Impact of Cryopreservation on Motile Subpopulations and Tyrosine-Phosphorylated Regions of Ram Spermatozoa during Capacitating Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Cryopreservation
2.2. Sperm Capacitation and Experimental Design
2.3. Estimation of Sperm Motility Variables Using CASA
2.4. Estimation of Mitochondrial Activity, Viability and Protein Tyrosine Phosphorylation by Flow Cytometry
2.5. Statistical Evaluation
3. Results
3.1. Total Sperm Motility, Mitochondrial Activity and Tyrosine Phosphorylation in Different Regions during Capacitating and Non-Capacitating Conditions in Fresh and Frozen-Thawed Spermatozoa
3.2. Characterization of Motile Sperm Subpopulations Present in Fresh and Frozen-Thawed Semen Samples during Capacitating and Non-Capacitating Conditions
3.3. Variations in the Distribution of Motile Sperm Subpopulations throughout the Incubation of Fresh and Frozen-Thawed Ram Spermatozoa
3.4. Relationships between Motile Sperm Subpopulations, Mitochondrial Activity and Tyrosine Phosphorylation in Fresh and Frozen-Thawed Semen Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abaigar, T.; Holt, W.V.; Harrison, R.A.P.; Del Barrio, G. Sperm subpopulations in Boar (Sus scrofa) and Gazelle (Gazella dama mhorr) semen as revealed by pattern analysis of computer-assisted motility assessments. Biol. Reprod. 1999, 60, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Bravo, J.A.; Montanero, J.; Calero, R.; Roy, T.J. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Ile de France rams. Anim. Reprod. Sci. 2011, 129, 22–29. [Google Scholar] [CrossRef]
- Colas, C.; James, P.; Howes, L.; Jones, R.; Cebrian-Perez, J.A.; Muiño-Blanco, T. Cyclic-AMP initiates protein tyrosine phosphorylation independent of cholesterol efflux during ram sperm capacitation. Reprod. Fertil. Dev. 2008, 20, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Martínez, I.; Moran, J.M.; Peña, F.J. A three-step statistical procedure to identify sperm kinematic subpopulations in canine ejaculates: Changes after cryopreservation. Reprod. Domest. Anim. 2006, 41, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.; Pérez-Guzmán, M.D.; Jiménez-Rabadán, P.; Esteso, M.C.; García-Álvarez, O.; Maroto-Morales, A.; Anel-López, L.