The BPH/5 Mouse Model of Superimposed Preeclampsia Is Not a Model of HELLP Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Mouse Experiments
2.3. Clinicopathologic Parameters
2.4. Liver Histology
2.5. Quantitative Reverse Transcription PCR
2.6. Statistical Analyses
3. Results
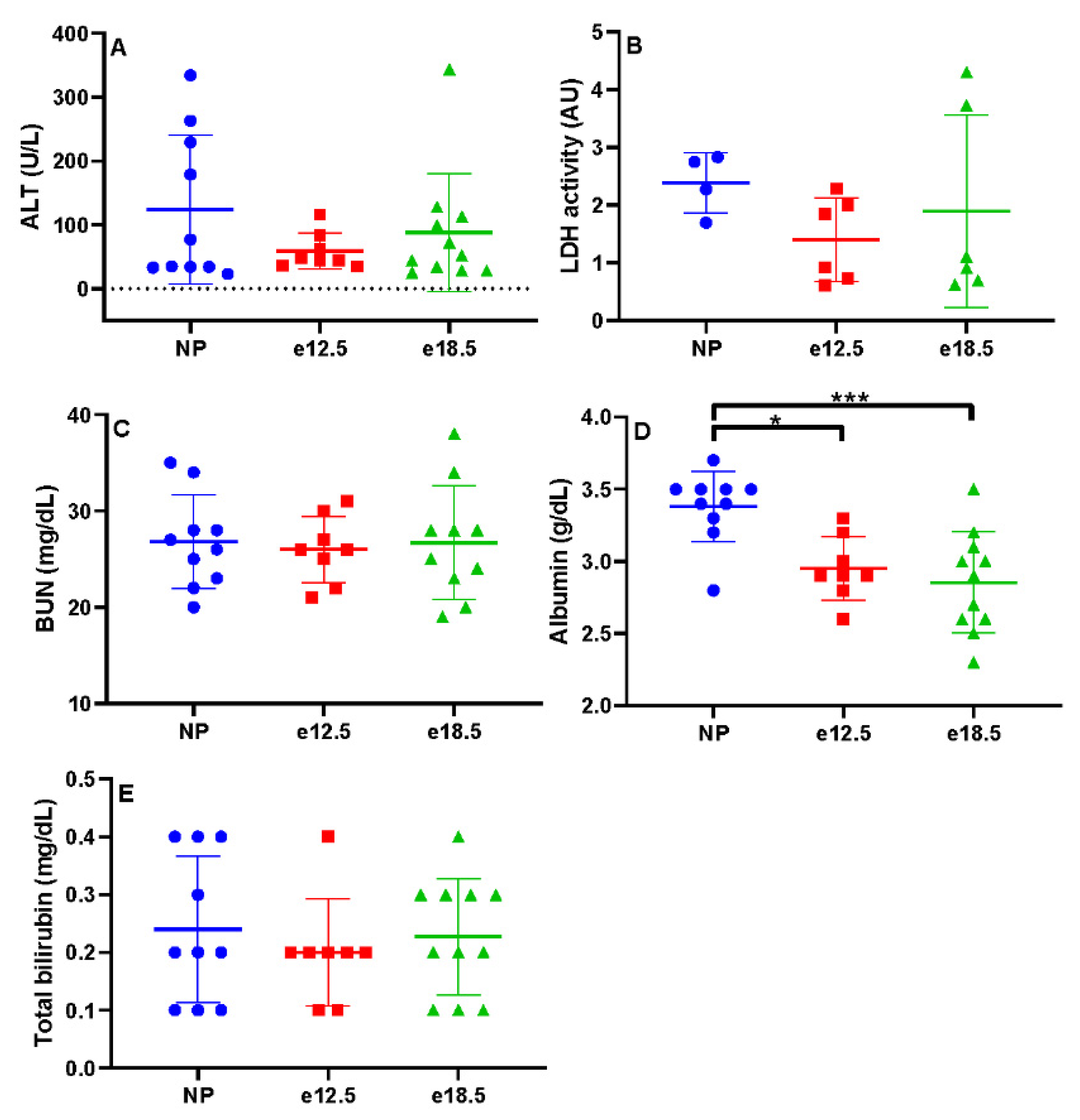
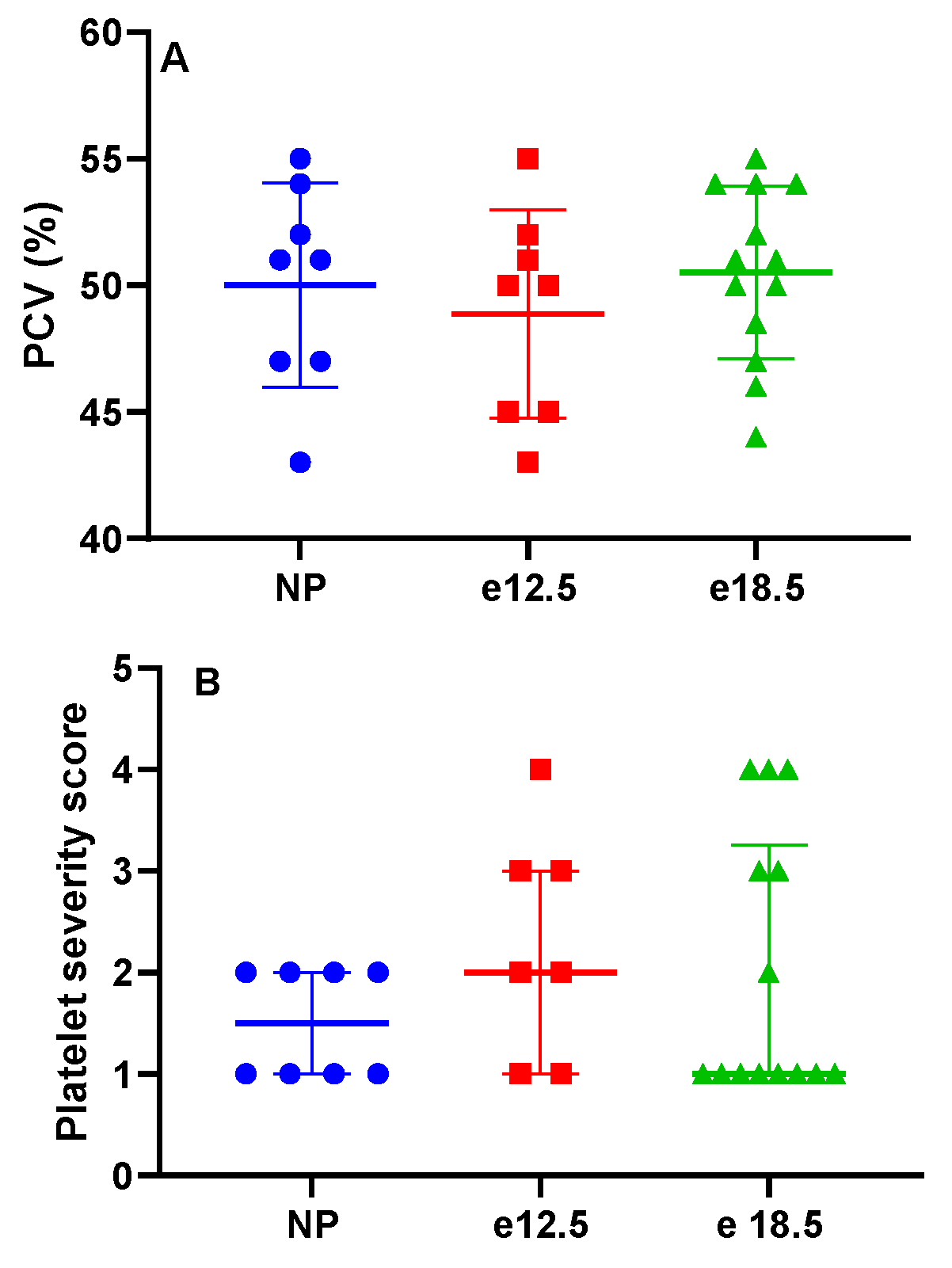
3.1. Clinicopathologic Parameters
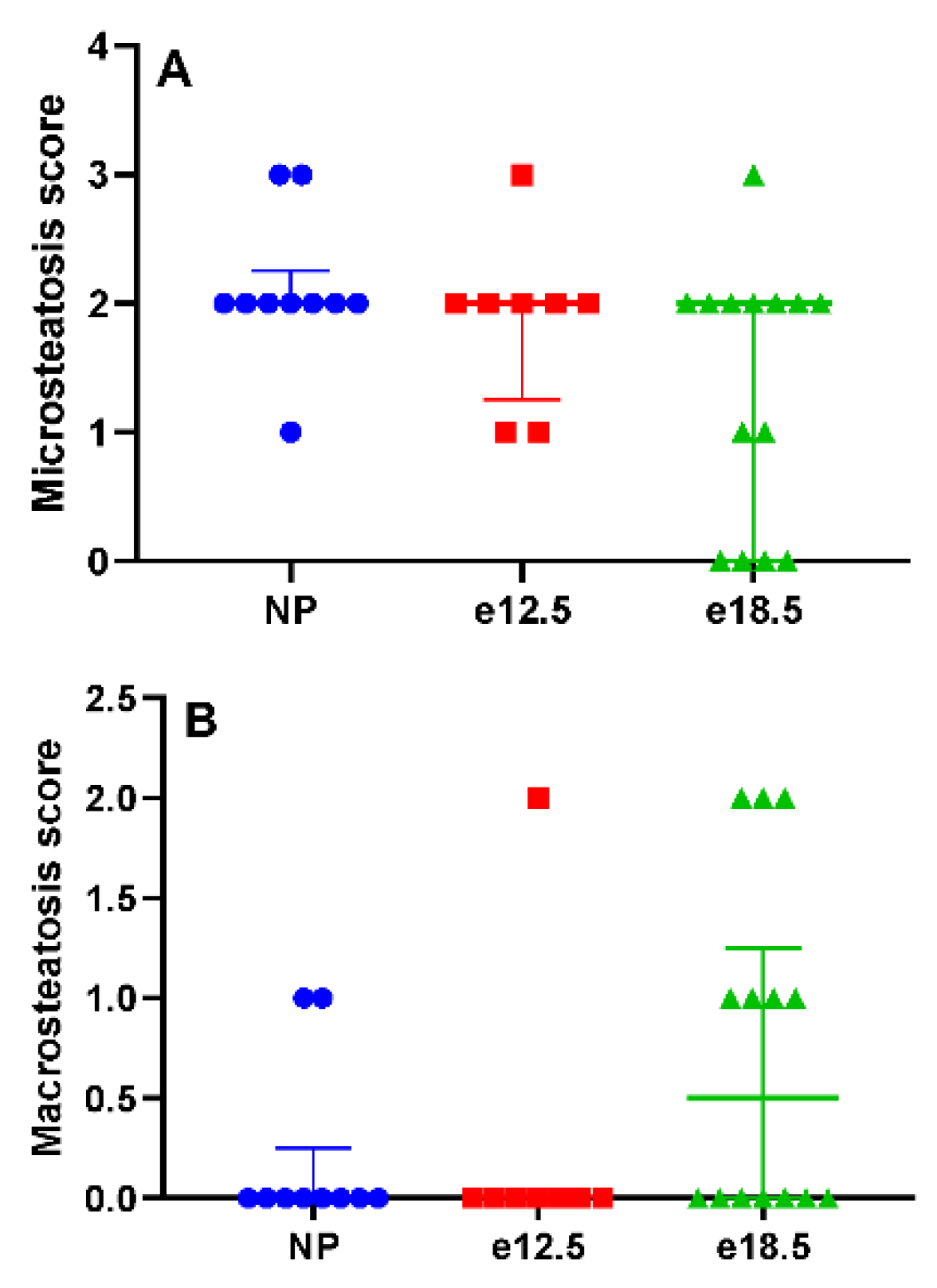
3.2. Liver Histology
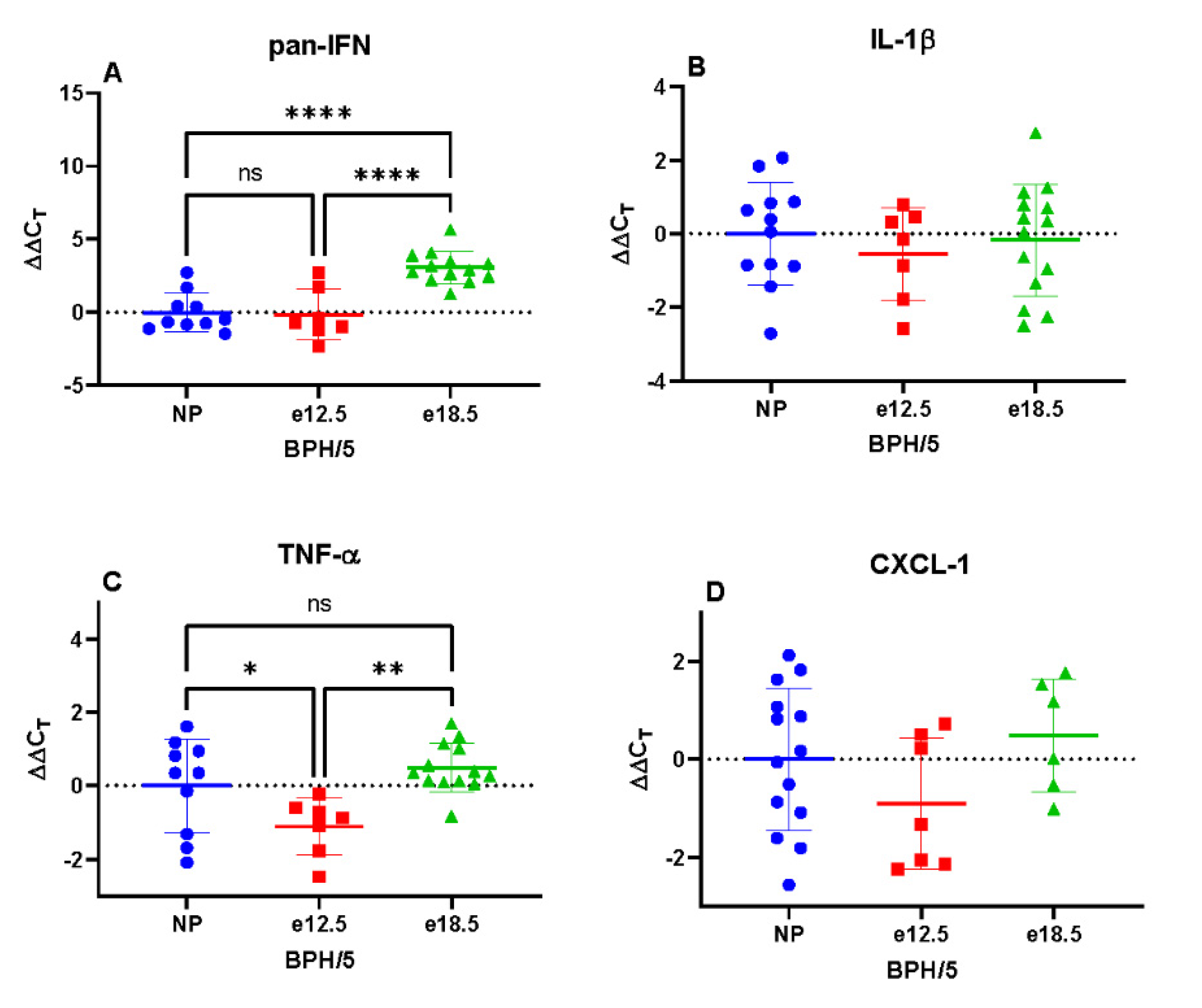
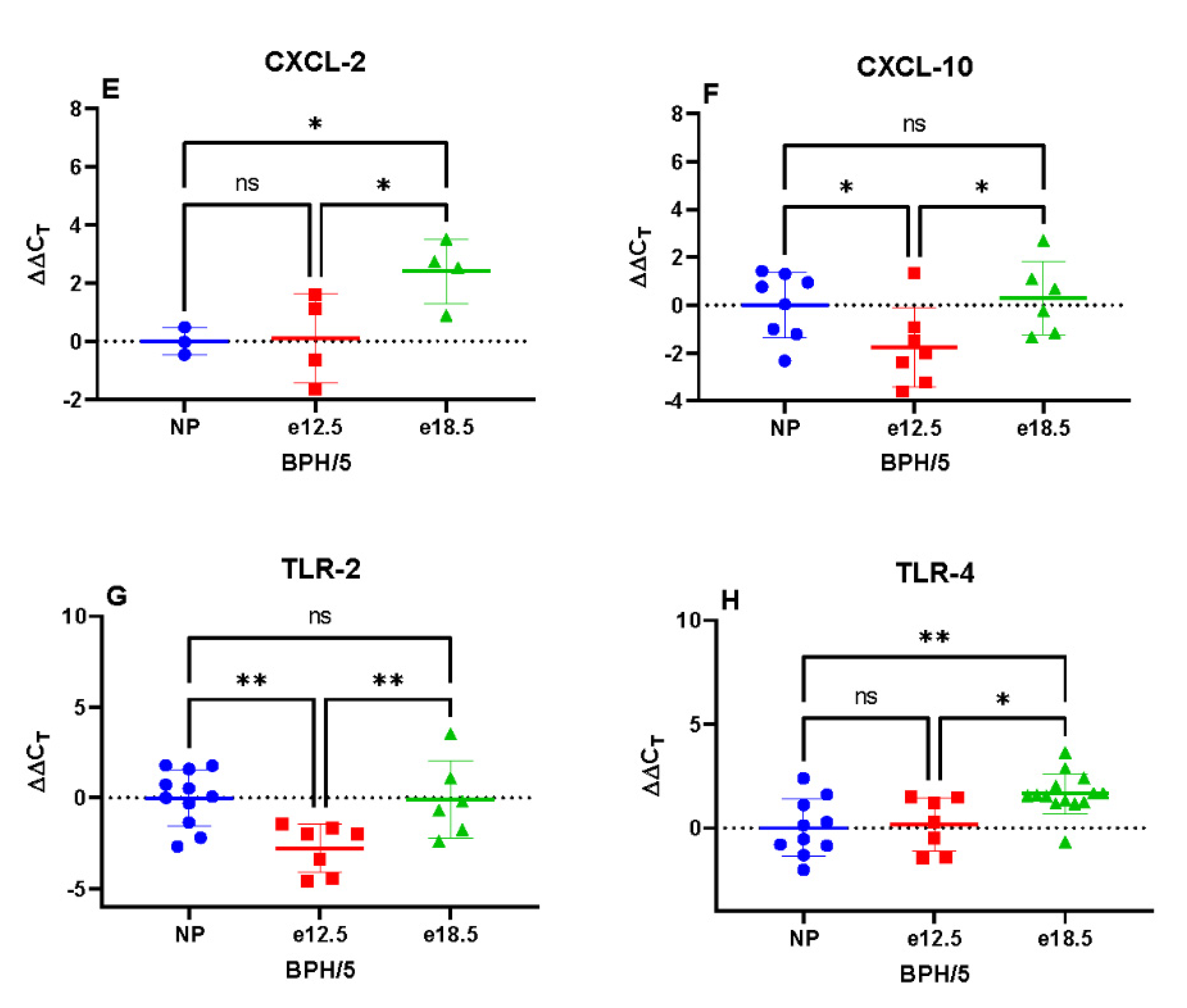
3.3. Transcriptional Markers of Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Primers | qPCR Primer Sequences (5′→3′) |
|---|---|
| Pan Interferon (pan-IFN) | CAACCCTCCTAGACTCATTCTGCA |
| TATTTCCTCACAGCCAGCAG | |
| Interleukin 1 beta (IL-1β) | TCCAGAGTGTGGATCCCAAGCAAT |
| TGTCCTGACCACTGTTGTTTCCCA | |
| Tumor necrosis factor alpha (TNF-α) | AGCCGATGGGTTGTACCTTGTTCTA |
| TGAGATAGCAAATCGGCTGACGGT | |
| C-X-C motif chemokine ligand-1 (CXCL-1) | TGAGCTGCGCTGTCAGTGCCT |
| AGAGCCAGCGTTCACCAGA | |
| C-X-C motif chemokine ligand-2 (CXCL-2) | GCTGGCCACCAACCACCAGG |
| AGCGAGGCACATCAGGTACG | |
| C-X-C motif chemokine ligand-10 (CXCL-10 | ACCATGAACCCAAGTGCTGCCGTC |
| GCTTCACTCCAGTTAAGGAGCCCT | |
| Toll like receptor 2 (TLR-2) | TGCTTTCCTGCTGGAGATTT |
| TGTAACGCAACAGCTTCAGG | |
| Toll like receptor 4 (TLR-4) | ACCTGGCTGGTTTACACGTC |
