Understanding Nutrition and Metabolism of Threatened, Data-Poor Rheophilic Fishes in Context of Riverine Stocking Success- Barbel as a Model for Major European Drainages?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
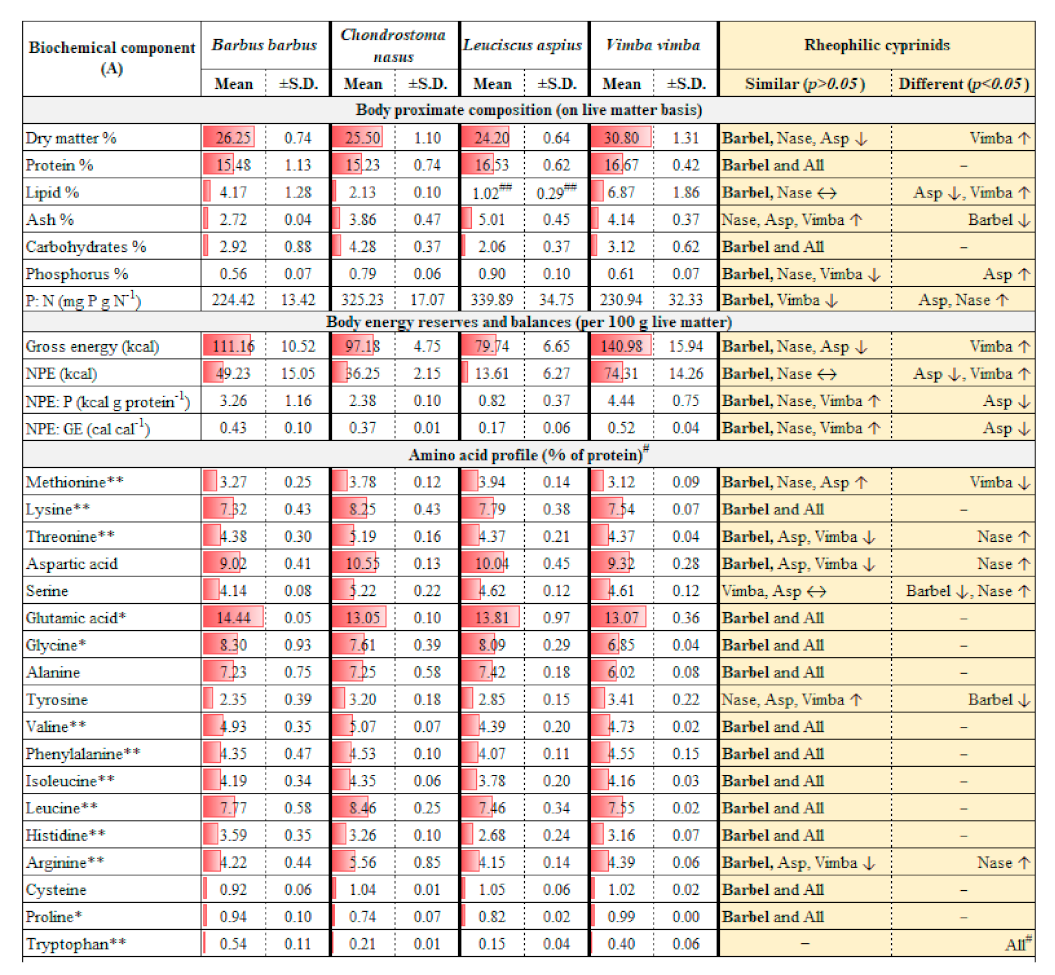
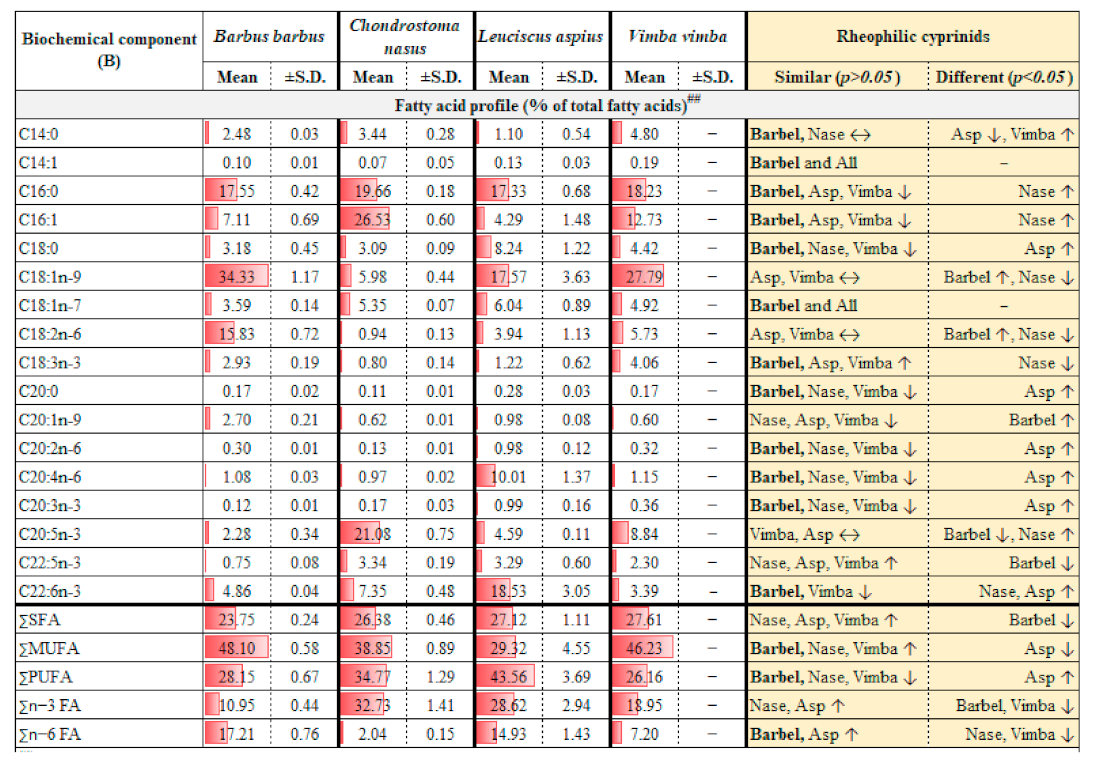
2.1. Assessment and Comparison of Wild Body Composition
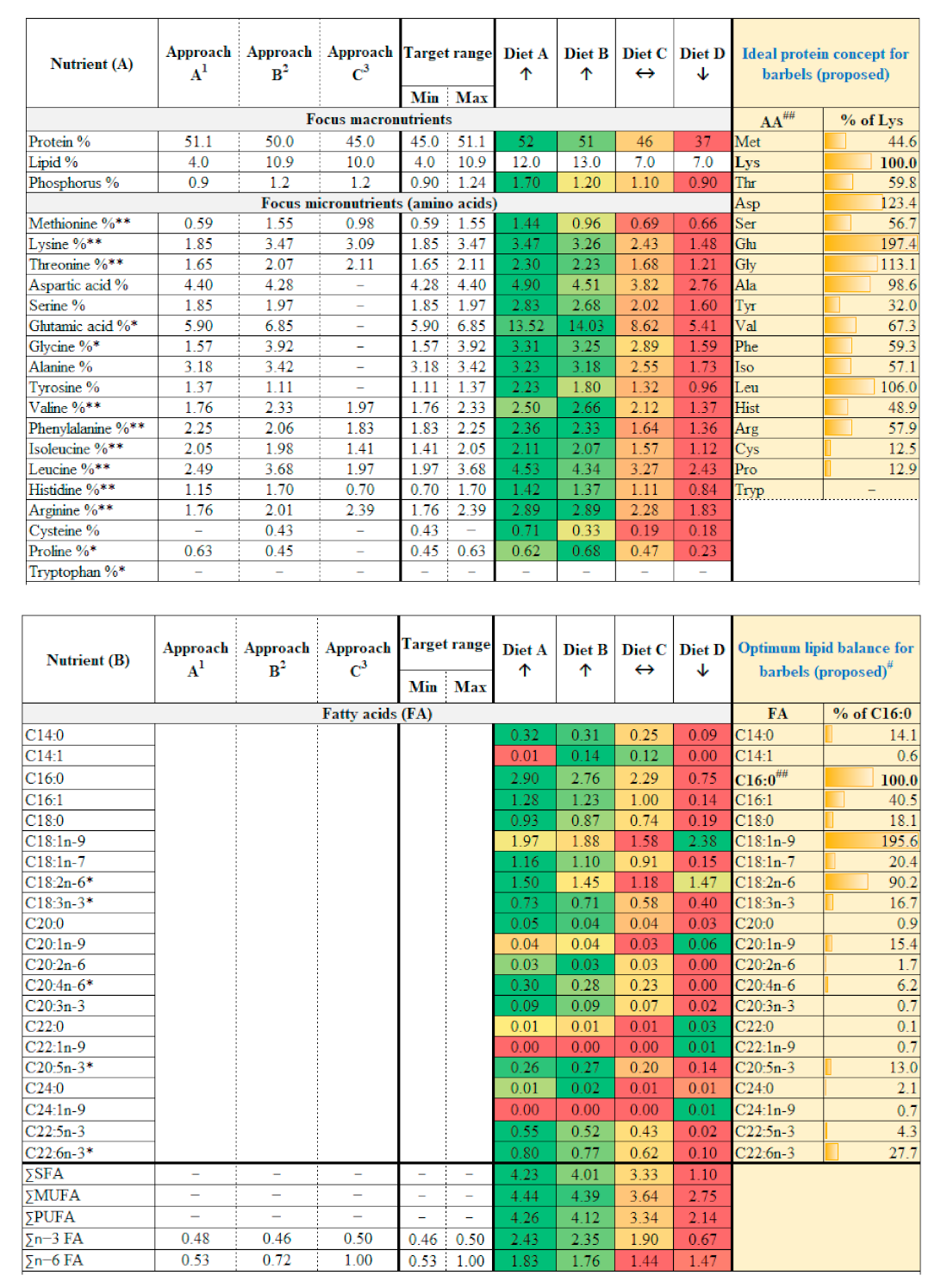
2.2. Selection of Model Species and Calculation of Target Nutrition
2.3. Selection of Experimental Diets and Feed Preparation
2.4. Growth Trial-I (Mid 0+ to Late 0+; 100 Days)
2.5. Validation Growth Trial-II (Late 0+ to Early 1+; 50 Days + 36 Days + 64 Days)
2.6. Evaluation of Growth, Factors Affecting Growth, and Physiological Performance
2.6.1. Evaluation of Experimental Results
2.6.2. Retrospective Evaluation against Reviewed Metadata
3. Results
3.1. Assessment and Comparison of Wild Body Composition
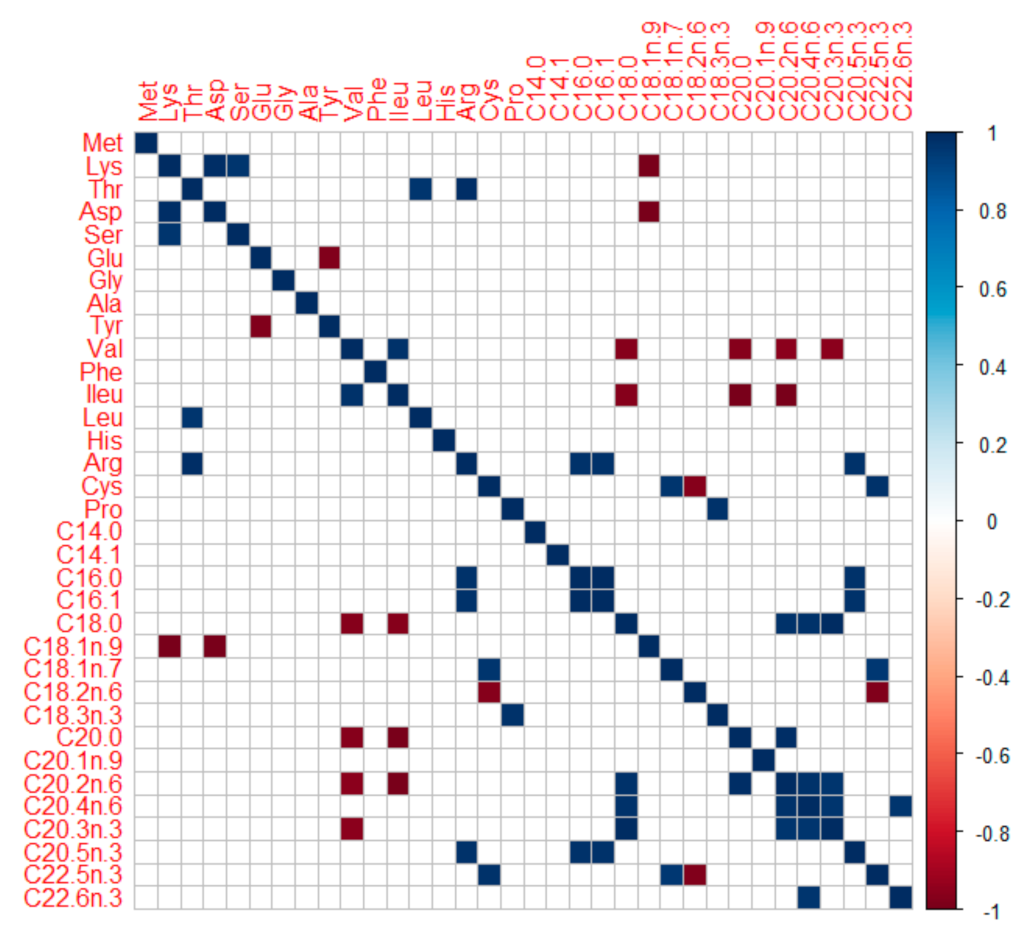
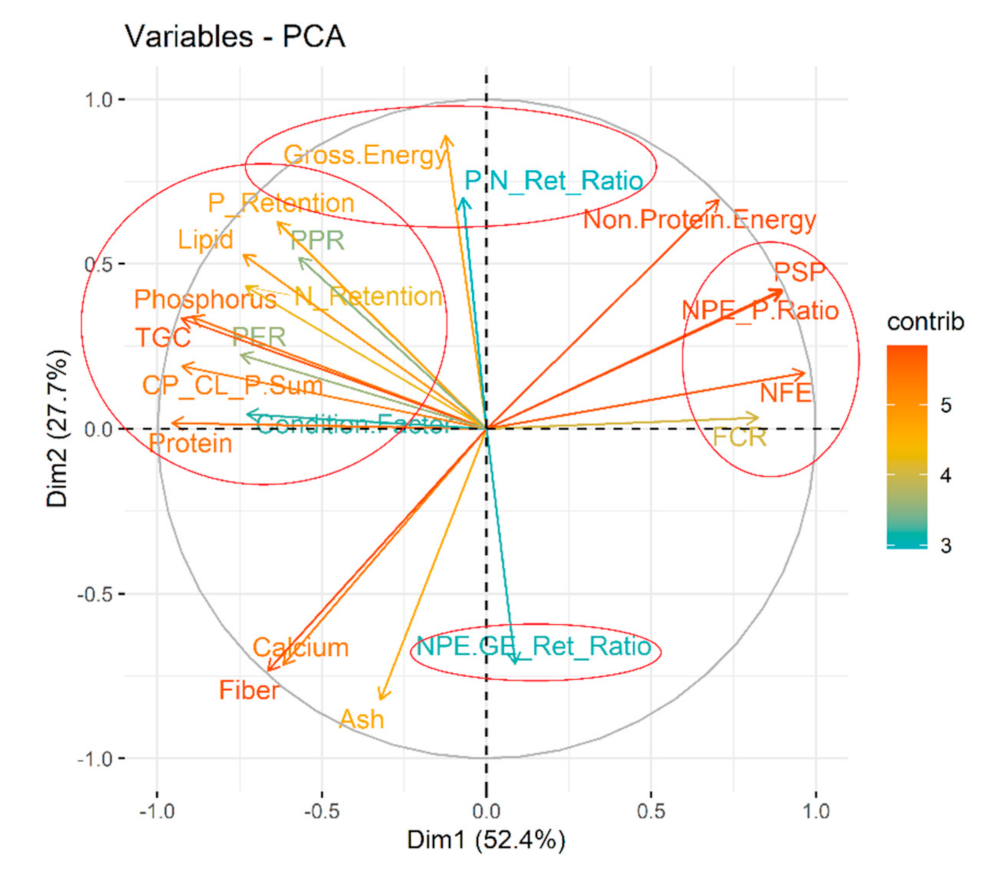
3.2. Optimum Nutrition for Achieving Steepest Growth Trajectory
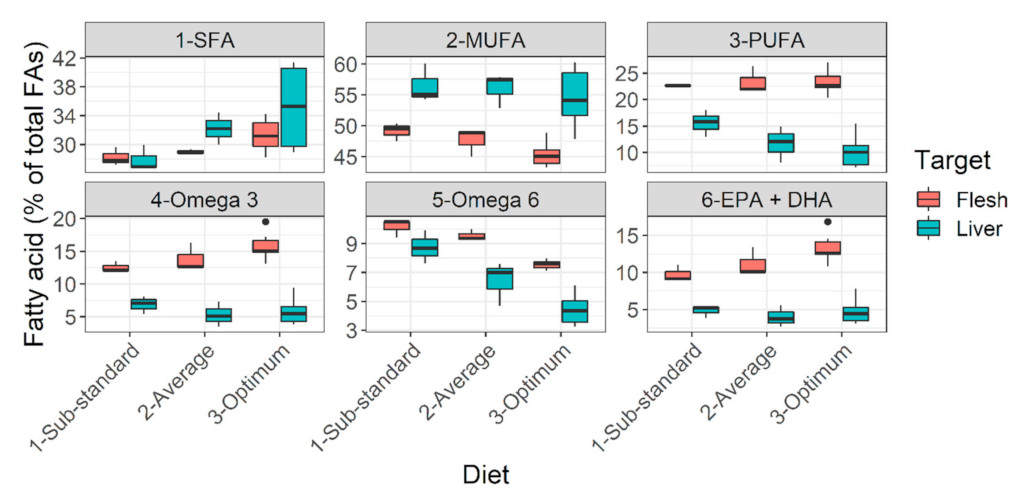
3.3. Understanding Nutritional Physiology and Conditions for Achieving Maximum Growth
4. Discussions
4.1. Bigger and Faster Is Better for Riverine Stocking Success
4.2. Status Quo Feeding Management of Riverine Fish Seeds and Their Fallacies
4.3. Importance of Optimum Nutrition and Metabolism for River Stocking Cohorts
4.4. Management around Optimum Nutrition to Achieve Goals of Riverine Stocking
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, M.; Pander, J.; Geist, J. Comprehensive analysis of >30 years of data on stream fish population trends and conservation status in Bavaria, Germany. Biol. Conserv. 2018, 226, 311–320. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The ecological value of stream restoration measures: An evaluation on ecosystem and target species scales. Ecol. Eng. 2014, 62, 129–139. [Google Scholar] [CrossRef]
- Deinet, S.; Scott-Gatty, K.; Rotton, H.; Twardek, W.M.; Marconi, V.; McRae, L.; Baumgartner, L.J.; Brink, K.; Claussen, J.E.; Cooke, S.J. The Living Planet Index (lpi) for Migratory Freshwater Fish: Technical Report; World Fish Migration Foundation: Groningen, The Netherlands, 2020. [Google Scholar]
- Barbarossa, V.; Schmitt, R.J.; Huijbregts, M.A.; Zarfl, C.; King, H.; Schipper, A.M. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. USA 2020, 117, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- García-Vega, A.; Leunda, P.M.; Ardaiz, J.; Sanz-Ronda, F.J. Effect of restoration measures in Atlantic rivers: A 25-year overview of sea and riverine brown trout populations in the River Bidasoa. Fish. Manag. Ecol. 2020, 27, 580–590. [Google Scholar] [CrossRef]
- Bartoň, D.; Bretón, F.; Blabolil, P.; Souza, A.T.; Vejřík, L.; Sajdlová, Z.; Kolařík, T.; Kubečka, J.; Šmejkal, M. Effects of hydropeaking on the attached eggs of a rheophilic cyprinid species. Ecohydrology 2021, 14, e2280. [Google Scholar] [CrossRef]
- Einum, S.; Fleming, I. Implications of stocking: Ecological interactions between wild and released salmonids. Nord. J. Freshw. Res. 2001, 75, 56–70. [Google Scholar]
- Brown, C.; Day, R.L. The future of stock enhancements: Lessons for hatchery practice from conservation biology. Fish Fish. 2002, 3, 79–94. [Google Scholar] [CrossRef]