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J. Sperm cell population dynamics in ram semen during the cryopreservation process. PLoS ONE 2013, 8, e59189. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, O.; Maroto-Morales, A.; Ramón, M.; Del Olmo, E.; Jiménez-Rabadán, P.; Fernández-Santos, M.R.; Anel-López, L.; Garde, J.J.; Soler, A.J. Dynamics of sperm subpopulations based on motility and plasma membrane status in thawed ram spermatozoa incubated under conditions that support in vitro capacitation and fertilisation. Reprod. Fertil. Dev. 2014, 26, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.A.M.M.; Morató, R.; Yeste, M.; Arcarons, N.; Pena, A.I.; Tamargo, C.; Hidalgo, C.O.; Muiño, R.; Mogas, T. Evaluation of sperm subpopulation structure in relation to invitro sperm-oocyte interaction of frozen-thawed semen from Holstein bulls. Theriogenology 2014, 81, 1067–1072. [Google Scholar] [CrossRef]
- Peña, A.I.; Adán, S.; Quintela, L.A.; Becerra, J.J.; Herradón, P.G. Relationship between motile sperm subpopulations identified in frozen-thawed dog semen samples and their ability to bind to the zona pellucida of canine oocytes. Reprod. Domest. Anim. 2018, 53, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ramón, M.; Soler, A.J.; Ortiz, J.A.; García-Alvarez, O.; Maroto-Morales, A.; Roldan, E.R.S.; Garde, J.J. Sperm population structure and male fertility: An intraspecific study of sperm design and velocity in red deer. Biol. Reprod. 2013, 89, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, J.M. Hyperactivated motility patterns of ram spermatozoa recovered from the oviducts of mated ewes. Gamete Res. 1982, 6, 53–63. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. Hyperactivation of mammalian spermatozoa: Function and regulation. Reproduction 2001, 122, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.J.; Vijayaraghavan, S.; Fardilha, M. Signaling mechanisms in mammalian sperm motility. Biol. Reprod. 2017, 96, 2–12. [Google Scholar] [PubMed] [Green Version]