| CTGCCAGAGACATTGCAGAA | |
| 18s RNA (18s) | GTAACCCGTTGAACCCCATT |
| CCATCCAATCGGTAGTAGCG |
References
- Ghulmiyyah, L.; Sibai, B. Maternal Mortality from Preeclampsia/Eclampsia. Semin. Perinatol. 2012, 36, 56–59. [Google Scholar] [CrossRef]
- Benedetto, C.; Marozio, L.; Tancredi, A.; Picardo, E.; Nardolillo, P.; Tavella, A.M.; Salton, L. Biochemistry of Hellp Syndrome. Adv. Clin. Chem. 2011, 53, 85–104. [Google Scholar] [PubMed]
- Alese, M.O.; Moodley, J.; Naicker, T. Preeclampsia and Hellp Syndrome, the Role of the Liver. J. Matern. Fetal. Neonatal Med. 2019, 34, 1–7. [Google Scholar] [CrossRef]
- Smulian, J.; Shen-Schwarz, S.; Scorza, W.; Kinzler, W.; Vintzileos, A. A Clinicohistopathologic Comparison between Hellp Syndrome and Severe Preeclampsia. J. Matern. Fetal. Neonatal Med. 2004, 16, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Bussen, S.; Sutterlin, M.; Steck, T. Plasma Endothelin and Big Endothelin Levels in Women with Severe Preeclampsia or Hellp-Syndrome. Arch. Gynecol. Obstet. 1999, 262, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hulstein, J.J.; van Runnard Heimel, P.J.; Franx, A.; Lenting, P.J.; Bruinse, H.W.; Silence, K.; de Groot, P.G.; Fijnheer, R. Acute Activation of the Endothelium Results in Increased Levels of Active Von Willebrand Factor in Hemolysis, Elevated Liver Enzymes and Low Platelets (Hellp) Syndrome. J. Thromb. Haemost. 2006, 4, 2569–2575. [Google Scholar] [CrossRef]