- Geist, J. Seven Steps Towards Improving Freshwater Conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 447–453. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat recovery and restoration in aquatic ecosystems: Current progress and future challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Bilton, H.; Alderdice, D.; Schnute, J. Influence of time and size at release of juvenile coho salmon (Oncorhynchus kisutch) on returns at maturity. Can. J. Fish. Aquat. Sci. 1982, 39, 426–447. [Google Scholar] [CrossRef]
- Hasegawa, K.; Honda, K.; Yoshiyama, T.; Suzuki, K.; Fukui, S. Small biased body size of salmon fry preyed upon by piscivorous fish in riverine and marine habitats. Can. J. Fish. Aquat. Sci. 2021, 78, 631–638. [Google Scholar] [CrossRef]
- Hyvärinen, P.; Vehanen, T. Effect of brown trout body size on post-stocking survival and pike predation. Ecol. Freshw. Fish 2004, 13, 77–84. [Google Scholar] [CrossRef]
- Huntingford, F.A. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 2004, 65, 122–142. [Google Scholar] [CrossRef]
- Thompson, B.C.; Porak, W.F.; Pouder, W.F.; Camp, E.V. Survival of Advanced-Fingerlings of Florida Largemouth Bass Stocked in Small Florida Lakes. N. Am. J. Fish. Manag. 2020, 40, 1532–1544. [Google Scholar] [CrossRef]
- Turek, J.; Sampels, S.; Khalili Tilami, S.; Červený, D.; Kolářová, J.; Randák, T.; Mráz, J.; Másílko, J.; Steinbach, C.; Burkina, V. Insects in the feed of rainbow trout, oncorhynchus mykiss (actinopterygii, salmonidae): Effect on growth, fatty acid composition, and sensory attributes. Acta Ichthyol. Piscat. 2020, 50, 171–181. [Google Scholar] [CrossRef]
- Shiau, J.; Watson, J.R.; Cramp, R.L.; Gordos, M.A.; Franklin, C.E. Interactions between water depth, velocity and body size on fish swimming performance: Implications for culvert hydrodynamics. Ecol. Eng. 2020, 156, 105987. [Google Scholar] [CrossRef]
- NRC, National Research Council. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Guillaume, J.; Kaushik, S.; Bergot, P.; Metailler, R. Nutrition and Feeding of Fish and Crustaceans; Springer Praxis Publishing: Chichester, UK, 2001. [Google Scholar]
- Alavi, S.M.H.; Pšenička, M.; Policar, T.; Rodina, M.; Hamáčková, J.; Kozák, P.; Linhart, O. Sperm quality in male Barbus barbus L. fed different diets during the spawning season. Fish Physiol. Biochem. 2009, 35, 683–693. [Google Scholar] [CrossRef]
- Policar, T.; Podhorec, P.; Stejskal, V.; Hamackova, J.; Alavi, S. Fertilization and hatching rates and larval performance in captive common barbel (Barbus barbus L.) throughout the spawning season. J. Appl. Ichthyol. 2010, 26, 812–815. [Google Scholar] [CrossRef]
- Policar, T.; Podhorec, P.; Stejskal, V.; Kozák, P.; Švinger, V.; Alavi, S.H. Growth and survival rates, puberty and fecundity in captive common barbel (Barbus barbus L.) under controlled conditions. Czech J. Anim. Sci. 2011, 56, 433–442. [Google Scholar] [CrossRef]
- Fiala, J.; Spurny, P. Intensive rearing of juvenile barbel (Barbus barbus) under controlled conditions. In Proceedings of the 4. Czech Ichthyological Conference, Vodnany, Czech Republic, 10–12 May 2000. [Google Scholar]
- Grund, S.; Keiter, S.; Böttcher, M.; Seitz, N.; Wurm, K.; Manz, W.; Hollert, H.; Braunbeck, T. Assessment of fish health status in the Upper Danube River by investigation of ultrastructural alterations in the liver of barbel Barbus barbus. Dis. Aquat. Org. 2010, 88, 235–248. [Google Scholar] [CrossRef][Green Version]