- Suarez, S.S. Interactions of spermatozoa with the female reproductive tract: Inspiration for assisted reproduction. Reprod. Fertil. Dev. 2007, 19, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Balbach, M.; Gervasi, M.G.; Hidalgo, D.M.; Visconti, P.E.; Levin, L.R.; Buck, J. Metabolic changes in mouse sperm during capacitation. Biol. Reprod. 2020, 103, 791–801. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- De Lamirande, E.; San Gabriel, M.C.; Zini, A. Human sperm chromatin undergoes physiological remodeling during in vitro capacitation and acrosome reaction. J. Androl. 2012, 33, 1025–1035. [Google Scholar] [CrossRef]
- Grasa, P.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Signal transduction mechanisms involved in in vitro ram sperm capacitation. Reproduction 2006, 132, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Fu, Q.; Huang, Y.; Zhang, P.; Chen, F.; Li, M.; Xu, Z.; Yao, S.; Chen, D.; Zhang, M. Comparative proteomic identification buffalo spermatozoa during in vitro capacitation. Theriogenology 2019, 126, 303–309. [Google Scholar] [CrossRef]
- Visconti, P.E.; Krapf, D.; De La Vega-Beltrán, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Cesari, A.; Garde, J.J.; Villar, M.; Soler, A.J. Cryopreservation of ram sperm alters the dynamic changes associated with in vitro capacitation. Theriogenology 2020, 145, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Bogle, O.A.; Jodar, M.; Torabi, F.; Delgado-Dueñas, D.; Estanyol, J.M.; Ballescà, J.L.; Miller, D.; Oliva, R. Proteomic changes in human sperm during sequential in vitro capacitation and acrosome reaction. Front. Cell Dev. Biol. 2019, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Pastor, F.; Garcia-Macias, V.; Alvarez, M.; Herraez, P.; Anel, L.; De Paz, P. Sperm subpopulations in Iberian red deer epididymal sperm and their changes through the cryopreservation process. Biol. Reprod. 2005, 72, 316–327. [Google Scholar] [CrossRef]
- Holt, W.V.; Van Look, K.J.W. Concepts in sperm heterogeneity, sperm selection and sperm competition as biological foundations for laboratory test of semen quality. Reproduction 2004, 127, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Escoffier, J.; Navarrete, F.; Haddad, D.; Santi, C.M.; Darszon, A.; Visconti, P.E. Flow Cytometry Analysis Reveals That Only a Subpopulation of Mouse Sperm Undergoes Hyperpolarization During Capacitation. Biol. Reprod. 2015, 92, 1–11. [Google Scholar] [CrossRef]