- Tranquilli, A.L.; Landi, B.; Corradetti, A.; Giannubilo, S.R.; Sartini, D.; Pozzi, V.; Emanuelli, M. Inflammatory Cytokines Patterns in the Placenta of Pregnancies Complicated by Hellp (Hemolysis, Elevated Liver Enzyme, and Low Platelet) Syndrome. Cytokine 2007, 40, 82–88. [Google Scholar] [CrossRef]
- Khalid, F.; Tonismae, T. Hellp Syndrome. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Wallace, K.; Harris, S.; Addison, A.; Bean, C. Hellp Syndrome: Pathophysiology and Current Therapies. Curr. Pharm. Biotechnol. 2018, 19, 816–826. [Google Scholar] [CrossRef]
- Shen, F.; Wei, J.; Snowise, S.; DeSousa, J.; Stone, P.; Viall, C.; Chen, Q.; Chamley, L. Trophoblast Debris Extruded from Preeclamptic Placentae Activates Endothelial Cells: A Mechanism by Which the Placenta Communicates with the Maternal Endothelium. Placenta 2014, 35, 839–847. [Google Scholar] [CrossRef]
- Haram, K.; Svendsen, E.; Abildgaard, U. The Hellp Syndrome: Clinical Issues and Management. A Review. BMC Pregnancy Childbirth 2009, 9, 8. [Google Scholar] [CrossRef]
- Davisson, R.L.; Hoffmann, D.S.; Butz, G.M.; Aldape, G.; Schlager, G.; Merrill, D.C.; Sethi, S.; Weiss, R.M.; Bates, J.N. Discovery of a Spontaneous Genetic Mouse Model of Preeclampsia. Hypertension 2002, 39, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Heyward, C.Y.; Sones, J.L.; Lob, H.E.; Yuen, L.C.; Abbott, K.E.; Huang, W.; Begun, Z.R.; Butler, S.D.; August, A.; Leifer, C.A.; et al. The Decidua of Preeclamptic-Like Bph/5 Mice Exhibits an Exaggerated Inflammatory Response During Early Pregnancy. J. Reprod. Immunol. 2017, 120, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lacko, L.A.; Massimiani, M.; Sones, J.L.; Hurtado, R.; Salvi, S.; Ferrazzani, S.; Davisson, R.L.; Campagnolo, L.; Stuhlmann, H. Novel Expression of Egfl7 in Placental Trophoblast and Endothelial Cells and Its Implication in Preeclampsia. Mech. Dev. 2014, 133, 163–176. [Google Scholar] [CrossRef]
- Olson, K.N.; Reijnders, D.; Gomes, V.C.L.; Hebert, R.C.; Liu, C.C.; Stephens, J.M.; Redman, L.M.; Douglas, N.C.; Sones, J.L. Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like Bph/5 Mouse Prior to and During Pregnancy. Biology 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, D.; Liu, C.C.; Xu, X.; Zhao, A.M.; Olson, K.N.; Butler, S.D.; Douglas, N.C.; Sones, J.L. Celecoxib Restores Angiogenic Factor Expression at the Maternal-Fetal Interface in the Bph/5 Mouse Model of Preeclampsia. Physiol. Genom. 2018, 50, 385–392. [Google Scholar] [CrossRef]
- Sones, J.L.; Cha, J.; Woods, A.K.; Bartos, A.; Heyward, C.Y.; Lob, H.E.; Isroff, C.E.; Butler, S.D.; Shapiro, S.E.; Dey, S.K.; et al. Decidual Cox2 Inhibition Improves Fetal and Maternal Outcomes in a Preeclampsia-Like Mouse Model. JCI Insight 2016, 1, e75351. [Google Scholar] [CrossRef][Green Version]