- Poncin, P. Effects of different photoperiods on the reproduction of the barbel, Barbus barbus (L.), reared at constant temperature. J. Fish Biol. 1989, 35, 395–400. [Google Scholar] [CrossRef]
- Mráz, J.; Pickova, J. Differences between lipid content and composition of different parts of fillets from crossbred farmed carp (Cyprinus carpio). Fish Physiol. Biochem. 2009, 35, 615. [Google Scholar] [CrossRef] [PubMed]
- Lunda, R.; Roy, K.; Dvorak, P.; Kouba, A.; Mraz, J. Recycling biofloc waste as novel protein source for crayfish with special reference to crayfish nutritional standards and growth trajectory. Sci. Rep. 2020, 10, 19607. [Google Scholar] [CrossRef] [PubMed]
- Team, R. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Sommerwerk, N.; Hein, T.; Schneider-Jakoby, M.; Baumgartner, C.; Ostojić, A.; Paunović, M.; Bloesch, J.; Siber, R.; Tockner, K.; Robinson, C. The Danube river basin. In Rivers of Europe, 1st ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 59–112. [Google Scholar]
- Antony Jesu Prabhu, P.; Schrama, J.; Kaushik, S. Quantifying dietary phosphorus requirement of fish—A meta-analytic approach. Aquac. Nutr. 2013, 19, 233–249. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Guoyao, W. Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 2020, 52, 523–542. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Sogard, S.M. Size-selective mortality in the juvenile stage of teleost fishes: A review. Bull. Mar. Sci. 1997, 60, 1129–1157. [Google Scholar]
- Kamiński, R.; Kamler, E.; Wolnicki, J.; Sikorska, J.; WaŁowski, J. Condition, growth and food conversion in barbel, Barbus barbus (L.) juveniles under different temperature/diet combinations. J. Therm. Biol. 2010, 35, 422–427. [Google Scholar] [CrossRef]
- Wolnicki, J.; Górny, W. Survival and growth of larval and juvenile barbel (Barbus barbus L.) reared under controlled conditions. Aquaculture 1995, 129, 258–259. [Google Scholar] [CrossRef]
- Piria, M.; Treer, T.; Aničić, I.; Safner, R.; Odak, T. The natural diet of five cyprinid fish species. Agric. Conspec. Sci. 2005, 70, 21–28. [Google Scholar]
- Myszkowski, L. Compensatory growth, condition and food utilization in barbel Barbus barbus juveniles reared at different feeding periodicities with a dry diet. J. Fish Biol. 2013, 82, 347–353. [Google Scholar] [CrossRef]
- Pegg, J.; Britton, J.R. Effects of inter-and intra-specific competition on the growth rates of juvenile European barbel Barbus barbus used in the stock enhancement of UK fisheries. Fish. Res. 2011, 112, 8–12. [Google Scholar] [CrossRef]
- Policar, T.; Kozák, P.; Hamáčková, J.; Lepičová, A.; Musil, J.; Kouřil, J. Effects of short-time Artemia spp. feeding in larvae and different rearing environments in juveniles of common barbel (Barbus barbus) on their growth and survival under intensive controlled conditions. Aquat. Living Resour. 2007, 20, 175–183. [Google Scholar] [CrossRef]
- Baras, E.; Philippart, J.-C. Adaptive and evolutionary significance of a reproductive thermal threshold in Barbus barbus. J. Fish Biol. 1999, 55, 354–375. [Google Scholar] [CrossRef]
- Penaz, M.; Barus, V.; Prokes, M.; Homolka, M. Movements of barbel, Barbus barbus (Pisces: Cyprinidae). Folia Zool. 2002, 51, 55–66. [Google Scholar]