- Ramió-LLuch, L.; Novell-Fernández, J.M.; Peña, A.; Colás, C.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Ramírez, A.; Concha, I.I.; Rigau, T.; Rodríguez-Gil, J. ‘In vitro’ capacitation and acrosome reaction are concomitant with specific changes in mitochondrial activity in boar sperm: Evidence for a nucleated mitochondrial activation and for the existence of a capacitation-sensitive subpopulational structure. Reprod. Domest. Anim. 2011, 46, 664–673. [Google Scholar] [CrossRef]
- Peña, A.I.; Barrio, M.; Becerra, J.J.; Quintela, L.A.; Herradón, P.G. Motile sperm subpopulations in frozen-thawed dog semen: Changes after incubation in capacitating conditions and relationship with sperm survival after osmotic stress. Anim. Reprod. Sci. 2012, 133, 214–223. [Google Scholar] [CrossRef]
- Ramió, L.; Rivera, M.M.; Ramírez, A.; Concha, I.I.; Peña, A.; Rigau, T.; Rodríguez-Gil, J.E. Dynamics of motile-sperm subpopulation structure in boar ejaculates subjected to “in vitro” capacitation and further “in vitro” acrosome reaction. Theriogenology 2008, 69, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Goodson, S.G.; Zhang, Z.; Tsuruta, J.K.; Wang, W.; O’Brien, D.A. Classification of mouse sperm motility patterns using an automated multiclass support vector machines model. Biol. Reprod. 2011, 84, 1207–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbas, J.P.; Leahy, T.; Horta, A.E.; García-Herreros, M. Sperm kinematics and subpopulational responses during the cryopreservation process in caprine ejaculates. Cryobiology 2018, 82, 137–147. [Google Scholar] [CrossRef]
- Flores, E.; Taberner, E.; Rivera, M.M.; Peña, A.; Rigau, T.; Miró, J.; Rodríguez-Gil, J.E. Effects of freezing/thawing on motile sperm subpopulations of boar and donkey ejaculates. Theriogenology 2008, 70, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Muiño, R.; Tamargo, C.; Hidalgo, C.O.; Peña, A.I. Identification of sperm subpopulations with defined motility characteristics in ejaculates from Holstein bulls: Effects of cryopreservation and between-bull variation. Anim. Reprod. Sci. 2008, 109, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldan, E.R.S. Assessments of sperm quality integrating morphology, swimming patterns, bioenergetics and cell signalling. Theriogenology 2020, 150, 388–395. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing–thawing procedures remodel the proteome of ram sperm before and after in vitro capacitation. Int. J. Mol. Sci. 2019, 20, 4596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peris-Frau, P.; Álvarez-Rodríguez, M.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Garde, J.J.; Villar, M.; Rodríguez-Martínez, H.; Soler, A.J. Unravelling how in vitro capacitation alters ram sperm chromatin before and after cryopreservation. Andrology 2021, 9, 414–425. [Google Scholar] [CrossRef]
- Takahashi, Y.; First, N.L. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992, 37, 963–978. [Google Scholar] [CrossRef]
- Huneau, D.; Crozet, N.; Ahmed-Ali, M. Estrous sheep serum as a potent agent for ovine IVF: Effect on cholesterol efflux from spermatozoa and the acrosome reaction. Theriogenology 1994, 42, 1017–1028. [Google Scholar] [CrossRef]
- García-Álvarez, O.; Maroto-Morales, A.; Jiménez-Rabadán, P.; Ramón, M.; Del Olmo, E.; Iniesta-Cuerda, M.; Anel-López, L.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of different media additives on capacitation of frozen-thawed ram spermatozoa as a potential replacement for estrous sheep serum. Theriogenology 2015, 84, 948–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cognié, A.; Poulin, N.; Locatelli, Y.; Pascal, M. State-of-the-art production, conservation and transfer of in-vitro -produced embryos in small ruminants. Reprod. Fertil. Dev. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Matamoros-Volante, A.; Moreno-Irusta, A.; Torres-Rodriguez, P.; Giojalas, L.; Gervasi, M.G.; Visconti, P.E.; Treviño, C.L. Semi-automatized segmentation method using image-based flow cytometry to study sperm physiology: The case of capacitation-induced tyrosine phosphorylation. Mol. Hum. Reprod. 2018, 24, 64–73. [Google Scholar] [CrossRef]
- Maroto-Morales, A.; Ramón, M.; García-Álvarez, O.; Soler, A.J.; Fernández-Santos, M.R.; Roldan, E.R.S.; Gomendio, M.; Pérez-Guzmán, M.D.; Garde, J.J. Morphometrically-distinct sperm subpopulations defined by a multistep statistical procedure in Ram ejaculates: Intra- and interindividual variation. Theriogenology 2012, 77, 1529–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseeuw, P.J. Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Halkidi, M.; Batistakis, Y.; Vazirgiannis, M. Cluster Validity Methods: Part I. SIGMOD Rec. 2002, 31, 40–45. [Google Scholar] [CrossRef]