- Sones, J.L.; Merriam, A.A.; Seffens, A.; Brown-Grant, D.A.; Butler, S.D.; Zhao, A.M.; Xu, X.; Shawber, C.J.; Grenier, J.K.; Douglas, N.C. Angiogenic Factor Imbalance Precedes Complement Deposition in Placentae of the Bph/5 Model of Preeclampsia. FASEB J. 2018, 32, 2574–2586. [Google Scholar] [CrossRef]
- Hoffmann, D.S.; Weydert, C.J.; Lazartigues, E.; Kutschke, W.J.; Kienzle, M.F.; Leach, J.E.; Sharma, J.A.; Sharma, R.V.; Davisson, R.L. Chronic Tempol Prevents Hypertension, Proteinuria, and Poor Feto-Placental Outcomes in Bph/5 Mouse Model of Preeclampsia. Hypertension 2008, 51, 1058–1065. [Google Scholar] [CrossRef]
- Woods, A.K.; Hoffmann, D.S.; Weydert, C.J.; Butler, S.D.; Zhou, Y.; Sharma, R.V.; Davisson, R.L. Adenoviral Delivery of Vegf121 Early in Pregnancy Prevents Spontaneous Development of Preeclampsia in Bph/5 Mice. Hypertension 2011, 57, 94–102. [Google Scholar] [CrossRef]
- Reijnders, D.; Olson, K.N.; Liu, C.C.; Beckers, K.F.; Ghosh, S.; Redman, L.M.; Sones, J.L. Dyslipidemia and the Role of Adipose Tissue in Early Pregnancy in the Bph/5 Mouse Model for Preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R49–R58. [Google Scholar] [CrossRef]