- Bischoff, A.; Freyhof, J. Seasonal shifts in day-time resource use of 0+ barbel, Barbus barbus. Environ. Biol. Fishes 1999, 56, 199–212. [Google Scholar] [CrossRef]
- Britton, J.; Pegg, J. Ecology of European barbel Barbus barbus: Implications for river, fishery, and conservation management. Rev. Fish. Sci. 2011, 19, 321–330. [Google Scholar] [CrossRef]
- Bašić, T.; Britton, J.R. Characterizing the trophic niches of stocked and resident cyprinid fishes: Consistency in partitioning over time, space and body sizes. Ecol. Evol. 2016, 6, 5093–5104. [Google Scholar] [CrossRef] [PubMed]
- Benitez, J.P.; Ovidio, M. The influence of environmental factors on the upstream movements of rheophilic cyprinids according to their position in a river basin. Ecol. Freshw. Fish 2018, 27, 660–671. [Google Scholar] [CrossRef]
- Carosi, A.; Ghetti, L.; La Porta, G.; Lorenzoni, M. Ecological effects of the European barbel Barbus barbus (L., 1758) (Cyprinidae) invasion on native barbel populations in the Tiber River basin (Italy). Eur. Zool. J. 2017, 84, 420–435. [Google Scholar] [CrossRef]
- Hunt, P.; Jones, J. A population study of Barbus barbus L. in the River Severn, England: III. Growth. J. Fish Biol. 1975, 7, 361–376. [Google Scholar] [CrossRef]
- Prokes, M.; Sovcik, P.; Penaz, M.; Barus, V.; Spurny, P.; Vilizzi, L. Growth of barbel, Barbus barbus, in the River Jihlava following major habitat alteration and estimated by two methods. Folia Zool. 2006, 55, 86. [Google Scholar]
- Roberts, C.G.; Britton, J.R. Spawning strategies in cypriniform fishes in a lowland river invaded by non-indigenous European barbel Barbus barbus. Hydrobiologia 2020, 847, 4031–4047. [Google Scholar] [CrossRef]
- Roy, K.; Vrba, J.; Kaushik, S.J.; Mraz, J. Feed-based common carp farming and eutrophication: Is there a reason for concern? Rev. Aquac. 2020, 12, 1736–1758. [Google Scholar] [CrossRef]
- Cherghou, S.; Khodari, M.; Yaâkoubi, F.; Benabid, M.; Badri, A. Contribution à l’étude du régime alimentairedu barbeau (Barbus barbus callensis Valenciennes, 1842) d’un cours d’eau du Moyen-Atlas (Maroc): Oued Boufekrane. Rev. Sci. L’eau/J. Water Sci. 2002, 15, 153–163. [Google Scholar] [CrossRef][Green Version]
- Sullam, K.E.; Dalton, C.M.; Russell, J.A.; Kilham, S.S.; El-Sabaawi, R.; German, D.P.; Flecker, A.S. Changes in digestive traits and body nutritional composition accommodate a trophic niche shift in Trinidadian guppies. Oecologia 2015, 177, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Zandonà, E.; Auer, S.K.; Kilham, S.S.; Reznick, D.N. Contrasting population and diet influences on gut length of an omnivorous tropical fish, the Trinidadian guppy (Poecilia reticulata). PLoS ONE 2015, 10, e0136079. [Google Scholar]
- Baras, E.; Cherry, B. Seasonal activities of female barbel Barbus barbus (L.) in the River Ourthe (Southern Belgium), as revealed by radio tracking. Aquat. Living Resour. 1990, 3, 283–294. [Google Scholar] [CrossRef]
- Cardeilhac, P.; Childress, K.; Townsend, H.; Szabo, N.; Samuelson, D.; Stout, R. Dietary associated incidence of hepatic lesions and tumors in largemouth bass Micropterus salmoides floridanus. In Proceedings of the 39th Annual Conference of the International Association for Aquatic Animal Medicine, Rome, Italy, 10–14 May 2008; Reidarson, T., Ed.; pp. 133–134. [Google Scholar]
