild/weedy relatives. Environ. Mon. Ass. 2009, 149, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Warwick, S.I. Persistence of an oilseed rape transgene in the environment. Crop Prot. 2010, 29, 509–512. [Google Scholar] [CrossRef]
- Beckie, H.J.; Hall, L.M.; Simard, M.-J.; Leeson, J.Y.; Willenborg, C.J. A framework for postrelease environmental monitoring of second-generation crops with novel traits. Crop Sci. 2010, 50, 1587–1604. [Google Scholar] [CrossRef]
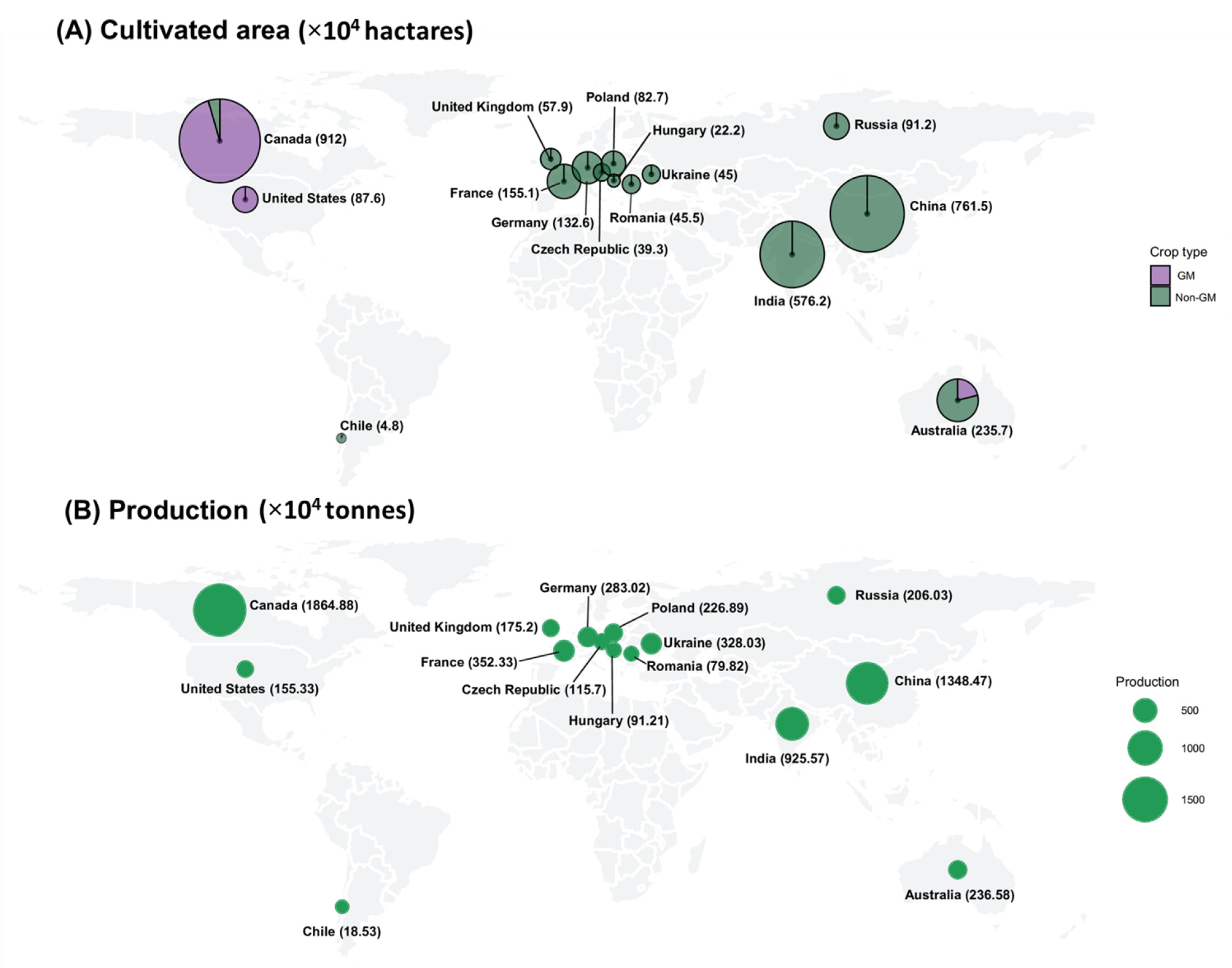
| S. No | Countries | Importing Quantity (104 Tonnes) |
|---|---|---|
| 1. | Germany | 574.637 |
| 2. | China | 475.6582 |
| 3. | Belgium | 258.8239 |
| 4. | Japan | 233.74 |
| 5. | Mexico | 143.6321 |
| 6. | France | 94.0338 |
| 7. | Pakistan | 80.8421 |
| 8. | United Arab Emirates | 73.6002 |
| 9. | Poland | 71.7704 |
| 10. | Netherlands | 68.7646 |
| 11. | United States of America | 62.917 |
| 12. | Czechia | 28.8407 |
| 13. | Austria | 28.6828 |
| 14. | Belarus | 26.1836 |
| 15. | United Kingdom | 19.7132 |
| 16. | Denmark | 16.614 |
| 17. | Portugal | 15.8598 |
| 18. | Canada | 15.5105 |
| 19. | Sweden | 12.4454 |
| 20. | Nepal | 9.0375 |
| 21. | Bangladesh | 8.9847 |
| 22. | Hungary | 7.7505 |
| 23. | Republic of Korea | 0.5601 |
| 24. | Switzerland | 0.4906 |
| 25. | Australia | 0.1176 |
| Nation | Year of Study | Region | Escaped Transgene | Hybridization | Comments | References |
|---|---|---|---|---|---|---|
| Japan | 2004 | Kashima, Kobe, Kanto R51 Kanto R124 | PAT, EPSPS | N/A | First published example of feral, transgenic populations occurring in a nation where the transgenic crop has not been cultivated commercially | [46] |
| Chiba, Nagoya Yokkaichi | EPSPS | |||||
| 2005 | Kashima, Chiba, Yokohama, Shimizu, Nagoya, Yokkaichi, Sakai-senboku, Kobe, Uno, Mizushima, Kita-Kyushu, Hakata | PAT, EPSPS | Inter-Specific Hybridization with B. rapa, B. juncea | First report identifying the outcrossing between different Brassica species. | [47] | |
| 2004–2007 | Fukushima, Mizushima | EPSPS | N/A | Seed spillage during transportation is the main cause for the gene transfer | [48] | |
| Kashima, Chiba, Nagoya, Yokkaichi, Hakata | PAT, EPSPS | |||||
| Yokohama, Shimizu, Ooita, Nagasaki | PAT | |||||
| 2005–2007 | Kanto Route 51 | EPSPS (2005~2007) PAT(2005) | N/A | Detailed report on seed spillage during transportation as the main cause for the gene transfer | [49] | |
| 2004–2005 | 19 sites around Kashima sea port | PAT, EPSPS | N/A | Found GM rapeseed in only 2 sites | [50] | |
| 2005–2008 | Kashima, Chiba, Yokohama, Shimizu, Nagoya, Yokkaichi, Sakai-senboku, Kobe, Uno, Mizushima, Kita-Kyushu, Hakata | EPSPS | Inter-Specific Hybridization with B. rapa | Origin of double resistance unclear | [51] | |
| 2006–2011 | Kashima, Chiba, Yokohama, Shimizu, Nagoya, Yokkaichi, Sakai-senboku, Kobe, Uno, Mizushima, Tobato, Hakata | EPSPS | N/A | Chiba, Yokkaichi, and Hakata were the hotspots for the feral rapeseed populations | [40] | |