- Halkidi, M.; Batistakis, Y.; Vazirgiannis, M. Clustering validity checking methods: Part II. SIGMOD Rec. 2002, 31, 19–27. [Google Scholar] [CrossRef]
- Salvador, S.; Chan, P. Determining the number of clusters/segments in hierarchical clustering/segmentation algorithms. In Proceedings of the 16th IEEE International Conference on Tools with Artificial Intelligence, Boca Raton, FL, USA, 15–17 November 2004; pp. 576–584. [Google Scholar]
- Luna, C.; Colás, C.; Casao, A.; Serrano, E.; Domingo, J.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Ram seminal plasma proteins contribute to sperm capacitation and modulate sperm-zona pellucida interaction. Theriogenology 2015, 83, 670–678. [Google Scholar] [CrossRef]
- Flores, E.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. The degree of resistance to freezing-thawing is related to specific changes in the structures of motile sperm subpopulations and mitochondrial activity in boar spermatozoa. Theriogenology 2009, 72, 784–797. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Bailey, J.L.; Leclerc, P. Cryopreservation affects bovine sperm intracellular parameters associated with capacitation and acrosome exocytosis. Reprod. Fertil. Dev. 2009, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Muiño, R.; Peña, A.I.; Rodríguez, A.; Tamargo, C.; Hidalgo, C.O. Effects of cryopreservation on the motile sperm subpopulations in semen from Asturiana de los Valles bulls. Theriogenology 2009, 72, 860–868. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Martinez, H. Role of the oviduct in sperm capacitation. Theriogenology 2007, 68, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, S.T.; Maxwell, W.M.C. Kinematic definition of ram sperm hyperactivation. Reprod. Fertil. Dev. 1999, 11, 25–30. [Google Scholar] [CrossRef]