- Dinken, C.P.; Keretz, K.R.; Schramm, H.L., Jr.; Petrie-Hanson, L.; Wes Schilling, M.; Allen, P.J. Changes in Physiology and Stress Responses of Pellet-Reared Largemouth Bass Fed Live-Forage Diets. N. Am. J. Aquac. 2020, 82, 3–23. [Google Scholar] [CrossRef]
- Porak, W.; Johnson, W.; Crawford, S.; Renfro, D.; Schoeb, T.; Stout, R.; Krause, R.; DeMauro, R. Factors affecting survival of largemouth bass raised on artificial diets and stocked into Florida lakes. In American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2002; pp. 649–666. [Google Scholar]
- Wintzer, A.; Motta, P. Diet-induced phenotypic plasticity in the skull morphology of hatchery-reared Florida largemouth bass, Micropterus salmoides floridanus. Ecol. Freshw. Fish 2005, 14, 311–318. [Google Scholar] [CrossRef]
- Mikavica, D.; Grujic, R.; Komic, J. Comparative growth analysis of the nase Chondrostoma nasus L. 1758, chub Leuciscus cephalus L. 1758 and barbel Barbus barbus L. 1758 in the river Drina [Republic of Srpska, Bosnia and Herzegovina]. Ichthyology 1997, 29, 1–17. [Google Scholar]
- Taylor, A.; Britton, J.; Cowx, I. Does the stock density of stillwater catch and release fisheries affect the growth performance of introduced cultured barbel? J. Fish Biol. 2004, 65, 308–313. [Google Scholar] [CrossRef]
- Brix, O.; Grüner, R.; Rønnestad, I.; Gemballa, S. Whether depositing fat or losing weight, fish maintain a balance. Proc. R. Soc. B Biol. Sci. 2009, 276, 3777–3782. [Google Scholar] [CrossRef] [PubMed]
- Zajic, T.; Mraz, J.; Sampels, S.; Pickova, J. Fillet quality changes as a result of purging of common carp (Cyprinus carpio L.) with special regard to weight loss and lipid profile. Aquaculture 2013, 400, 111–119. [Google Scholar] [CrossRef]
- Rombenso, A.N.; Turchini, G.M.; Trushenski, J.T. The omega-3 sparing effect of saturated fatty acids: A reason to reconsider common knowledge of fish oil replacement. Rev. Aquac. 2021. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon Salmo salar. J. Anim. Ecol. 1997, 66, 425–436. [Google Scholar] [CrossRef]
- Jørgensen, C.; Ernande, B.; Fiksen, Ø.; Dieckmann, U. The logic of skipped spawning in fish. Can. J. Fish. Aquat. Sci. 2006, 63, 200–211. [Google Scholar] [CrossRef]
- Eliasen, K.; Patursson, E.J.; McAdam, B.J.; Pino, E.; Morro, B.; Betancor, M.; Baily, J.; Rey, S. Liver colour scoring index, carotenoids and lipid content assessment as a proxy for lumpfish (Cyclopterus lumpus L.) health and welfare condition. Sci. Rep. 2020, 10, 8927. [Google Scholar] [CrossRef]
- Kirchner, S.; Panserat, S.; Lim, P.L.; Kaushik, S.; Ferraris, R.P. The role of hepatic, renal and intestinal gluconeogenic enzymes in glucose homeostasis of juvenile rainbow trout. J. Comp. Physiol. B 2008, 178, 429–438. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Lall, S.P. The minerals. In Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 259–308. [Google Scholar]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 17, 2711. [Google Scholar] [CrossRef]
- Kaushik, S.J.; Seiliez, I. Protein and amino acid nutrition and metabolism in fish: Current knowledge and future needs. Aquac. Res. 2010, 41, 322–332. [Google Scholar] [CrossRef]