| 2005–2014 | Kanto Route 51 | EPSPS, PAT | N/A | Ten years of seed spillage during transportation is the main cause for the gene transfer | [52] | |
| Canada | 1996–1998 | Alberta | EPSPS | N/A | Neighboring field, multiple herbicide resistance | [53] |
| 2002 | Saskatchewan | PAT, EPSPS | N/A | Neighboring field, multiple herbicide resistance, double resistance in seed lots | [33] | |
| 2002 | Western Canada | PAT, EPSPS | N/A | Double-resistant seed lots | [54] | |
| 2000 | Québec | EPSPS | Inter-Specific Hybridization with B. rapa | Commercial fields, no escape to Raphanus raphanistrum, Sinapis arvensis, or Erucastrum gallicum | [22] | |
| 2005 | Vancouver | EPSPS | Inter-Specific Hybridization with B. rapa | High probability of hybridization between these two Brassica species | [30] | |
| 2003 | Québec | PAT, EPSPS | Inter-Specific Hybridization with B. rapa | Double resistance by transgene flow in escaped populations | [20] | |
| 2005 | Québec | EPSPS | Inter-Specific Hybridization with B. rapa | Persistence over 6 years | [55] | |
| 2004–2006 | Manitoba | PAT, EPSPS | N/A | Double resistance by transgene flow in escaped populations | [56] | |
| 2005–2007 | Manitoba | PAT, EPSPS | N/A | Agricultural transport and landscape-scale cropping pattern are the key determinants. | [17] | |
| USA | 2008–2009 | North Dakota | PAT, EPSPS | N/A | Double resistance in feral rapeseed at the roadways | [57] |
| 2007–2011 | Butte county farm (California) | EPSPS | N/A | Glyphosate-resistant rapeseed in the fields | [58] | |
| Switzerland | 2011 | Swiss railway station, Basel, Liechtenstein | EPSPS | N/A | Four GM rapeseed were identified in 2 sites | [59] |
| 2012 | Basel’s Rhine port | PAT, EPSPS | N/A | Discovered glufosinate-resistant GM events MS8xRF3, MS8, and RF3 | [60] | |
| 2010–2012 | Rail roads along the country (Basel) | PAT, EPSPS | N/A | Strain GT73 carrying the glyphosate resistance transgene, gox, and CP4-EPSPS were detected | [61] | |
| Argentina | 2012 | Southeast of Buenos Aires province | EPSPS | N/A | Transgenic rapeseed (GT73) was identified | [62] |
| Nation | Year of Study | Region | Comments | References |
|---|---|---|---|---|
| Belgium | 2007–2008 | Roadsides in Wallonia | - | [63,64] |
| 2009 | Port areas of Antwerpen, Gent, Izegem, and Kluisbergen | - | [65] | |
| Austria | 1998–1999 | Burgenland, Waldviertel, and Innviertel | Field evaluation and genetic variation analysis among the feral populations | [66,67] |
| 2015–2016 | 60 sites all over Austria considers transportation routes (railways, roads) and loading sites such as railway stations, switch yards, ports, oil mills, and processing companies. | Feral rapeseed found in 44 of the 60 sites surveyed | [68] | |
| Denmark | 2005–2006 | Mid-Jutland | Population dynamics of feral rapeseed | [12] |