- Sutton, E.F.; Lob, H.E.; Song, J.; Xia, Y.; Butler, S.; Liu, C.C.; Redman, L.M.; Sones, J.L. Adverse Metabolic Phenotype of Female Offspring Exposed to Preeclampsia in Utero: A Characterization of the Bph/5 Mouse in Postnatal Life. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R485–R491. [Google Scholar] [CrossRef]
- Dokras, A.; Hoffmann, D.S.; Eastvold, J.S.; Kienzle, M.F.; Gruman, L.M.; Kirby, P.A.; Weiss, R.M.; Davisson, R.L. Severe Feto-Placental Abnormalities Precede the Onset of Hypertension and Proteinuria in a Mouse Model of Preeclampsia. Biol. Reprod. 2006, 75, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a General Nafld Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef]
- Young, C.N.; Cao, X.; Guruju, M.R.; Pierce, J.P.; Morgan, D.A.; Wang, G.; Iadecola, C.; Mark, A.L.; Davisson, R.L. Er Stress in the Brain Subfornical Organ Mediates Angiotensin-Dependent Hypertension. J. Clin. Investig. 2012, 122, 3960–3964. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Oe, Y.; Ko, M.; Fushima, T.; Sato, E.; Karumanchi, S.A.; Sato, H.; Sugawara, J.; Ito, S.; Takahashi, N. Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase. Sci. Rep. 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Isler, C.M.; Bennett, W.A.; Rinewalt, A.N.; Cockrell, K.L.; Martin, J.N.; Morrison, J.C.; Granger, J.P. Evaluation of a rat model of preeclampsia for HELLP syndrome characteristics. J. Soc. Gynecol. Investig. 2003, 10, 151–153. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Giuggioli, D.; Ferrannini, E.; Ferri, C.; Fallahi, P. Chemokine (C-X-C Motif) Ligand (Cxcl)10 in Autoimmune Diseases. Autoimmun. Rev. 2014, 13, 272–280. [Google Scholar] [CrossRef]
- Sibai, B.M. The Hellp Syndrome (Hemolysis, Elevated Liver Enzymes, and Low Platelets): Much Ado About Nothing? Am. J. Obstet. Gynecol. 1990, 162, 311–316. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Schenker, J.G. Hellp Syndrome--a Syndrome of Hemolysis, Elevated Liver Enzymes and Low Platelet Count--Complicating Preeclampsia-Eclampsia. Int. J. Gynaecol. Obstet. 1991, 36, 95–102. [Google Scholar] [CrossRef]
- Vinnars, M.T.; Wijnaendts, L.C.; Westgren, M.; Bolte, A.C.; Papadogiannakis, N.; Nasiell, J. Severe Preeclampsia with and without Hellp Differ with Regard to Placental Pathology. Hypertension 2008, 51, 1295–1299. [Google Scholar] [CrossRef]
- Gibbens, J.; Morris, R.; Bowles, T.; Spencer, S.K.; Wallace, K. Dysregulation of the Fas/Fasl System in an Experimental Animal Model of Hellp Syndrome. Pregnancy Hypertens. 2017, 8, 26–30. [Google Scholar] [CrossRef]
- Gibbens, J.; Spencer, S.K.; Solis, L.; Bowles, T.; Kyle, P.B.; Szczepanski, J.L.; Dumas, J.P.; Robinson, R.; Wallace, K. Fas Ligand Neutralization Attenuates Hypertension, Endothelin-1, and Placental Inflammation in an Animal Model of Hellp Syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R195–R202. [Google Scholar] [CrossRef] [PubMed]