- Rollin, X.; Mambrini, M.; Abboudi, T.; Larondelle, Y.; Kaushik, S.J. The optimum dietary indispensable amino acid pattern for growing Atlantic salmon (Salmo salar L.) fry. Br. J. Nutr. 2003, 90, 865–876. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.; Turner, C.; Øvrebø, B.; Bastani, N.E.; Refsum, H.; Vinknes, K.J. Postprandial effects of a meal low in sulfur amino acids and high in polyunsaturated fatty acids compared to a meal high in sulfur amino acids and saturated fatty acids on stearoyl CoA-desaturase indices and plasma sulfur amino acids: A pilot study. BMC Res. Notes 2020, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Poloni, S.; Blom, H.J.; Schwartz, I.V. Stearoyl-CoA desaturase-1: Is it the link between sulfur amino acids and lipid metabolism? Biology 2015, 4, 383–396. [Google Scholar] [CrossRef]
- Perera, E.; Turkmen, S.; Simó-Mirabet, P.; Zamorano, M.J.; Xu, H.; Naya-Català, F.; Izquierdo, M.; Pérez-Sánchez, J. Stearoyl-CoA desaturase (scd1a) is epigenetically regulated by broodstock nutrition in gilthead sea bream (Sparus aurata). Epigenetics 2020, 15, 536–553. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22: 5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Salamanca, N.; Giráldez, I.; Morales, E.; de La Rosa, I.; Herrera, M. Phenylalanine and Tyrosine as Feed Additives for Reducing Stress and Enhancing Welfare in Gilthead Seabream and Meagre. Animals 2021, 11, 45. [Google Scholar] [CrossRef]
- Braasch, I.; Schartl, M.; Volff, J.-N. Evolution of pigment synthesis pathways by gene and genome duplication in fish. BMC Evol. Biol. 2007, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Krauss, J.; Geiger-Rudolph, S.; Koch, I.; Nüsslein-Volhard, C.; Irion, U. A dominant mutation in tyrp1 A leads to melanophore death in zebrafish. Pigment Cell Melanoma Res. 2014, 27, 827–830. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [PubMed]
- van Overveld, F.W.; Haenen, G.R.; Rhemrev, J.; Vermeiden, J.P.; Bast, A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem.-Biol. Interact. 2000, 127, 151–161. [Google Scholar] [CrossRef]
- Antanasijević, D.; Pocajt, V.; Perić-Grujić, A.; Ristić, M. Modelling of dissolved oxygen in the Danube River using artificial neural networks and Monte Carlo Simulation uncertainty analysis. J. Hydrol. 2014, 519, 1895–1907. [Google Scholar] [CrossRef]
- Csábrági, A.; Molnár, S.; Tanos, P.; Kovács, J.; Molnár, M.; Szabó, I.; Hatvani, I.G. Estimation of dissolved oxygen in riverine ecosystems: Comparison of differently optimized neural networks. Ecol. Eng. 2019, 138, 298–309. [Google Scholar] [CrossRef]
- Simčič, T.; Jesenšek, D.; Brancelj, A. Effects of increased temperature on metabolic activity and oxidative stress in the first life stages of marble trout (Salmo marmoratus). Fish Physiol. Biochem. 2015, 41, 1005–1014. [Google Scholar] [CrossRef]
- He, W.; Li, P.; Wu, G. Amino acid nutrition and metabolism in chickens. Amino Acids Nutr. Health Amino Acids Nutr. Companion Zoo Farm Anim. 2021, 1285, 109–131. [Google Scholar]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Marques, V.H.; Moreira, R.G.; Branco, G.S.; Honji, R.M.; Rombenso, A.N.; Viana, M.T.; de Mello, P.H.; Mata-Sotres, J.A.; Araújo, B.C. Different saturated and monounsaturated fatty acids levels in fish oil-free diets to cobia (Rachycentron canadum) juveniles: Effects in growth performance and lipid metabolism. Aquaculture 2021, 541, 736843. [Google Scholar] [CrossRef]