| France | 1996–1997 | Roadways in Selommes | Origin and persistence of feral rapeseed populations | [43] |
| 2000–2003 | Village in Selommes | Population dynamics of feral rapeseed and modeling studies | [16,44] | |
| 2000–2005 | Roadways and field edges in Selommes | Population dynamics of feral rapeseed | [12,69] | |
| 2002–2006 | Village in Selommes | Population dynamics of feral rapeseed | [70] | |
| 2010 | Village in Selommes | Genetic variation analysis among the feral populations | [71] | |
| Germany | 2001–2003 2005 | Bremen | Population dynamics of feral rapeseed | [12,72,73] |
| 2002–2005 | Braunschweig | Population dynamics of feral rapeseed | [12,74,75] | |
| 2004–2007 | Lower Saxony | Population dynamics of feral rapeseed | [76] | |
| 1998–2015 | Saxony-Anhalt | Dynamics of feral GM rapeseed events (MS8/RF3, GT73, GS 40/90, and MS1/RF1) in different time periods and long-term persistence | [21] | |
| United Kingdom | 1993–2002 | Roadways in southern England | Dynamics of feral rapeseed in roadways | [77,78] |
| 1993–1994 2004 | Tayside region (Scotland) | Field survey in roadways and genetic variation analysis | [12,79,80,81] | |
| 1994–2000 | Fields across the England | Distribution and dynamics of feral rapeseed | [82] | |
| Australia | 2009–2011 | Fields in western Australia and Albany Highway | Step wise adoption of GM rapeseed in agricultural fields and their persistence | [83] |
| 2009–2013 | Roadsides of western Australia | Occurrence of feral rapeseed in roadsides and grain-receiving sites | [84] | |
| The Netherlands | 2008–2009 | Ports of Rotterdam and Amsterdam | Distribution and dynamics of feral rapeseed | [85] |
| New Zealand | 2003, 2005 | Canterbury (South Island) | Distribution of feral rapeseed in road verges, drainage ditches, channels, natural watercourses, shelterbelts, and wasteland | [86,87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, S.-I.; Pandian, S.; Oh, Y.-J.; Kang, H.-J.; Ryu, T.-H.; Cho, W.-S.; Shin, E.-K.; Shin, K.-S. A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment. Biology 2021, 10, 1264. https://doi.org/10.3390/biology10121264
Sohn S-I, Pandian S, Oh Y-J, Kang H-J, Ryu T-H, Cho W-S, Shin E-K, Shin K-S. A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment. Biology. 2021; 10(12):1264. https://doi.org/10.3390/biology10121264
Chicago/Turabian StyleSohn, Soo-In, Subramani Pandian, Young-Ju Oh, Hyeon-Jung Kang, Tae-Hun Ryu, Woo-Suk Cho, Eun-Kyoung Shin, and Kong-Sik Shin. 2021. "A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment" Biology 10, no. 12: 1264. https://doi.org/10.3390/biology10121264
APA StyleSohn, S.-I., Pandian, S., Oh, Y.-J., Kang, H.-J., Ryu, T.-H., Cho, W.-S., Shin, E.-K., & Shin, K.-S. (2021). A Review of the Unintentional Release of Feral Genetically Modified Rapeseed into the Environment. Biology, 10(12), 1264. https://doi.org/10.3390/biology10121264