- Yeste, M.; Bonet, S.; Rodríguez-Gil, J.E.; Del Álamo, M.M.R. Evaluation of sperm motility with CASA-Mot: Which factors may influence our measurements? Reprod. Fertil. Dev. 2018, 30, 789–798. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. Biomed. Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.; Brito, M.; Mendes, C.; Kawai, G.; Vannucchi, C.; Assumpção, M.; Barnabe, V.; et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reprod. Domest. Anim. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Harayama, H. Roles of intracellular cyclic AMP signal transduction in the capacitation and subsequent hyperactivation of mouse and boar spermatozoa. J. Reprod. Dev. 2013, 59, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Petrunkina, A.M.; Simon, K.; Günzel-Apel, A.R.; Töpfer-Petersen, E. Specific order in the appearance of protein tyrosine phosphorylation patterns is functionally coordinated with dog sperm hyperactivation and capacitation. J. Androl. 2003, 24, 423–437. [Google Scholar] [CrossRef]
- Si, Y.; Okuno, M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol. Reprod. 1999, 61, 240–246. [Google Scholar] [CrossRef]
- Luconi, M.; Porazzi, I.; Ferruzzi, P.; Marchiani, S.; Forti, G.; Baldi, E. Tyrosine phosphorylation of the A Kinase Anchoring Protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol. Reprod. 2005, 72, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.F. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef] [PubMed]
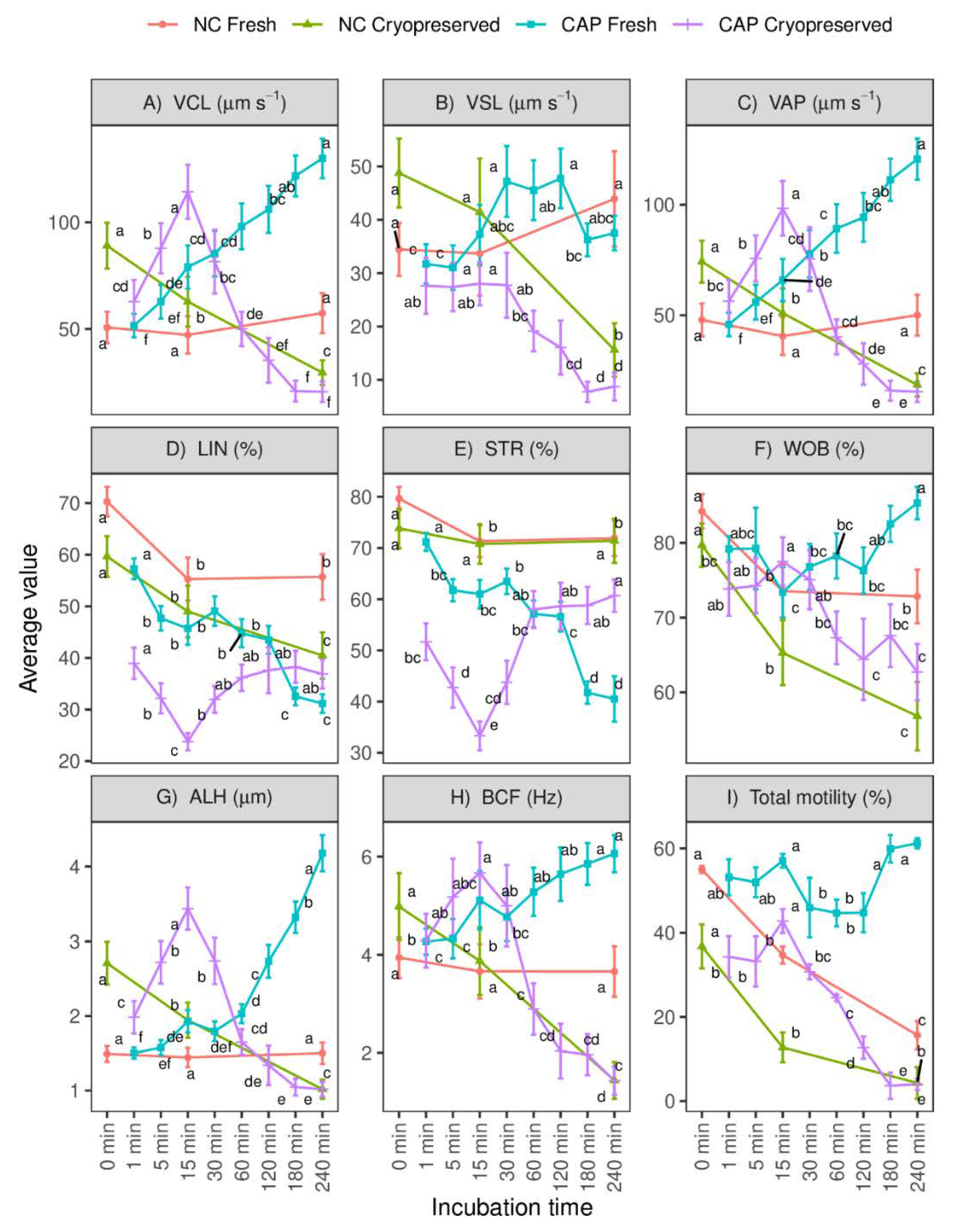
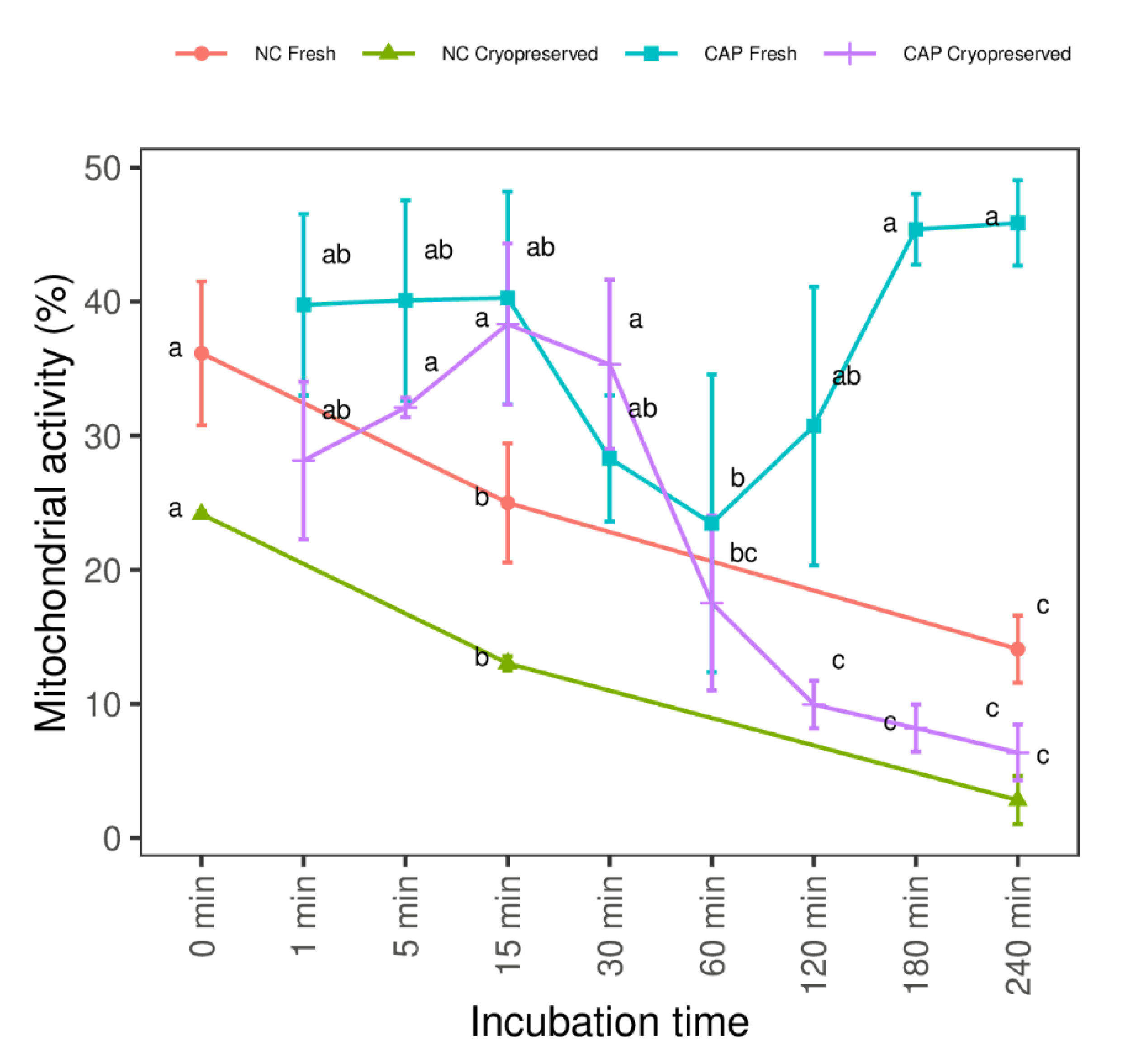
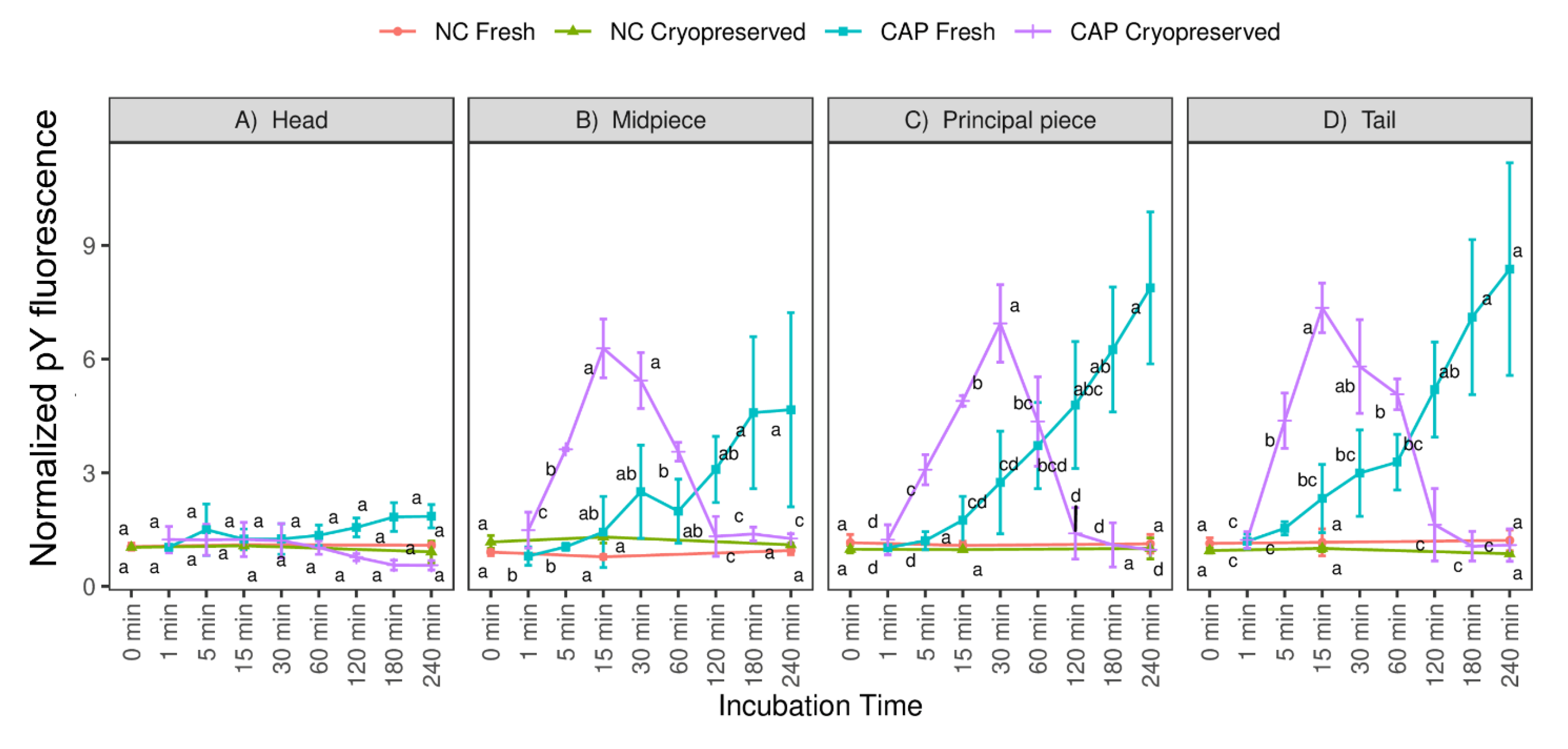

| Kinematic Parameters | Subpopulations | |||
|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | |
| VCL (µm/s) | 26.38 ± 1.77 c | 75.78 ± 1.85 b | 162.12 ± 1.26 a | 183.64 ± 3.50 a |
| VSL (µm/s) | 15.35 ± 1.85 b | 46.45 ± 1.14 ab | 87.91 ± 9.55 c | 54.22 ± 1.05 a |
| VAP (µm/s) | 21.32 ± 2.09 c | 66.49 ± 1.88 b | 151.93 ± 1.64 a | 163.15 ± 3.34 a |
| LIN (%) | 56.45 ± 3.02 a | 44.04 ± 4.56 a | 52.75 ± 3.01 a | 29.49 ± 0.16 b |
| STR (%) | 75.44 ± 1.22 a | 59.79 ± 1.74 a | 58.55 ± 1.84 a | 35.43 ± 0.26 b |
| WOB (%) | 72.78 ± 2.91 b | 74.31 ± 5.78 a,b | 87.00 ± 0.29 a | 90.18 ± 2.63 a |
| ALH (µm) | 1.23 ± 0.02 d | 2.02 ± 0.15 c | 2.91 ± 0.08 b | 5.42 ± 0.08 a |
| BCF (Hz) | 2.56 ± 0.35 b | 5.73 ± 0.88 a | 7.26 ± 0.11 a | 7.83 ± 0.66 a |
| CAP Conditions | Fresh Sperm | Frozen-Thawed Sperm | ||||||