- Guan, X.; Fierke, C.A. Understanding protein palmitoylation: Biological significance and enzymology. Sci. China Chem. 2011, 54, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Yoshizawa, F. Effects of leucine and isoleucine on glucose metabolism. In Branched Chain Amino Acids in Clinical Nutrition; Springer: Berlin/Heidelberg, Germany, 2015; pp. 63–73. [Google Scholar]
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel metabolic and physiological functions of branched chain amino acids: A review. J. Anim. Sci. Biotechnol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Greene, E.; Li, W.; Kidd, M.T.; Dridi, S. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol. Nutr. Food Res. 2015, 59, 1171–1181. [Google Scholar] [CrossRef]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar]
- Ma, C.; Liu, Y.; Liu, S.; Lévesque, C.L.; Zhao, F.; Yin, J.; Dong, B. Branched chain amino acids alter fatty acid profile in colostrum of sows fed a high fat diet. J. Anim. Sci. Biotechnol. 2020, 11, 9. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Liu, N.; Chen, J.; Guo, L.; Dai, Z.; Wang, C.; Wu, Z.; Wu, G. Dietary L-arginine supplementation reduces lipid accretion by regulating fatty acid metabolism in Nile tilapia (Oreochromis niloticus). J. Anim. Sci. Biotechnol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Liang, H.; Habte-Tsion, H.-M.; Ge, X.; Ren, M.; Xie, J.; Miao, L.; Zhou, Q.; Lin, Y.; Pan, W. Dietary arginine affects the insulin signaling pathway, glucose metabolism and lipogenesis in juvenile blunt snout bream Megalobrama amblycephala. Sci. Rep. 2017, 7, 7864. [Google Scholar] [CrossRef]
- Hamani, D.; Kuhn, M.; Charrueau, C.; Waligora-Dupriet, A.-J.; Neveux, N.; Butel, M.-J.; Cynober, L.; Moinard, C. Interactions between ω3 polyunsaturated fatty acids and arginine on nutritional and immunological aspects in severe inflammation. Clin. Nutr. 2010, 29, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Won, E.T.; Borski, R.J. Endocrine regulation of compensatory growth in fish. Front. Endocrinol. 2013, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Philippart, J.-C.; Mélard, C.; Poncin, P. Intensive culture of the common barbel, Barbus barbus (L.) for restocking. In Aquaculture: A Biotechnology in Progress; European Aquaculture Society: Bredene, Belgique, 1989; pp. 483–491. [Google Scholar]
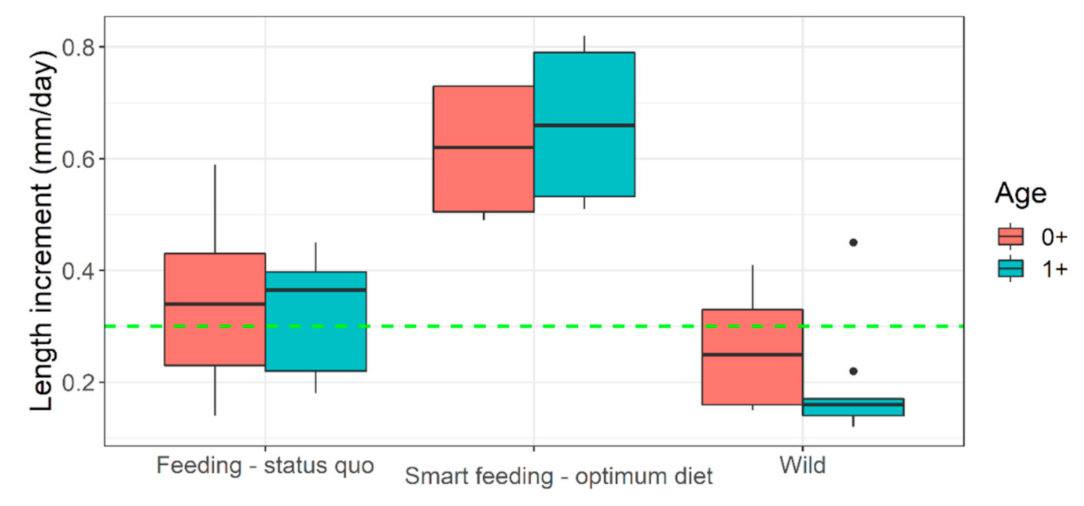
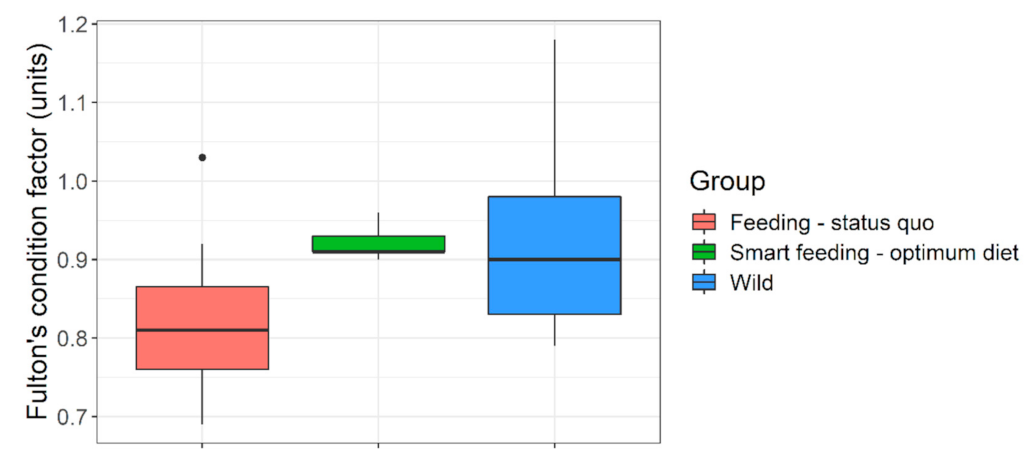
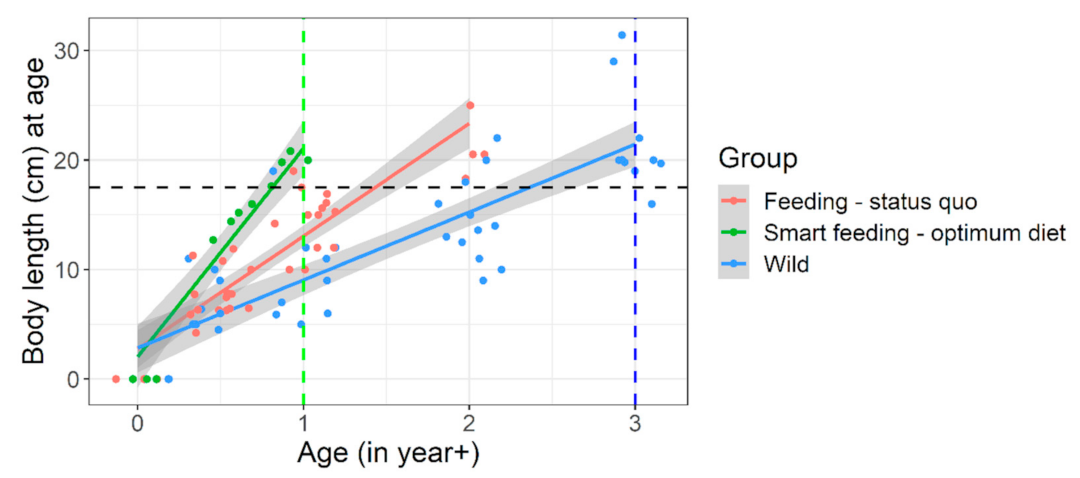

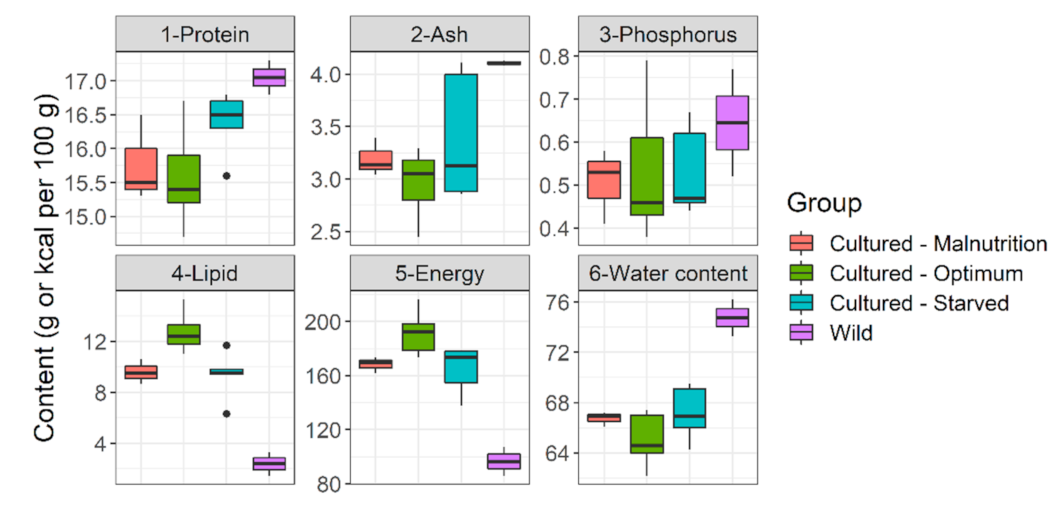

| Parameters | Diet A | Diet B | Diet C | Diet D |
|---|---|---|---|---|
| Morphometrics | ||||
| Age (years) * | Late 0+ (8+ months) | Late 0+ (8+ months) | Late 0+ (8+ months) | Late 0+ (8+ months) |
| Final TL (cm) | 15.2 ± 1.1 (14.5–16; 7.1%) a | 12.7 ± 1.9 (11.5–14.4; 14.8%) b | 11.3 ± 1.1 (10.7–11.9; 9.7%) c | 10.0 ± 1.1 (9.2–10.8; 10.8%) d |
| Final BW (g) | 34.4 ± 8.1 (29.0–41.7; 23.5%) a | 20.0 ± 9.7 (13.7–23.8; 48.3%) b | 13.3 ± 4.1 (11.0–14.9; 30.7%) c | 8.9 ± 3.0 (7.2–11.2; 33.5%) d |
| CF (units) | 0.96 ± 0.05 a | 0.92 ± 0.10 b | 0.91 ± 0.06 b | 0.86 ± 0.07 c |
| Growth indicators | ||||
| TGC (unit) | 0.77 ± 0.04 a | 0.49 ± 0.06 b | 0.36 ± 0.02 c | 0.23 ± 0.03 d |
| LI (mm day−1) | 0.74 ± 0.03 a | 0.51 ± 0.08 b | 0.35 ± 0.02 c | 0.23 ± 0.04 d |
| Yield (kg m−3 day−1) | 0.23 ± 0.01 a | 0.15 ± 0.03 b | 0.08 ± 0.016 c | 0.04 ± 0.007 d |
| Feed utilization | ||||
| Protein retention (%) | 39.2 ± 0.6 a | 26 ± 1.6 a,b | 22.6 ± 0.7 b | 23.3 ± 1.9 b |