- Trottmann, F.; Baumann, M.; Amylidi-Mohr, S.; Surbek, D.; Risch, L.; Mosimann, B.; Raio, L. Angiogenic Profiling in Hellp Syndrome Cases with or without Hypertension and Proteinuria. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 93–96. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnston, A.N.; Batts, T.L.; Langohr, I.M.; Moeller, C.; Liu, C.-C.; Sones, J.L. The BPH/5 Mouse Model of Superimposed Preeclampsia Is Not a Model of HELLP Syndrome. Biology 2021, 10, 1179. https://doi.org/10.3390/biology10111179
Johnston AN, Batts TL, Langohr IM, Moeller C, Liu C-C, Sones JL. The BPH/5 Mouse Model of Superimposed Preeclampsia Is Not a Model of HELLP Syndrome. Biology. 2021; 10(11):1179. https://doi.org/10.3390/biology10111179
Chicago/Turabian StyleJohnston, Andrea N., Tifini L. Batts, Ingeborg M. Langohr, Cambri Moeller, Chin-Chi Liu, and Jennifer L. Sones. 2021. "The BPH/5 Mouse Model of Superimposed Preeclampsia Is Not a Model of HELLP Syndrome" Biology 10, no. 11: 1179. https://doi.org/10.3390/biology10111179
APA StyleJohnston, A. N., Batts, T. L., Langohr, I. M., Moeller, C., Liu, C.-C., & Sones, J. L. (2021). The BPH/5 Mouse Model of Superimposed Preeclampsia Is Not a Model of HELLP Syndrome. Biology, 10(11), 1179. https://doi.org/10.3390/biology10111179