|---|---|---|---|---|---|---|---|---|
| SP1 | SP2 | SP3 | SP4 | SP1 | SP2 | SP3 | SP4 | |
| Mitochondrial activity | −0.18 | −0.60 | 0.83 * | 0.79 * | −0.99 * | −0.24 | 0.63 * | 0.91 * |
| pY head | −0.84 * | −0.90 * | 0.29 | 0.88 * | −0.93 * | −0.12 | 0.59 * | 0.79 * |
| pY midpiece | −0.93 * | −0.91 * | 0.21 | 0.91 * | −0.84 * | −0.56 | 0.40 | 0.90 * |
| pY principal piece | −0.97 * | −0.92 * | 0.21 | 0.93 * | −0.69 * | −0.38 | 0.64 | 0.69 * |
| pY tail | −0.96 * | −0.95 * | 0.26 | 0.96 * | −0.81 * | −0.60 | 0.51 | 0.86 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris-Frau, P.; Sánchez-Ajofrín, I.; Martín Maestro, A.; Maside, C.; Medina-Chávez, D.A.; García-Álvarez, O.; Fernández-Santos, M.d.R.; Montoro, V.; Garde, J.J.; Ramón, M.; et al. Impact of Cryopreservation on Motile Subpopulations and Tyrosine-Phosphorylated Regions of Ram Spermatozoa during Capacitating Conditions. Biology 2021, 10, 1213. https://doi.org/10.3390/biology10111213
Peris-Frau P, Sánchez-Ajofrín I, Martín Maestro A, Maside C, Medina-Chávez DA, García-Álvarez O, Fernández-Santos MdR, Montoro V, Garde JJ, Ramón M, et al. Impact of Cryopreservation on Motile Subpopulations and Tyrosine-Phosphorylated Regions of Ram Spermatozoa during Capacitating Conditions. Biology. 2021; 10(11):1213. https://doi.org/10.3390/biology10111213
Chicago/Turabian StylePeris-Frau, Patricia, Irene Sánchez-Ajofrín, Alicia Martín Maestro, Carolina Maside, Daniela Alejandra Medina-Chávez, Olga García-Álvarez, María del Rocío Fernández-Santos, Vidal Montoro, José Julián Garde, Manuel Ramón, and et al. 2021. "Impact of Cryopreservation on Motile Subpopulations and Tyrosine-Phosphorylated Regions of Ram Spermatozoa during Capacitating Conditions" Biology 10, no. 11: 1213. https://doi.org/10.3390/biology10111213
APA StylePeris-Frau, P., Sánchez-Ajofrín, I., Martín Maestro, A., Maside, C., Medina-Chávez, D. A., García-Álvarez, O., Fernández-Santos, M. d. R., Montoro, V., Garde, J. J., Ramón, M., & Soler, A. J. (2021). Impact of Cryopreservation on Motile Subpopulations and Tyrosine-Phosphorylated Regions of Ram Spermatozoa during Capacitating Conditions. Biology, 10(11), 1213. https://doi.org/10.3390/biology10111213