| Lipid retention (%) | 117.2 ± 3 a,b,# | 95.5 ± 5.7 b,# | 169.4 ± 2.7 a,# | 77.4 ± 8.2 b |
| Phosphorus retention (%) | 53.3 ± 2.3 a | 35.5 ± 3.8 b | 22.3 ± 1.7 c | 32.2 ± 3 b |
| Physiological performance markers | ||||
| PER | 0.80 ± 0.01 a | 0.65 ± 0.04 b | 0.59 ± 0.01 b,c | 0.52 ± 0.04 c |
| P: N retention ratio | 274.3 ± 8.0 a | 200.5 ± 9.4 b | 149.3 ± 15.4 c | 217.2 ± 18.8 b |
| NPE: GE retention ratio | 0.63 ± 0.01 a | 0.71 ± 0.01 b,c | 0.74 ± 0.01 b | 0.65 ± 0.01 a,c |
| Feed economics | ||||
| Feed cost per kg yield | 9.6€ | 8.4€ | 5.36€ | 6.7€ |
| Parameters | Diet A | Diet B | Diet C | Diet D |
|---|---|---|---|---|
| Morphometrics | ||||
| Age (years) * | Early 1+ (13+ month) | Early 1+ (13+ month) | Early 1+ (13+ month) | Early 1+ (13+ month) |
| Final TL (cm) | 19.8 ± 2.1 (18.7–20.8; 10.5%) a | 17.6 ± 2.5 (16.2–20; 14.5%) b | 16.9 ± 2.9 (16.8–19; 17.3%) b,c | 15.6 ± 2.6 (13.8–17.5; 16.7%) c |
| Final BW (g) | 73 ± 23.1 (57.8–81.3; 31.7%) a | 52.6 ± 20.7 (39.6–68.1; 39.4%) b | 46 ± 24.3 (27.3–57.7; 52.8%) b | 33.7 ± 17.9 (32.2–45.7; 53.1%) c |
| CF (units) | 0.90 ± 0.06 a | 0.91 ± 0.08 a | 0.87 ± 0.06 b | 0.81 ± 0.06 c |
| Growth indicators | ||||
| TGC (unit) | 0.74 ± 0.04 a | 0.49 ± 0.05 b | 0.33 ± 0.03 c | 0.14 ± 0.04 d |
| LI (mm day−1) | 0.81 ± 0.02 a | 0.55 ± 0.03 b | 0.39 ± 0.02 c | 0.21 ± 0.03 d |
| Yield (kg m−3 day−1) | 0.16 ± 0.01 a | 0.10 ± 0.02 b | 0.06 ± 0.013 c | 0.02 ± 0.008 d |
| Feed utilization | ||||
| Protein retention (%) | 36.8 ± 3.8 a | 18.9 ± 4.8 a,b | 11.9 ± 3.4 b,c | 7.9 ± 1.4 c |
| Lipid retention (%) | 121.5 ± 8 a,# | 94.9 ± 12.2 a,# | 169.2 ± 16.6 b,# | 36 ± 7.8 c |
| Phosphorus retention (%) | 56.8 ± 4.3 a | 25.8 ± 7 a,b | 10.4 ± 5.6 b | 15.8 ± 6.3 b |
| Physiological performance markers | ||||
| PER | 0.76 ± 0.06 a | 0.57 ± 0.09 b | 0.44 ± 0.06 b | 0.25 ± 0.05 c |
| P: N retention ratio | 313.4 ± 10.0 a | 200.1 ± 8.1 a | 148.9 ± 23.2 b | 295.8 ± 137.9 a |
| NPE: GE retention ratio | 0.65 ± 0.01 a | 0.78 ± 0.03 b | 0.84 ± 0.03 c | 0.74 ± 0.02 b |
| Feed economics | ||||
| Feed cost per kg yield | 10€ | 9.8€ | 7.2€ | 14.8€ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, K.; Podhorec, P.; Dvorak, P.; Mraz, J. Understanding Nutrition and Metabolism of Threatened, Data-Poor Rheophilic Fishes in Context of Riverine Stocking Success- Barbel as a Model for Major European Drainages? Biology 2021, 10, 1245. https://doi.org/10.3390/biology10121245
Roy K, Podhorec P, Dvorak P, Mraz J. Understanding Nutrition and Metabolism of Threatened, Data-Poor Rheophilic Fishes in Context of Riverine Stocking Success- Barbel as a Model for Major European Drainages? Biology. 2021; 10(12):1245. https://doi.org/10.3390/biology10121245
Chicago/Turabian StyleRoy, Koushik, Peter Podhorec, Petr Dvorak, and Jan Mraz. 2021. "Understanding Nutrition and Metabolism of Threatened, Data-Poor Rheophilic Fishes in Context of Riverine Stocking Success- Barbel as a Model for Major European Drainages?" Biology 10, no. 12: 1245. https://doi.org/10.3390/biology10121245
APA StyleRoy, K., Podhorec, P., Dvorak, P., & Mraz, J. (2021). Understanding Nutrition and Metabolism of Threatened, Data-Poor Rheophilic Fishes in Context of Riverine Stocking Success- Barbel as a Model for Major European Drainages? Biology, 10(12), 1245. https://doi.org/10.3390/biology10121245







